Abstract
The Cdc25 phosphatase promotes entry into mitosis through the removal of inhibitory phosphorylations on the Cdc2 subunit of the Cdc2/CyclinB complex. During interphase, or after DNA damage, Cdc25 is suppressed by phosphorylation at Ser287 (Xenopus numbering; Ser216 of human Cdc25C) and subsequent binding of the small acidic protein, 14-3-3. As reported recently, at the time of mitotic entry, 14-3-3 protein is removed from Cdc25 and S287 is dephosphorylated by protein phosphatase 1 (PP1). After the initial activation of Cdc25 and consequent derepression of Cdc2/CyclinB, Cdc25 is further activated through a Cdc2-catalyzed positive feedback loop. Although the existence of such a loop has been appreciated for some time, the molecular mechanism for this activation has not been described. We report here that phosphorylation of S285 by Cdc2 greatly enhances recruitment of PP1 to Cdc25, thereby accelerating S287 dephosphorylation and mitotic entry. Moreover, we show that two other previously reported sites of Cdc2-catalyzed phosphorylation on Cdc25 are required for maximal biological activity of Cdc25, but they do not contribute to PP1 regulation and do not act solely through controlling S287 phosphorylation. Therefore, multiple mechanisms, including enhanced recruitment of PP1, are used to promote full activation of Cdc25 at the time of mitotic entry.
INTRODUCTION
Entry into mitosis in all eukaryotic cells is driven by activation of the Cdc2/Cyclin B kinase complex (Solomon et al., 1990; Lew and Kornbluth, 1996). Before entry into mitosis, this kinase complex is held inactive by inhibitory phosphorylations at Thr 14 and Tyr 15 of Cdc2 (Gautier et al., 1991; Mueller et al., 1995a,b). These phosphorylations are catalyzed by the dual-specificity kinase Myt1 and the tyrosine kinase Wee1, residing at intracellular membranes and within the nucleus, respectively (Gould and Nurse, 1989; Parker et al., 1992; Heald et al., 1993; Atherton-Fessler et al., 1994; Kornbluth et al., 1994; Mueller et al., 1995b; Liu et al., 1997). At the end of interphase, activation of the dual (Thr/Tyr) specificity phosphatase Cdc25 leads, in concert with inactivation of Wee1 and Myt1, to the dephosphorylation and activation of Cdc2 (Kumagai and Dunphy, 1992; Borgne and Meijer, 1996; Lew and Kornbluth, 1996).
Interestingly, the activation of Cdc2/Cyclin B is, in part, influenced by its own ability to regulate its regulators in both negative and positive feedback loops (Karaiskou et al., 1998, Xiong and Ferrell, 2003; Pomerening et al., 2003, 2005). In Cdc25, the presence of a Cdc2/Cyclin B–mediated positive feedback activation loop has been suggested to be critical for promoting entry into mitosis (Murray and Kirschner, 1989; Ducommun et al., 1990; Solomon et al., 1990; Gautier and Maller, 1991; Hoffmann et al., 1993; Karaiskou et al., 1998; Stanford and Ruderman, 2005). However, the precise mechanism that drives this positive feedback loop activation has not been fully elucidated.
Before entry into mitosis, Cdc25 is inhibited by several kinases that phosphorylate Serine 287 of Cdc25 (Xenopus numbering; S216 in humans), thereby promoting binding of 14-3-3 protein (Peng et al., 1997; Kumagai et al., 1998b). In response to blocks in replication or DNA damage, DNA-responsive checkpoints are thought to suppress entry into mitosis by promoting the formation and maintenance of the Cdc25/14-3-3 complex (Sanchez et al., 1997; Kumagai et al., 1998b). In support of this view, a mutant variant of Cdc25 that can no longer be phosphorylated at S287 (S287A) cannot bind 14-3-3 and thus promotes rapid entry into mitosis even in the presence of unreplicated or damaged DNA or in the face of elevated Myt1/Wee1 activity (Peng et al., 1997; Kumagai et al., 1998a; Yang et al., 1999; Graves et al., 2001). S287 phosphorylation is dynamically regulated during the cell cycle, but it is sustained by DNA checkpoint activation. Although activation of checkpoint function during normal DNA replication may contribute to Cdc25 suppression, it is clear that other, non-checkpoint kinases (c-Tak1, PKA) also can phosphorylate S287 and may contribute to Cdc25 suppression during normal interphase. Because no other Cdc25 mutant has been identified that so potently activates mitosis, controlling the status of S287 phosphorylation is considered critical for maintaining proper cell cycle regulation of Cdc25.
Recent work from our laboratory demonstrated that suppression of Cdc25 is relieved at the time of mitotic entry by Cdk2-regulated removal of 14-3-3 from S287 followed by PP1-mediated S287 dephosphorylation (Margolis et al., 2003). Specifically, phosphorylation of Thr 138 on Cdc25 (T138), catalyzed by Cdk2, is required for the release of 14-3-3 from phospho-S287, which permits subsequent access of PP1 to its target. Consistent with these observations, mutation of T138 to Ala (T138A) abrogates the ability of Cdc25 to induce mitotic entry. Once active, Cdc2/Cyclin B promotes further phosphorylation of Cdc25 at multiple sites. Indeed, in vitro experiments using purified components have suggested that the catalytic activity of Cdc25 can be enhanced by Cdc2-mediated phosphorylation and that Cdc25 can physically interact with the Cdc2/Cyclin B complex (Russell and Nurse, 1986; Enoch and Nurse, 1990; Izumi and Maller, 1993).
It has recently been reported that Cdc2–Cyclin B–mediated phosphorylation of Cdc25 at serine 285 (S285 Xenopus numbering; S214 in human Cdc25c) prevents rephosphorylation of S287 by checkpoint kinases once cells have entered mitosis (Bulavin et al., 2003a,b). This suggested that one role of Cdc2/Cyclin B was to prevent Cdc25 from being immediately inactivated once mitosis had commenced. In considering these data, we wanted to determine whether S285 phosphorylation might also play some more direct role in promoting the positive feedback activation (rather than maintenance of activity) of Cdc25 at the time of mitotic entry. Interestingly, we have determined that phosphorylation of S285 markedly enhances PP1-mediated S287 dephosphorylation by promoting the increased binding of Cdc25 to PP1. In addition, phosphorylation of S285 by Cdc2/Cyclin B can occur only after 14-3-3 has been released through T138 phosphorylation. Thus, 14-3-3 binding protects phospho-S287 not only by sterically blocking access of PP1 to Cdc25 but also by preventing phospho-S285–mediated recruitment of PP1. We have also found that two additional sites of Cdc2-catalyzed phosphorylation, Thr 48 (T48) and Thr 67 (T67), are required for maximal biological activity of Cdc25, but unlike phospho-S285, these phosphorylated residues do not seem to act solely through modulation of S287 phosphorylation. These data suggest that Cdc2 augments Cdc25 activation by acting on multiple sites, one of which (S285) enables the efficient reversal of Cdc25 suppression mediated by S287 phosphorylation.
MATERIALS AND METHODS
Plasmid Cloning, Site-directed Mutagenesis, and Protein Expression
Full-length WT and N-terminal (1-322) Cdc25 were PCR amplified from the Xenopus Cdc25 cDNA and cloned into pGEX-KG as described previously (Margolis et al., 2003). T48V, T67V, and T138V mutant Cdc25 cDNAs, generously provided by J. Maller (Howard Hughes Medical Institute and Department of Pharmacology, University of Colorado School of Medicine, Denver, CO), were used as templates to generate N-terminal clones in pGEX-KG using primers described in Yang et al. (1999)). S285A and S285D mutant N-terminal Cdc25 proteins were generated using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The primers used for S285A were 5′-CAGAAGCCGCCTTTACCGCGCA CCTTCTATGCCAGAGAAAC-3′ and its complement, and 5′-CAGAAGCCGCCTTTAC CGCGACCCTTCTATGCCAGAGAAAC-3′ and its complement were used to generate S285D.
For oocyte expression, full-length FLAG-tagged wild-type (WT) Cdc25 was cloned into pSP64T as described in Margolis et al. (2003). The QuikChange site-directed mutagenesis kit (Stratagene) was used to generate T48V, T67V, S285A, S287A, P289A, T48V/T67V, T48V/T67V/S287A, S285A/S287A, and S285A/P289A mutant Cdc25 constructs in pSP64T. T48V primers were 5′-CACCGGAACAGCCTTTGGTA CCTGTGACTGACCTTGC-3′ and its complement, and T67V primers were 5′-CCTAAGTACTTTCAGTGGTGAAGTACCCAAACGCTGCCTGGACTTGTCC-3′ and its complement. The T48V/T67V double mutant was generated by sequential mutation using the primers listed above. The primers listed above were used to make the S285A mutant. The S287A and P289A single mutants were produced as described in Margolis et al. (2003). The S285A/S287A and S285A/P289A double mutants were produced using the following primers and their compliments: 5′-CAGAAGCCGCCTTTACCGCGCACCTGCTATGCCAGAGAAAC-3′ and 5′-GCCGCCTTTACCGCGCACCTTCTATGGCAGAGAAACTTGACAG-3′. The T48V/T67V/S287A triple mutant was generated by mutating S287A in the background of the T48V/T67V mutant. mRNA was generated from XbaI-linearized pSP64T clones using the Stratagene mCAP RNA capping kit. Cyclin B1Δ13 was made as described previously (Walsh et al., 2003). Recombinant PP1 and PKA were generous gifts from S. Shenolikar (Pfizer Global Research and Development, Ann Arbor, MI).
Antibodies
Rabbit polyclonal antibody specific for Xenopus Cdc25 phosphorylated at Thr 138 was raised against the Cdc25C phosphopeptide Ac-LPHLLCSpTPSFKKACNH2. Serum from immunized rabbits was used for immunoblotting. Xenopus anti-Cdc2 was produced as described in Walsh et al. (2003). Anti-14-3-3 antibody and anti-PP1 antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and BD Transduction Laboratories (Lexington, KY), respectively. S287 (S216) phospho-specific antibodies were purchased from Cell Signaling Technology (Beverly, CA).
Kinase Assays
The rates of phosphorylation of S285 and S287 were examined by incubating glutathione S-transferase (GST)-Cdc25 protein in interphase egg extracts supplemented with CyclinB1Δ13. Samples were taken at indicated times, washed with egg lysis buffer (ELB; 250 mM sucrose, 2.5 mM MgCl2, 1 mM dithiothreitol [DTT], 50 mM KCl, and 10 mM HEPES, pH 7.7) plus 300 mM NaCl and 0.5% Triton X-100, analyzed by SDS-PAGE and immunoblotted with anti-pS285 or anti-pS287.
The phosphorylation status of T48, T67, T138, S285, and S287 were measured by incubating GST-Cdc25 WT or mutant proteins in interphase extracts or mitotic extracts for 60 min at 4°C. Where indicated, 10 μM okadaic acid (OA) was added to the extracts to inhibit phosphatases. Samples were washed with ELB plus 300 mM NaCl and 0.5% Triton X-100, resolved by SDS-PAGE, and immunoblotted with phospho-specific antibodies.
To generate in vitro phosphorylated GST-Cdc25, recombinant Cdc2/Cyclin B1 (Calbiochem, San Diego, CA) and 2 μg of WT, T138V, or S285A Cdc25 was incubated in kinase buffer (10 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 1 mM DTT, and 100 μM ATP) for 30 min at 30°C. Proteins were retrieved on glutathione-Sepharose and washed with kinase buffer. Proteins were then analyzed by SDS-PAGE and immunoblotting with anti-pS285 antibody.
To assess whether PKA could phosphorylate S287 when S285 was phosphorylated, GST-Cdc25 was incubated with recombinant Cdc2/Cyclin B1 as described above, washed with kinase buffer, and then incubated in fresh kinase buffer supplemented with recombinant PKA and 1 μM OA for 30 min at 37°C. Samples were then washed, resolved by SDS-PAGE, and immunoblotted with anti-pS287 antibody. Alternatively, samples were analyzed by two-dimensional (2D) gel analysis.
2D Electrophoresis
First-Dimension Isoelectrofocusing. Samples were resuspended in 200 μl of rehydration buffer [8.5 M urea, 4% 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate, 2 mM tributyl phosphine, and 0.2% carrier ampholytes [1:1 mixture of pH 4–6 and pH 5–7]) and incubated at room temperature for 30 min with agitation. The beads were pelleted (16,000 × g for 1 min), the supernatants were applied to ReadyStrip IPG strips (11 cm; pH 4–7; Bio-Rad, Hercules, CA), and actively rehydrated at 50 V for 12 h at 20°C. Samples were focused at 250 V for 20 min, gradually ramped up to 8000 V for 2.5 h, and maintained at 8000 V for a total of 35000 V-h per gel. Focused strips were either processed immediately for second dimensional analysis or stored at –80°C.
Second-Dimension SDS-PAGE. Focused ReadyStrips were incubated in 2.5 ml of equilibration buffer (6 M urea, 2% SDS, 0.05 M Tris-HCl, and 20% glycerol) with 2% DTT for 10 min with agitation, followed by a 10-min incubation in equilibration buffer with 2.5% iodoacetamide. Equilibrated strips were rinsed in Tris-glycine SDS running buffer, inserted into the IPG well of a precast 10% Tris-HCl Criterion Gel (Bio-Rad), and covered with low-melting point agarose (0.5%). Gels were run at 200 V for 60 min and prepared for Western transfer.
Sample Preparation and Mass Spectrometry
Cdc25 was phosphorylated in the presence of [γ-32P]ATP with either Cdc2, PKA, or by Cdc2 and PKA sequentially as described above. The phosphorylated proteins were separated by SDS-PAGE and silver stained by standard methods. The radioactive proteins were excised from the gel, treated with trypsin, and tryptic peptides were extracted as described in Graves et al. (2005). The extracted tryptic peptides were separated by reverse phase high-performance liquid chromatography (HPLC) and phosphopeptides identified in column fractions by Cerenkov counting. Radioactive peptides were concentrated and analyzed by electrospray mass spectrometry.
Preparation of Xenopus Oocytes and Extracts
Egg extracts were prepared as described by Smythe and Newport (1991)). Stage VI oocytes were prepared for microinjection as described previously (Walsh et al., 2003). Twenty oocytes were injected with 40 ng of each mRNA and then incubated for 1 h before adding 200 nM leptomycin B. To determine protein expression levels, 10 oocytes were lysed manually in 200 μl of lysis buffer (20 mM HEPES, pH 7.5, 20 mM β-glycerophosphate, 15 mM MgCl2, 20 mM EGTA, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, and 10 μg of aprotonin/leupeptin] and spun at 14,000 × g for 5 min. FLAG-Cdc25 proteins in the supernatant were immunoprecipitated using anti-FLAG M2 Agarose (Sigma-Aldrich, St. Louis, MO), washed with ELB plus 300 mM NaCl and 0.5% Triton X-100, and analyzed by SDS-PAGE and Western blotting.
Coprecipitation and Immunodepletion Experiments
To analyze interactions between WT and mutant Cdc25 proteins with 14-3-3 or PP1, N-terminal GST-Cdc25 proteins were incubated in Xenopus interphase egg extract for 1 h at 4°C. Extracts were moved to room temperature and supplemented with Cyclin B1Δ13 to drive the extracts into mitosis. Samples were taken at indicated times. The GST fusion proteins were retrieved on glutathione-Sepharose, washed with ELB plus 300 mM NaCl and 0.5% Triton X-100, analyzed by SDS-PAGE, and immunoblotted with anti-14-3-3 or anti-PP1.
To analyze the effect of Cdc2 phosphorylation on PP1 binding to Cdc25, phosphorylated GST-Cdc25 was prepared as described above, washed, and then incubated in fresh kinase buffer supplemented with recombinant PP1 and 1 μM OA for 30 min at 37°C. Samples were washed and processed for immunoblotting with anti-PP1 antibody.
To deplete Cdc2 from extract, ultraspun interphase extract was incubated with His-Cyclin B1Δ13 to drive the extract into mitosis. The extract, in the presence of 10 μM OA, was depleted of Cdc2 by three 30-min incubations with polyclonal anti-cdc2 serum coupled to protein A-Sepharose and Ni-NTA agarose to remove the His-Cyclin B1Δ13. Cdc2 and Cdk2 were depleted from mitotic extracts by three 30-min incubations with recombinant His-p13 coupled to CnBr-activated Sepharose beads. GST-Cdc25 proteins were incubated in mock or depleted extracts for 5 min at room temperature or 1 h at 4°C before being retrieved on glutathione-Sepharose, washed, resolved by SDS-PAGE, and immunoblotted with phospho-specific antibodies.
RESULTS
Serine 285 Controls Dephosphorylation of S287
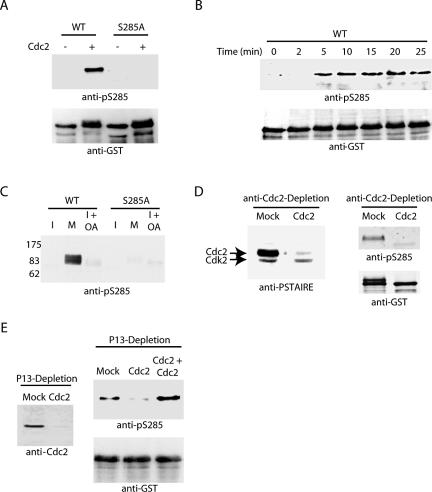
Recently, it was reported that phosphorylation of S285 on Cdc25 contributes to maintenance of the mitotic state by preventing Chk1-mediated rephosphorylation of S287 (Bulavin et al., 2003a,b). They hypothesized that S285 phosphorylation might be a critical point for positive feedback activation of Cdc25 by Cdc2/Cyclin B. To approach this question, we acquired a phospho-specific antibody directed against S285. As shown in Figure 1A, this antibody recognized recombinant Cdc25 only after in vitro phosphorylation by Cdc2/Cyclin B. Moreover, Cdc25 in an interphase extract prepared from Xenopus eggs was not immunoreactive with the anti-pS285 antibody until the extract was driven into mitosis by the addition of recombinant Cyclin B (Figure 1B). However, the inhibition of phosphatases with okadaic acid was not sufficient to promote the phosphorylation of S285 during interphase, suggesting that a mitotic kinase(s) is required for S285 phosphorylation (Figure 1C). Furthermore, depletion of Cdc2/Cyclin B from mitotic egg extracts completely removed kinase activity targeted against S285, whereas readdition of Cdc2/Cyclin B restored it fully (Figure 1, D and E). These data are consistent with previous reports that S285 phosphorylation is catalyzed by Cdc2 and also suggest that Cdc2 is likely to be the only S285-directed kinase in mitotic egg extracts.
Figure 1.
Cdc2/Cyclin B mediates phosphorylation of S285. (A) Recombinant WT and serine 285 to alanine mutant (S285A) N-terminal GST-Cdc25 proteins were incubated in buffer containing ATP with and without recombinant Cdc2/Cyclin B1 for 30 min at 37°C. Samples were collected, washed, and analyzed by SDS-PAGE for immunoblotting with anti-pS285 antibody. Protein loading levels are shown by anti-GST immunoblotting (bottom). (B) N-terminal GST-Cdc25 protein linked to glutathione-Sepharose was added to interphase extract supplemented with nondegradable cyclin. Samples were collected by centrifugation at the indicated times, washed, and analyzed by SDS-PAGE and immunoblotted with anti-pS285 sera and to control for loading, anti-GST sera. (C) Full-length Cdc25 proteins, WT or S285A, were incubated in interphase, mitotic, or interphase extracts treated with OA for 1 h at 4°C. Samples were washed and immunoblotted with anti-pS285. (D and E) Mitotic extract was mock depleted or affinity-depleted of Cdc2 using anti-Cdc2 antibody coupled to protein A-Sepharose (in conjunction with Cyclin B on nickel beads as described in Materials and Methods) or recombinant His-p13 coupled to CnBr-activated Sepharose beads. Recombinant GST-Cdc25 WT linked to glutathione-Sepharose was incubated in mock or Cdc2-depleted extracts, washed, and analyzed by immunoblotting with anti-S285 and anti-GST antibodies.
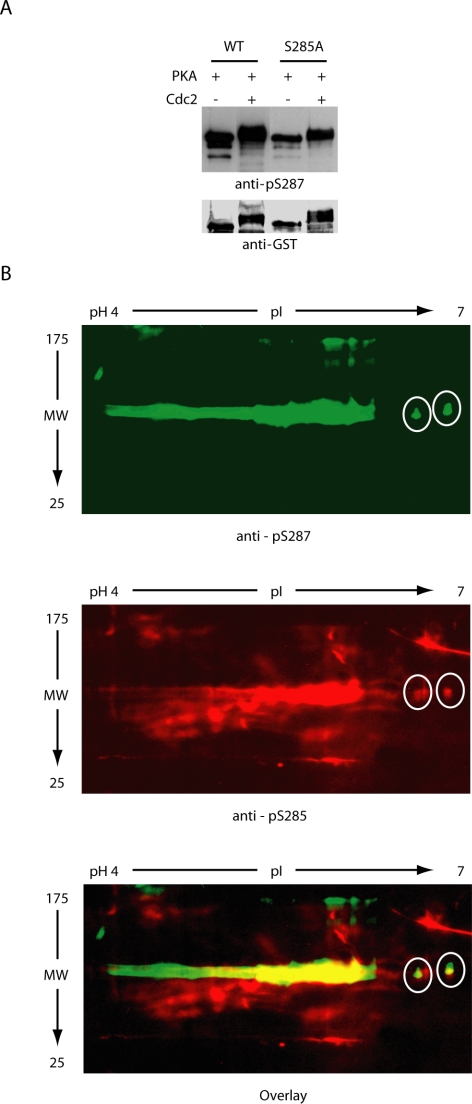
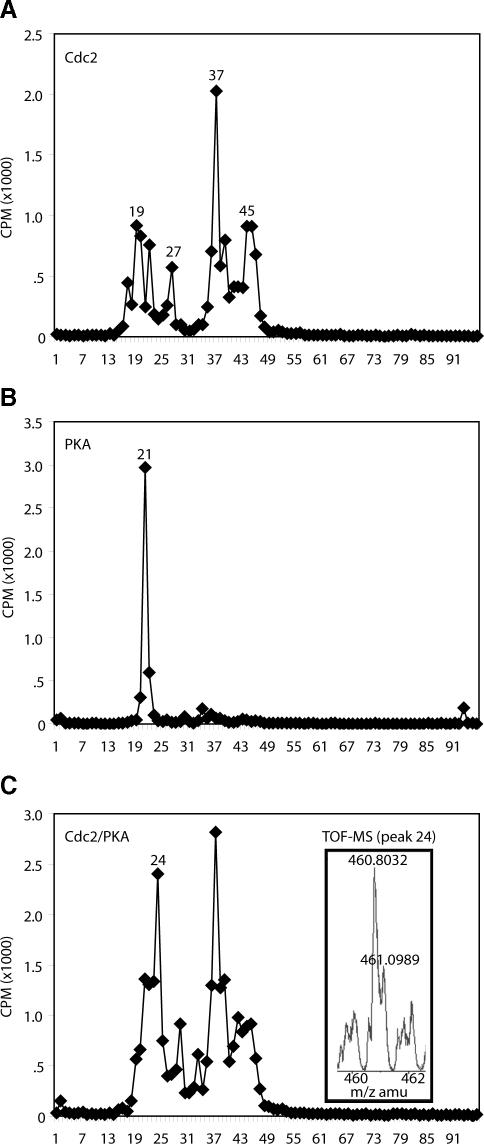
To evaluate the importance of S285 phosphorylation in Cdc25 regulation, we produced a mutant Cdc25 variant in which S285 had been changed to Ala (S285A). It had been reported that Cdc2 prephosphorylation could prevent subsequent in vitro phosphorylation of S287 on wild-type Cdc25 by Chk1, but it was unable to prevent similar Chk1-mediated phosphorylation of the S285A mutant. However, Chk1 is not the only kinase able to phosphorylate S287, and it was not clear whether S285 phosphorylation would prevent phosphorylation by other S287-directed kinases during mitosis (Peng et al., 1998; Bulavin et al., 2001; Duckworth et al., 2002; Isagawa et al., 2005; Manke et al., 2005). Indeed, we found that protein kinase A (PKA), previously reported to phosphorylate S287, could phosphorylate this site in vitro even after prephosphorylation of either wild-type or S285A Cdc25 protein with Cdc2/Cyclin B (Figure 2A). That dual phosphorylated molecules (rather than distinct populations of molecules singly phosphorylated on either site alone) existed after sequential phosphorylation with Cdc2/Cyclin B and PKA was demonstrated by the partially overlapping recognition of Cdc25 species on two-dimensional gels by the pS285 and pS287 antibodies (Figure 2B) as well as by mass spectrometric analyses revealing the presence of phosphopeptides dually phosphorylated on S285 and S287 (Figure 3). This is particularly evident on the far right (pH 7) of the gels, where individual resolved species are recognized by both antibodies (white circles).
Figure 2.
S285 phosphorylation does not prevent S287 rephosphorylation by PKA. (A) WT or S285A N-terminal GST-Cdc25 protein was incubated with or without recombinant Cdc2/Cyclin B1, retrieved on glutathione-beads, washed, and then incubated with recombinant PKA before being resolved by SDS-PAGE and immunoblotted with anti-pS287 antibodies. (B) Cdc25 phosphorylated as described in A was resolved by 2D SDS-PAGE and immunoblotted with either anti-pS285 or anti-pS287 antibodies (gels run in parallel).
Figure 3.
Phosphorylated S285 and S287 can exist on the same Cdc25 molecules. WT N-terminal GST-Cdc25 was phosphorylated with either Cdc2, PKA, or sequentially with Cdc2 and PKA in the presence of [γ-32P]ATP. Samples were separated by SDS-PAGE and silver stained. Radiolabeled proteins excised from the gel were digested with trypsin, and the extracted tryptic peptides were separated by reverse phase HPLC, and phosphopeptides were identified in column fractions by Cerenkov counting. Radioactive peptides were concentrated and analyzed by electrospray mass spectrometry. The radiogram, which has been normalized for protein concentration, shows phosphorylation by Cdc2 gave rise to several phosphopeptides eluting at 19, 27, 37, and 45 min. In contrast, PKA phosphorylated Cdc25 at a single peptide eluting at 21 min. Phosphorylation of the protein with both kinases gave largely a similar phosphopeptide profile as seen with Cdc2 alone, with the exception of a new peak eluting at 24 min. The phosphopeptide identified at 21 min after phosphorylation by PKA alone and the peptide eluting at 19 min after phosphorylation with Cdc2 were notably absent from the profile. These data suggest that the peak eluting at 24 min was the result of phosphorylation of Cdc25 by the protein kinases on a single phosphopeptide fragment. To investigate this further, peptide 24 was analyzed by electrospray ionization mass spectrometry. Analysis of the mass spectra demonstrates a single +3-charged peptide recovered at 460.8032 Da (inset). Theoretical tryptic peptide mass fingerprint analysis of the entire Cdc25 sequence unambiguously identified a single phosphopeptide with the amino acid sequence LYRS*PSM(SO3-)PEK containing two phosphates and an oxidized methionine. This is the only possible peptide that would explain the presence of +3-charged tryptic peptide and mass of 460.8032 Da in the mass spectra. No other phosphopeptide species were identified in spectra. The presence of a miscleavage at the R is consistent with presence of a phosphate at the adjacent Serine. Phosphorylation of serines or threonines next to R or K is known to prevent proteolytic cleavage by trypsin.
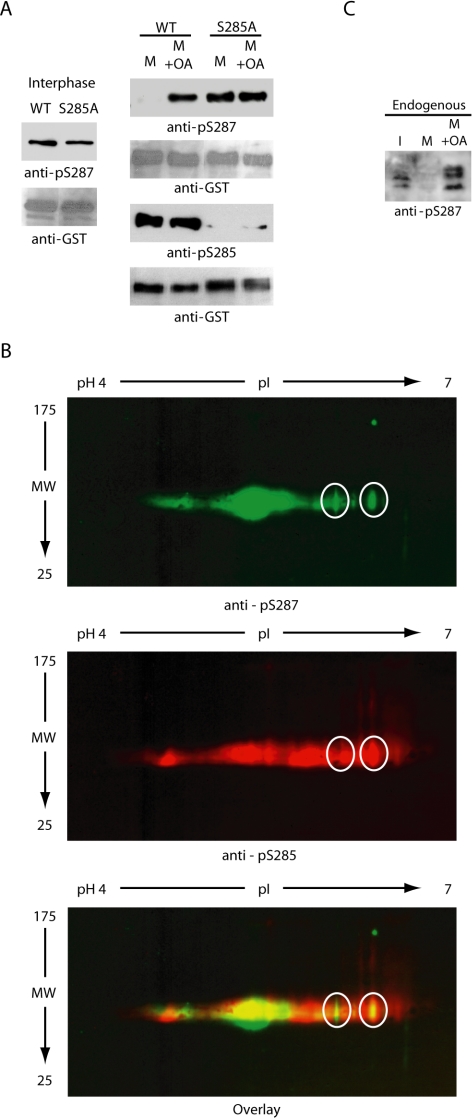
Consistent with the findings of Bulavin et al. (2003a,b), wild-type Cdc25 could not be phosphorylated at S287 upon incubation in mitotic egg extracts, whereas S285A was readily phosphorylated, even though both proteins could be equivalently phosphorylated in an interphase extract (Figure 4A). However, these proteins were equally phosphorylated at S287 in mitotic Xenopus egg extracts treated with okadaic acid to inhibit S287-directed phosphatase activity (Figure 4A). The okadaic acid treatment did not perturb S285 phosphorylation during mitosis, suggesting that Cdc25 could be doubly phosphorylated at S285 and S287 (Figure 4A). To confirm this, we performed two-dimensional gel analysis and immunoblots on GST-Cdc25 retrieved from mitotic extracts treated with okadaic acid to demonstrate, as with the in vitro experiments described above, that individual Cdc25 species could be recognized by both the pS285 and pS287 antibodies (Figure 4B). Similarly S287 phosphorylation was observed when endogenous Cdc25 was analyzed in interphase extracts driven into mitosis and incubated with or without okadaic acid (Figure 4C). Thus, although S285 phosphorylation was reported to inhibit in vitro phosphorylation of S287 by Chk1, it did not preclude phosphorylation of this site in a full egg extract treated with phosphatase inhibitors. Together, these data suggest that the absence of repressive S287 phosphorylation that we and others have observed in the mitotic egg extract did not result from an inability of S287-directed kinases to act on S285-phoshphorylated Cdc25. This raised the question of whether S285 might be regulating Cdc25 in some other manner. In particular, it raised the possibility that S285 might regulate S287 dephosphorylation.
Figure 4.
Phosphatase inhibition induces rephosphorylation of Cdc25 during mitosis. (A) Left, WT and S285A mutant N-terminal GST-Cdc25 proteins linked to glutathione-Sepharose (at levels approximately equal to endogenous Cdc25) were added to interphase egg extracts, incubated at 4°C for 1 h, retrieved by centrifugation, and immunoblotted with anti-phospho-S287 sera. Right, WT and S285A mutant N-terminal GST-Cdc25 proteins linked to glutathione-Sepharose were added to mitotic egg extracts (prepared by incubating an interphase extract with nondegradable Cyclin B) with or without OA and incubated at 4°C for 1 h. Samples were collected, washed, and immunoblotted for phospho-S285 or phospho-S287. (B) WT N-terminal GST-Cdc25 protein was incubated for 1 h in an interphase extract that was driven into mitosis by nondegradable Cyclin B and incubated with OA. Samples were retrieved on glutathione-beads, washed, and analyzed by 2D SDS-PAGE. Gels were run in parallel and blotted for either pS285 or pS287 antibodies. (C) Interphase extract (I) was incubated with nondegradable Cyclin B to push it into mitosis (M) for 30 min and treated with or without OA. Samples from these extracts or interphase extract were resolved by SDS-PAGE and immunoblotted with anti-pS287 antibody to determine the S287 phosphorylation status of endogenous Cdc25.
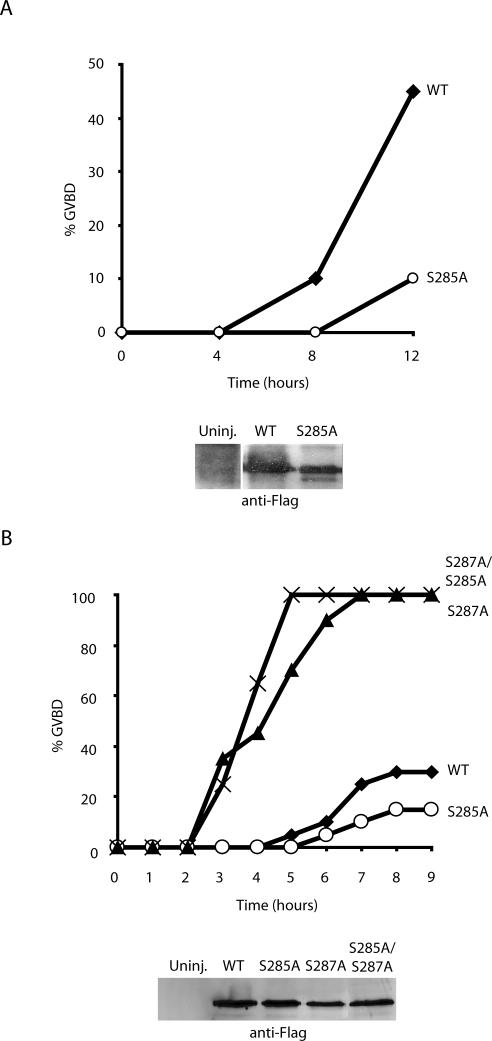
Previous reports had suggested that the effects of S285 phosphorylation were dependent upon the status of S287 phosphorylation (Bulavin et al., 2003a,b). To examine this issue further, we injected G2-arrested Xenopus oocytes with either WT or S285A Cdc25-encoding mRNA (in the presence of leptomycin B to inhibit nuclear export and thereby accelerate the effects of Cdc25). Remarkably, although WT and S285A proteins were produced at comparable levels, S285A was considerably less active in this assay, inducing little detectable M-phase entry (as monitored by breakdown of the oocyte nucleus, or germinal vesicle breakdown (GVBD); Figure 5, A and B). However, when we injected oocytes with S285A, S287A, or S285A/S287A doubly mutant Cdc25, we found that the double mutant promoted M-phase entry with the same kinetics as the S287A mutant alone (Figure 5B). Therefore, the apparently critical requirement for S285 phosphorylation to achieve efficient entry into M phase was bypassed by mutation of S287. Because S285 phosphorylation did not impede S287 phosphorylation in the presence of the full complement of cellular kinases (and phosphatase inhibitors), these data suggested that S285 might control dephosphorylation of S287 at the time of mitotic entry. To address this, we incubated recombinant wild-type or S285A mutant Cdc25 in interphase egg extract driven into mitosis by addition of nondegradable Cyclin B. As shown in Figure 6A, wild-type, but not S285A Cdc25, could be dephosphorylated at S287 by phosphatases in the mitotic egg extract. These data are fully consistent with the idea that S287 dephosphorylation is controlled by Cdc2-mediated S285 phosphorylation.
Figure 5.
Effects of S285 phosphorylation are mediated through S287 phosphorylation. (A and B) Stage VI Xenopus oocytes were injected with mRNA encoding full-length WT, S285A, S287A, or S285A/S287A FLAG-tagged Cdc25 in the presence of 200 nM leptomycin B. Oocytes were monitored for GVBD over time.
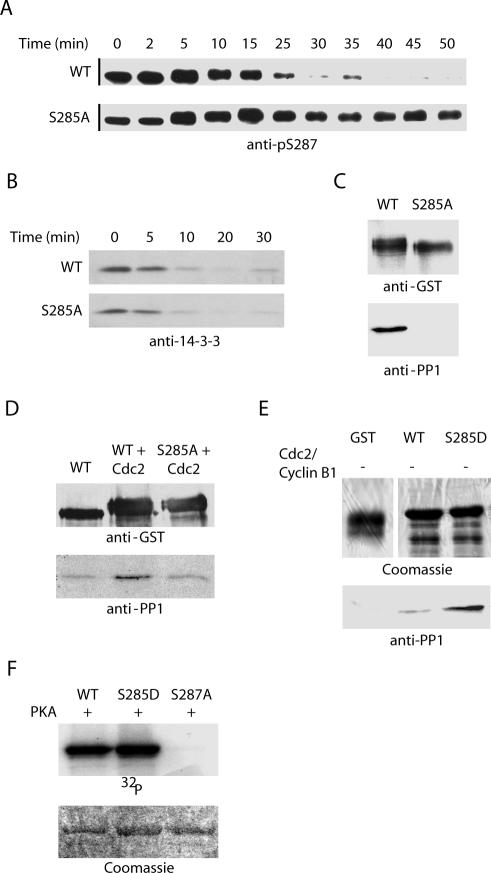
Figure 6.
PP1 binding to Cdc25 is mediated by S285 phosphorylation. (A and B) Recombinant GST-WT and S285A full-length Cdc25 proteins were incubated in interphase egg extract at 4°C for 1 h. Nondegradable cyclin was added, and samples were collected at the indicated times on glutathione-Sepharose, washed, and analyzed by immunoblotting with anti-pS287 or anti-14-3-3 antibodies. (C) WT and S285A full-length GST-Cdc25 proteins were dipped into mitotic egg extracts, retrieved by centrifugation, washed, and immunoblotted with anti-PP1 antibody. (D) WT and S285A N-terminal GST-Cdc25 proteins were incubated with or without recombinant Cdc2 in buffer containing ATP for 30 min at 37°C. Samples were washed and incubated in fresh kinase buffer supplemented with recombinant PP1 before being separated by SDS-PAGE and immunoblotted with anti-PP1 antibody. (E) WT and S285D N-terminal GST-Cdc25 proteins were incubated with recombinant PP1 before being separated by SDS-PAGE and immunoblotted with anti-PP1 antibody. (F) WT and S285D GST-Cdc25 N-terminal proteins were incubated in kinase buffer containing [γ-32P]ATP and recombinant PKA for 30 min at 37°C. Samples were resolved by SDS-PAGE and visualized by autoradiography.
S285 Phosphorylation Is Required for Docking of PP1 to Cdc25
As we reported recently, S287 dephosphorylation upon entry into mitosis is catalyzed by PP1 through direct docking to Cdc25 (Margolis et al., 2003). Moreover, efficient dephosphorylation of this site requires the prior removal of 14-3-3 from phosphorylated S287 to facilitate access of the phosphatase. Because S285A mutant Cdc25 seemed to be refractory to S287 dephosphorylation at mitosis, we suspected that either 14-3-3 removal or PP1-docking to Cdc25 was defective in the absence of Cdc2-mediated S285 phosphorylation. To address this issue, GST-wild type or S285A mutant Cdc25 proteins were incubated in interphase extracts to allow S287 phosphorylation and 14-3-3 binding followed by the addition of Cyclin B to drive the extract into mitosis. As shown in Figure 6B, 14-3-3 removal was unaffected by mutation of S285. However, when we examined docking of PP1 on to Cdc25, we found that the S285A mutant Cdc25 bound much less efficiently than the wild-type protein to PP1 (Figure 6C). In addition, preincubation of recombinant wild-type Cdc25 with Cdc2/Cyclin B enhanced in vitro PP1 binding, whereas preincubation of the S285A mutant protein with active Cdc2/Cyclin B did not (Figure 6D). We believe that the effect of phosphorylation on PP1 binding in Figure 6C is more striking than in 6D because we could achieve a higher stoichiometry of S285 phosphorylation in the egg extracts than in vitro using recombinant Cdc2/Cyclin B. That said, we cannot rule out the possibility that other factors present in the extract, but absent in vitro, synergize with Cdc2 phosphorylation in promoting PP1 recruitment to Cdc25. That phosphorylation at S285 can enhance PP1 recruitment was further suggested by the observation that mutation of S285 to D to mimic phosphorylation enhanced PP1 recruitment relative to wild-type protein, even in the absence of Cdc2/Cyclin B (Figure 6E). As predicted, mutation of S285 to D did not preclude in vitro phosphorylation at S287 by PKA (Figure 6F). Note that this is the only site phosphorylated in vitro by PKA as the S287A mutant was not phosphorylated by PKA in a parallel sample (Figure 6F). These data demonstrate that the role of S285 phosphorylation on Cdc25 is to promote PP1 docking and hence the efficient dephosphorylation of S287 that is required for mitotic entry.
Serine 285 Phosphorylation Requires Prior Phosphorylation of Threonine 138 and Removal of 14-3-3
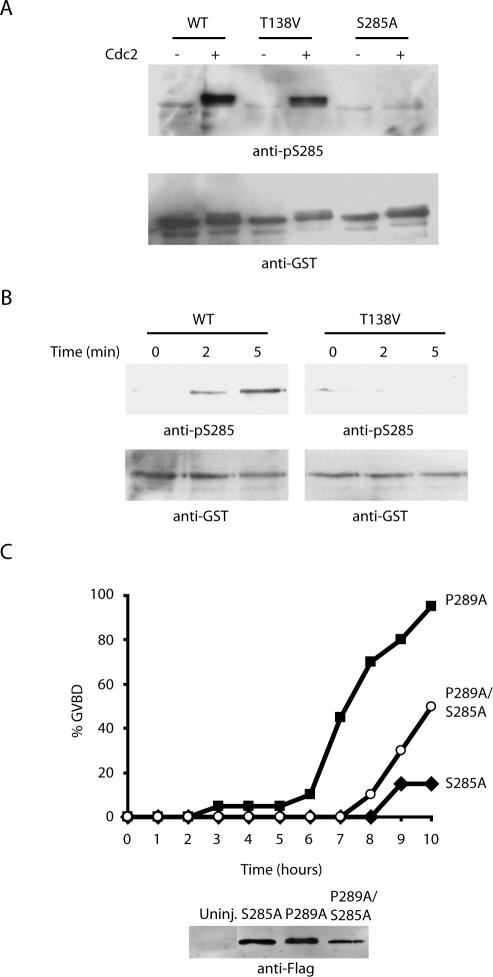
In seeking to place S285 phosphorylation in the ordering of events that occur as Cdc25 is activated at mitosis, we considered our previous data demonstrating that phosphorylation of T138 precedes 14-3-3 removal and dephosphorylation of S287. Because our data indicated that S285 phosphorylation was not required for 14-3-3 removal, yet it was required for PP1 docking, we postulated that S285 phosphorylation might occur between these two events in the activation of Cdc25. Specifically, we wished to determine whether 14-3-3 removal was required for the S285 phosphorylation that would enhance S287 phosphate removal. As we reported previously, mutation of T138 to Val prevents 14-3-3 removal at mitosis. Therefore, we examined the status of S285 phosphorylation in wild-type and T138V mutant Cdc25 proteins. As shown in Figure 7A, recombinant mutant T138V protein was susceptible to S285 phosphorylation using purified Cdc2 in vitro (in the absence of 14-3-3). However, T138V Cdc25 could not be phosphorylated at S285 if it was first incubated with interphase extracts (to acquire bound 14-3-3) and then exposed to active Cdc2 by addition of recombinant Cyclin B to the extracts (Figure 7B). These data suggest that 14-3-3 removal normally precedes S285 phosphorylation by Cdc2, which in turn accelerates PP1 docking, S287 dephosphorylation and consequent Cdc25 activation.
Figure 7.
T138 phosphorylation and 14-3-3 removal precede S285 phosphorylation by Cdc2. (A) Recombinant WT, T138V, and S285A N-terminal GST-Cdc25 proteins were incubated with recombinant Cdc2 in buffer containing ATP for 30 min at 37°C, washed, and analyzed for phosphorylation at S285 with antibody. Proteins were blotted with anti-GST antibody to control for loading. (B) N-terminal GST-Cdc25 WT and T138V proteins were incubated in interphase egg extracts followed by the addition of nondegradable cyclin. Proteins were retrieved from extracts with glutathione-Sepharose and immunoblotted with anti-p285 antibody or anti-GST. (C) Oocytes were injected with mRNA encoding full-length S285A, P289A, or S285A/P289A FLAG-tagged Cdc25 in the presence of 200 nM leptomycin B. Oocytes were monitored for GVBD over time. Protein extracted from injected or uninjected oocytes was resolved by SDS-PAGE and immunoblotted with anti-FLAG antibody to confirm equal levels of protein expression.
As we reported previously, when Cdc25 is mutated at P289 so as to disrupt the 14-3-3 binding consensus sequence without disrupting S287 phosphorylation itself, M phase entry in mRNA-injected oocytes occurs with kinetics faster than that of similarly injected WT Cdc25 mRNA, but slower than that induced by S287A Cdc25 (Margolis and Kornbluth, 2004). This is likely due to the phosphorylation of S287 suppressing Cdc25 in the absence of 14-3-3 binding, but this phosphate is more susceptible to dephosphorylation than in the wild-type context because 14-3-3 binding helps to shield this site from phosphatases. If S285 phosphorylation does indeed function to enhance PP1 recruitment to Cdc25, we reasoned that mutation of S285 ought to decrease the biological activity of the P289 mutant by helping to “protect” the vulnerable S287 phosphate and thereby maintain Cdc25 suppression. Consistent with this idea, the S285A, P289A doubly mutant Cdc25 was considerably less active than P289A mutant Cdc25 in promoting M-phase entry (Figure 7C). The retention of slight activity by this mutant (compared with Cdc25 bearing only the S285A mutation) most likely reflects that Cdc25 unprotected by 14-3-3 is more subject to the action of random phosphatases in the extract and thus could be dephosphorylated to some degree even in the absence of specific PP1 recruitment (see Discussion for details).
Other Sites on Cdc25 Subject to Positive Feedback by Cdc2/Cyclin B Do Not Act Solely through S287
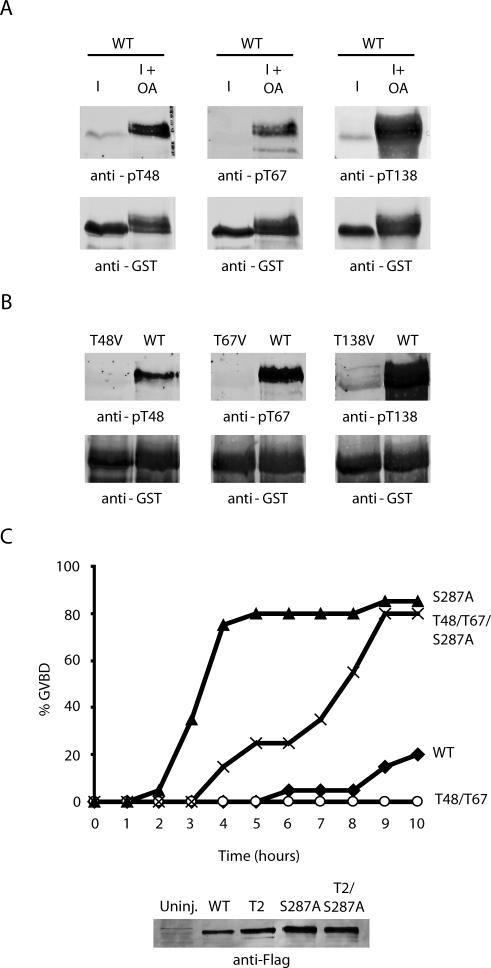
Before identification of S285 as a Cdc2 target, several sites of in vitro Cdc2-mediated Cdc25 phosphorylation, including Thr 48, 67, and 138, were identified by phosphopeptide mapping (Izumi and Maller, 1993). Our more recent analyses have revealed T138 to be a Cdk2 site not dependent upon Cdc2 for its phosphorylation (Margolis et al., 2003). Although we found that phosphorylations at Thr 48 and 67 in mitotic extracts were somewhat diminished by Cdc2 depletion (our unpublished data), we noted that okadaic acid treatment of cycloheximide-treated interphase extracts was able to induce phosphorylation of Thr 48, 67, and 138, but not 285, as detected by immunoblotting with specific phospho-antibodies (Figures 1C and 8A). The specificity of these antibodies is demonstrated in Figure 8B, where GST-Cdc25 WT, T48V, T67V, or T138V mutant proteins were incubated with mitotic extract, retrieved on glutathione-Sepharose, and then assayed for phosphorylation by immunoblotting with the appropriate phospho-antibodies (Figure 8B). Because interphase extracts contain no potentially active Cdc2 (because of the complete absence of Cyclin B), the data in Figure 8A suggest that kinases other than Cdc2 can contribute to phosphorylation of Thr 48, 67, and 138 and that S285 is the only one of these sites entirely dependent upon Cdc2 for its phosphorylation.
Figure 8.
T48 and T67 do not regulate S287 dephosphorylation. (A) Recombinant N-terminal WT GST-Cdc25 protein was incubated in interphase extract treated with or without okadaic acid for 1 h at 4°C. The samples were then washed, analyzed by SDS-PAGE, and immunoblotted with antibodies against pT48, pT67 or pT138. (B) Recombinant WT, T48V, T67V or T138V N-terminal GST-Cdc25 proteins were incubated in mitotic extract for 10 min at room temperature before being retrieved, washed, and immunoblotted with antibodies against pT48, pT67, or pT138. (C) Oocytes were injected with mRNA encoding full-length WT, S287A, T48V/T67V, or T48V/T67V/S287A FLAG-tagged Cdc25 in the presence of 200 nM leptomycin B. Oocytes were monitored for GVBD over time.
As we reported previously, mutation of T138 entirely abrogates Cdc25 biological activity as assayed in oocyte injections because 14-3-3 cannot be removed from Cdc25 and S287 is not dephosphorylated. To perform similar analyses for determining the importance of the T48 and T67 sites, we injected mRNA encoding either WT, T48V/T67V, S287A, or T48V/T67V/S287A Cdc25 into oocytes and monitored entry into M phase. As shown in Figure 8C, the T48/T67 mutant was significantly compromised in its ability to induce M phase. However, in sharp contrast to the results obtained with the S285A mutant protein, mutation of S287 could not fully compensate for the effects of T48/67 mutation (Figure 8C). Indeed, a significant lag in the induction of M phase was noted upon injection of the triple T48/T67/S287A mutant, relative to the rate observed after injection of the S287A mutant alone. These data suggest that T48 and T67 phosphorylation can modulate Cdc25 in a S287-independent manner and suggest that Cdc2 may use at least one alternative mechanism for controlling Cdc25 action in addition to modulating dephosphorylation of S287, consistent with early observations suggesting that Cdc2 could regulate Cdc25 catalytic activity in the absence of other cellular components. In aggregate, our data provide a novel mechanism for Cdc25 regulation by Cdc2 through the enhanced recruitment of PP1 and also suggest that Cdc25 is subject to multiple levels of positive feedback regulation by Cdc2/Cyclin B.
DISCUSSION
Mitotic activation of Cdc2/Cyclin B is, in part, controlled by its ability to phosphorylate and thereby activate or inhibit its own regulators in positive or negative feedback loops. In this study, we have examined the contribution of a previously identified Cdc2 target, S285 of Cdc25, to the positive feedback activation of mitotic entry and have established that this site is a target of Cdc2/Cyclin B feedback activation. Mechanistically, our data suggest that the effect of S285 phosphorylation is to enhance PP1 binding to Cdc25 and thereby promote S287 dephosphorylation. Furthermore, analysis of Cdc25 mutants has demonstrated the critical importance of this positive feedback site in promoting mitotic entry. We also show that additional sites of Cdc2 phosphorylation on Cdc25 contribute to its biological activity, although these sites are likely to act in manner distinct from S285. Indeed, S285 is likely to be the only site important for Cdc2-enhanced PP1 recruitment because mutation of S285 abrogated the ability of Cdc2 to enhance PP1 binding in vitro.
Serine 285 Phosphorylation Is Required for PP1 Binding and Serine 287 Dephosphorylation
Recently, Bulavin et al. (2003a, b) reported that Cdc2/Cyclin B–mediated phosphorylation of S285 blocks Chk1-mediated phosphorylation of S287 in vitro and that S287 phosphorylation is inappropriately high in mitosis when S285 is mutated. Although we, too, have found that S287 is highly phosphorylated when S285 is mutated, we interpret these results in a somewhat different manner. Our data demonstrating that WT Cdc25 could acquire S287 phosphorylation in mitotic extracts treated with okadaic acid, even though S285 was also well-phosphorylated, suggest that the primary effects of S285 phosphorylation may be to control S287 dephosphorylation, rather than rephosphorylation. Indeed, even if S285 phosphorylation prevents rephosphorylation of S287 by Chk1, as proposed by Bulavin et al. (2003a, b) the ability of other kinases to continue acting on S287 even after S285 phosphorylation (as demonstrated by our finding that PKA can phosphorylate S287 regardless of S285 phosphorylation status) makes it likely that the critical role of S285 phosphorylation is to enhance S287 dephosphorylation. Consistent with this interpretation, we showed that S285 phosphorylation did indeed enhance dephosphorylation of previously phosphorylated S287. In addition, the S285A mutant Cdc25 protein bound less well to the S287-directed phosphatase PP1. These data are in full agreement with the hypothesis that S285 phosphorylation plays a direct role in controlling S287 dephosphorylation through the recruitment of PP1.
Serine 285 Phosphorylation Is Controlled by 14-3-3 Removal
Recently, we reported that dephosphorylation of S287 requires the prior removal of 14-3-3, presumably to allow access to PP1 (Margolis et al., 2003). Given the proximity of S285 and S287, we speculated that the S285 site might also be occluded by 14-3-3 binding. In accordance with this, we demonstrated that removal of 14-3-3 both preceded and was required for phosphorylation of S285. Thus, 14-3-3 controls S287 dephosphorylation both because it physically preempts access of PP1 to S287 and because it prevents the enhanced PP1 recruitment that occurs when S285 is phosphorylated. Furthermore, our findings are in contrast to the idea that the importance of S285 phosphorylation at mitosis is to prevent 14-3-3 rebinding, given that 14-3-3 release is unperturbed in the S285 mutant. We note additionally that the Cdc25 protein bearing a mutation at P289, which prevented 14-3-3 binding by disruption of the 14-3-3 consensus site (without impairing S287 phosphorylation) was less impaired than the S285A/P289A double mutant in promoting oocyte GVBD. These findings are consistent with the idea that S285 phosphorylation has effects distinct from 14-3-3 removal because its mutation impairs Cdc25 function, even when 14-3-3 cannot bind, presumably by preventing efficient S287 dephosphorylation. Moreover, these data suggest that S287 phosphorylation alone (in the absence of 14-3-3 binding) must have some suppressive effect on Cdc25 function.
The Ordering of Events in Activation of Cdc25
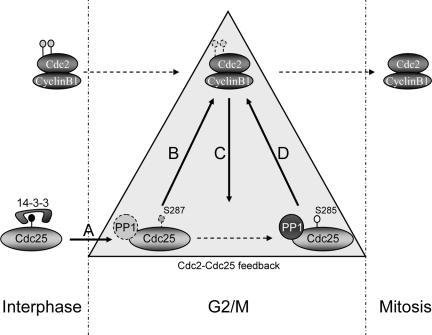
Together with our previously published results, the data presented here suggest a sequence of events that occur to promote full Cdc25 activation (Figure 9). Although some features of this model are still somewhat speculative, we propose that removal of 14-3-3 through a mechanism that has yet to be fully determined (but depends upon T138 phosphorylation) allows the inefficient dephosphorylation and activation of a small amount of Cdc25. That inefficient dephosphorylation can occur in the absence of specific PP1 recruitment once 14-3-3 has been removed is suggested (although not yet fully proven) by the fact that the P289A/S285A mutant protein retained some biological function, and, given a long enough incubation time, the S285A mutant can eventually be dephosphorylated at S287 (our unpublished data). Once activated, Cdc25 can promote dephosphorylation and activation of a proportion of the available Cdc2/Cyclin B, which then goes on to phosphorylate both S285 on Cdc25 as well as contributing to phosphorylation of Thr 48 and 67 (although not T138; we have found that even in vitro T138 phosphorylation is preferentially catalyzed by Cdk2). As reported here, the consequence of S285 phosphorylation is to greatly enhance recruitment of PP1, which accelerates S287 dephosphorylation, further promoting Cdc25 activation. Because Cdc2/Cyclin B can enhance Cdc25 catalytic activity in vitro in the absence of other cellular components, there must be effects of Cdc2 in addition to enhanced dephosphorylation of S287. Consistent with this idea, the effects of mutating Thr 48 and 67 could not be fully rectified by mutation of S287 to Ala.
Figure 9.
A model for activation of Cdc25. (A) During interphase, Cdc25 is phosphorylated on S287 and bound to 14-3-3. At the time of mitotic entry, Thr 138 is phosphorylated and 14-3-3 is released. (B) Based on data presented here, we speculate that PP1 binds weakly to Cdc25 and inefficient dephosphorylation of S287 is allowed after 14-3-3 removal. (C) The small pool of active Cdc25 promotes activation of a small pool of Cdc2/Cyclin B, which would then phosphorylate S285. (D) Once phosphorylated, S285 promotes the recruitment of PP1. We propose that this leads to dephosphorylation of S287 and activation of Cdc25 in a positive feedback loop.
The importance of Cdc2/Cyclin B feedback in promoting full activation of Cdc25 was clearly demonstrated by the mutation of S285, or T48/T67, markedly impairing the ability of Cdc25 to promote M-phase entry. Thus, although 14-3-3 removal from S287 at the time of mitotic entry can set Cdc25 activation in motion, efficient activation of Cdc25 at mitosis relies upon its positive feedback activation by Cdc2/Cyclin B.
Acknowledgments
We thank Shirish Shenolikar for many helpful discussions and members of the Shenolikar laboratory for reagents and advice. We thank Susan Walsh, Ayumi Yamada, Leta Nutt, and Zach Schafer for many helpful discussions and support. This work was supported by National Institutes of Health Grant R01 GM 067225 (to S. K.). S.S.M. is a recipient of the George H. Hitchings Fund for Health Research and Science Education of the Triangle Community Foundation. J.A.P. is a predoctoral fellow of the Breast Cancer Research Program of the U.S. Army Medical Research and Materiel Command. C.D.F. is a postdoctoral fellow of the Ruth L. Kirschstein National Research Service Awards Training Fellowship.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-08-0751) on February 8, 2006.
References
- Atherton-Fessler, S., Liu, F., Gabrielli, B., Lee, M. S., Peng, C.-Y., and Piwnica-Worms-H. (1994). Cell cycle Regulation of the p34cdc2 Inhibitory kinases. Mol. Biol. Cell 5, 989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgne, A., and Meijer, L. (1996). Sequential dephosphorylation of p34(cdc2) on Thr-14 and Tyr-15 at the prophase/metaphase transition. J. Biol. Chem. 271, 27847–27854. [DOI] [PubMed] [Google Scholar]
- Bulavin, D. V., Demidenko, Z. N., Phillips, C., Moody, S. A., and Fornace, A. J., Jr. (2003a). Phosphorylation of Xenopus Cdc25C at Ser285 interferes with ability to activate a DNA damage replication checkpoint in pre-midblastula embryos. Cell Cycle 2, 263–266. [PubMed] [Google Scholar]
- Bulavin, D. V., et al. (2003b). Dual phosphorylation controls Cdc25 phosphatases and mitotic entry. Nat. Cell Biol. 5, 545–551. [DOI] [PubMed] [Google Scholar]
- Bulavin, D. V., Higashimoto, Y., Popoff, I. J., Gaarde, W. A., Basrur, V., Potapova, O., Appella, E., and Fornace, A. J., Jr. (2001). Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature 411, 102–107. [DOI] [PubMed] [Google Scholar]
- Duckworth, B. C., Weaver, J. S., and Ruderman, J. V. (2002). G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc. Natl. Acad. Sci. USA 99, 16794–16799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducommun, B., Draetta, G., Young, P., and Beach, D. (1990). Fission yeast cdc25 is a cell-cycle regulated protein. Biochem. Biophys. Res. Commun. 167, 301–309. [DOI] [PubMed] [Google Scholar]
- Enoch, T., and Nurse, P. (1990). Mutation of fission yeast cell cycle control genes abolishes dependence of mitosis on DNA replication. Cell 60, 665–673. [DOI] [PubMed] [Google Scholar]
- Gautier, J., and Maller, J. L. (1991). Cyclin B in Xenopus oocytes: implications for the mechanism of pre-MPF activation. EMBO J. 10, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier, J., Solomon, M. J., Booher, R. N., Bazan, J. F., and Kirschner, M. W. (1991). cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell 647, 197–212. [DOI] [PubMed] [Google Scholar]
- Gould, K. L., and Nurse, P. (1989). Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature 342, 39–45. [DOI] [PubMed] [Google Scholar]
- Graves, P. R., Lovly, C. M., Uy, G. L., and Piwnica-Worms, H. (2001). Localization of human Cdc25C is regulated both by nuclear export and 14-3-3 protein binding. Oncogene 20, 1839–1851. [DOI] [PubMed] [Google Scholar]
- Graves, P. R., Winkfield, K. M., and Haystead, T. A. (2005). Regulation of zipper-interacting protein kinase activity in vitro and in vivo by multisite phosphorylation. J. Biol. Chem. 280, 9363–9374. [DOI] [PubMed] [Google Scholar]
- Heald, R., McLoughlin, M., and McKeon, F. (1993). Human wee1 maintains mitotic timing by protecting the nucleus from cytoplasmically activated cdc2 kinase. Cell 74, 463–474. [DOI] [PubMed] [Google Scholar]
- Hoffmann, I., Clarke, P. R., Marcote, M. J., Karsenti, E., and Draetta, G. (1993). Phosphorylation and activation of human cdc25-C by cdc2-cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J. 12, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isagawa, T., Takahashi, M., Kato, T., Jr., Mukai, H., and Ono, Y. (2005). Involvement of protein kinase PKN1 in G2/M delay caused by arsenite. Mol. Carcinog. 43, 1–12. [DOI] [PubMed] [Google Scholar]
- Izumi, T., and Maller, J. L. (1993). Elimination of cdc2 phosphorylation sites in the cdc25 phosphatase blocks initiation of M-phase. Mol. Biol. Cell 4, 1337–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaiskou, A., Cayla, X., Haccard, O., Jessus, C., and Ozon, R. (1998). MPF amplification in Xenopus oocyte extracts depends on a two-step activation of cdc25 phosphatase. Exp. Cell Res. 244, 491–500. [DOI] [PubMed] [Google Scholar]
- Kornbluth, S., Sebastian, B., Hunter, T., and Newport, J. (1994). Membrane localization of the kinase which phosphorylates p34cdc2 on Threonine 14. Mol. Biol. Cell 5, 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai, A., and Dunphy, W. G. (1992). Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell 70, 139–151. [DOI] [PubMed] [Google Scholar]
- Kumagai, A., Guo, Z., Emami, K. H., Wang, S. X., and Dunphy, W. G. (1998a). The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J. Cell Biol. 142, 1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai, A., Yakowec, P. S., and Dunphy, W. G. (1998b). 14-3-3 proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts. Mol. Biol. Cell 9, 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew, D. J., and Kornbluth, S. (1996). Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr. Opin. Cell Biol. 8, 795–804. [DOI] [PubMed] [Google Scholar]
- Liu, F., Stanton, J. J., Wu, Z., and Piwnica-Worms, H. (1997). The human Myt1 kinase preferentially phosphorylates cdc2 on threonine 14 and localizes to the endoplasmic reticulum and Golgi. Mol. Cell. Biol. 17, 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manke, I. A., Nguyen, A., Lim, D., Stewart, M. Q., Elia, A. E., and Yaffe, M. B. (2005). MAPKAP kinase-2 is a cell cycle checkpoint kinase that regulates the G2/M transition and S phase progression in response to UV irradiation. Mol. Cell 17, 37–48. [DOI] [PubMed] [Google Scholar]
- Margolis, S. S., and Kornbluth, S. (2004). When the checkpoints have gone: insights into Cdc25 functional activation. Cell Cycle 3, 425–428. [PubMed] [Google Scholar]
- Margolis, S. S., Walsh, S., Weiser, D. C., Yoshida, M., Shenolikar, S., and Kornbluth, S. (2003). PP1 control of M phase entry exerted through 14-3-3-regulated Cdc25 dephosphorylation. EMBO J. 22, 5734–5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, P. R., Coleman, T. R., and Dunphy, W. G. (1995a). Cell cycle regulation of a Xenopus Wee1-like kinase. Mol. Biol. Cell 6, 119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, P. R., Coleman, T. R., Kuamgai, A., and Dunphy, W. G. (1995b). Myt 1, a membrane-associated inhibitory kinase that phosphorylates cdc2 on both threonine-14 and tyrosine-15. Science 270, 86–90. [DOI] [PubMed] [Google Scholar]
- Murray, A., and Kirschner, M. (1989). Cyclin synthesis drives the early embryonic cell cycle. Nature 339, 275–280. [DOI] [PubMed] [Google Scholar]
- Parker, L. L., Atherton-Fessler, S., and Piwnica-Worms, H. (1992). p107wee1 is a dual specificity kinase that phosphorylates p34cdc2 on tyrosine 15. Proc. Natl. Acad. Sci. USA 89, 2917–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, C. Y., Graves, P. R., Ogg, S., Thoma, R. S., Byrnes, M. J., 3rd, Wu, Z., Stephenson, M. T., and Piwnica-Worms, H. (1998). C-TAK1 protein kinase phosphorylates human Cdc25C on serine 216 and promotes 14-3-3 protein binding. Cell Growth Differ. 9, 197–208. [PubMed] [Google Scholar]
- Peng, C. Y., Graves, P. R., Thoma, R. S., Wu, Z., Shaw, A. S., and Piwnica-Worms, H. (1997). Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277, 1501–1505. [DOI] [PubMed] [Google Scholar]
- Pomerening, J. R., Kim, S. Y., and Ferrell, J. E., Jr. (2005). Systems-level dissection of the cell-cycle oscillator: bypassing positive feedback produces damped oscillations. Cell 122, 565–578. [DOI] [PubMed] [Google Scholar]
- Pomerening, J. R., Sontag, E. D., and Ferrell, J. E., Jr. (2003). Building a cell cycle oscillator: hysteresis and bistability in the activation of Cdc2. Nat. Cell Biol. 5, 346–351. [DOI] [PubMed] [Google Scholar]
- Russell, P., and Nurse, P. (1986). cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell 45, 145–153. [DOI] [PubMed] [Google Scholar]
- Sanchez, Y., Wong, C., Thoma, R. S., Richman, R., Wu, Z., Piwnica-Worms, H., and Elledge, S. J. (1997). Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277, 1497–1501. [DOI] [PubMed] [Google Scholar]
- Smythe, C., and Newport, J. W. (1991). Systems for the study of nuclear assembly, DNA replication, and nuclear breakdown in Xenopus laevis egg extracts. Methods Cell Biol. 35, 449–468. [DOI] [PubMed] [Google Scholar]
- Solomon, M., Glotzer, M., Lee, T., Philippe, M., and Kirschner, M. (1990). Cyclin activation of p34cdc2. Cell 63, 1013–1024. [DOI] [PubMed] [Google Scholar]
- Stanford, J. S., and Ruderman, J. V. (2005). Changes in regulatory phosphorylation of Cdc25C Ser287 and Wee1 Ser549 during normal cell cycle progression and checkpoint arrests. Mol. Biol. Cell 16, 5749–5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, S., Margolis, S. S., and Kornbluth, S. (2003). Phosphorylation of the cyclin b1 cytoplasmic retention sequence by mitogen-activated protein kinase and Plx. Mol. Cancer Res. 1, 280–289. [PubMed] [Google Scholar]
- Xiong, W., and Ferrell, J. E., Jr. (2003). A positive-feedback-based bistable `memory module' that governs a cell fate decision. Nature 426, 460–465. [DOI] [PubMed] [Google Scholar]
- Yang, J., Winkler, K., Yoshida, M., and Kornbluth, S. (1999). Maintenance of G2 arrest in the Xenopus oocyte: a role for 14-3-3-mediated inhibition of Cdc25 nuclear import. EMBO J. 18, 2174–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]