Abstract
The transcription factor NFATc1 may be involved in slow skeletal muscle gene expression. NFATc1 translocates from cytoplasm to nuclei during slow fiber type electrical stimulation of skeletal muscle fibers because of activation of the Ca2+-dependent phosphatase calcineurin, resulting in nuclear factor of activated T-cells (NFAT) dephosphorylation and consequent exposure of its nuclear localization signal. Here, we find that unstimulated adult skeletal muscle fibers exhibit a previously unanticipated nucleocytoplasmic shuttling of NFATc1 without appreciable nuclear accumulation. In resting fibers, the nuclear export inhibitor leptomycin B caused nuclear accumulation of NFATc1 (but not of isoform NFATc3) and formation of NFATc1 intranuclear bodies independent of calcineurin. The rate of nuclear uptake of NFATc1 was 4.6 times lower in resting fibers exposed to leptomycin B than during electrical stimulation. Inhibitors of glycogen synthase kinase and protein kinase A or of casein kinase 1 slowed the decay of nuclear NFATc1 after electrical stimulation, but they did not cause NFATc1 nuclear uptake in unstimulated fibers. We propose that two nuclear translocation pathways, one pathway mediated by calcineurin activation and NFAT dephosphorylation and the other pathway independent of calcineurin and possibly independent of NFAT dephosphorylation, determine the distribution of NFATc1 between cytoplasm and nuclei in adult skeletal muscle.
INTRODUCTION
Nuclear factor of activated T-cells (NFAT) was originally identified as an inducible nuclear transcription factor that could bind to the interleukin-2 promoter in activated T-cells (Shaw et al., 1988). NFAT is a multigene family containing five members: NFAT1 (NFATp or NFATc2), NFAT2 (NFATc or NFATc1), NFAT3 (NFATc4), NFAT4 (NFATc3 or NFATx), and NFAT5. Except for NFAT5, which is activated in response to osmotic stress (Miyakawa et al., 1999), all NFAT family members are regulated by the calcium-activated protein phosphatase calcineurin (Hogan et al., 2003; Macian, 2005). NFAT possesses nuclear localization sequences (NLSs) and nuclear export signals (NESs) that mediate entry into and exit from the nucleus, respectively. In resting lymphocytes, NFAT is heavily phosphorylated in its regulatory domain, especially in the serine-rich region and the serine-proline–rich repeat regions (Beals et al., 1997a). Phosphorylation of serine residues on NFAT results in intramolecular masking of the NLSs, with consequent cytoplasmic sequestration of the transcription factor. In response to signals that increase intracellular calcium, NFAT is dephosphorylated by the calcium- and calmodulin-dependent phosphatase calcineurin, resulting in exposure of NLSs and nuclear import of the transcription factor. When calcium signaling is terminated, NFAT can be rephosphorylated by several protein kinases such as glycogen synthase kinase-3 (GSK), protein kinase A (PKA), casein kinase 1 (CK1), p38 MAP kinase (p38), and c-Jun NH2-terminal kinase (JNK), which promotes this transcription factor's nuclear exit (Beals et al., 1997b; Dong et al., 1998; Zhu et al., 1998; Sheridan et al., 2002; Braz et al., 2003; Liang et al., 2003; Okamura et al., 2004). NFAT isoforms exit the nucleus via the nuclear export receptor chromosome region maintenance 1 (CRM1; Kehlenbach et al., 1998; Zhu and McKeon, 1999).
We have recently used an in vitro system for maintaining and studying adult rodent fast-twitch skeletal muscle fibers in culture with or without stimulation (Liu and Schneider, 1998). After expressing an NFATc1-green fluorescent protein (GFP) fusion protein delivered by adenovirus infection in dissociated flexor digitorum brevis (FDB) single muscle fibers from adult mouse in culture, we found an activity-dependent cytoplasm-to-nuclear translocation of NFATc1 and focal accumulation in NFATc1 nuclear bodies (Liu et al., 2001). Electrical stimulation with patterns typical of slow-twitch muscle caused a calcineurin-dependent (cyclosporin A-sensitive) appearance of fluorescent foci of NFATc1 in all nuclei. Unexpectedly, very recent studies have indicated that NFAT is also shuttling in resting cells. Wild-type NFATc2 expressed in HeLa or baby hamster kidney (BHK) cells is predominantly cytoplasmic, but treatment with leptomyocin B (LMB), a highly specific inhibitor of CRM1-dependent export (Fukuda et al., 1997), promotes a slow nuclear import of NFATc2 that is independent of dephosphorylation by calcineurin, suggesting that NFATc2 moves between the cytoplasm and nucleus in unstimulated cells (Okamura et al., 2000; Terui et al., 2004). However, it is not known whether the resting shuttling of NFAT is a general phenomenon or whether it is restricted to distinct isoforms or cell types.
We now use cultured adult skeletal muscle fibers and leptomycin B to test the existence of shuttling of NFATc1 in resting muscle and to examine its mechanism of regulation. Previous studies indicated that NFATc1 and NFATc3 are the primary isoforms at the mRNA level expressed in human skeletal muscle (Hoey et al., 1995). NFATc1 may play a role in fast-twitch to slow-twitch fiber transformation (Chin et al., 1998). Here, we demonstrate that NFATc1, but not NFATc3, shuttles between cytoplasm and nuclei under resting conditions. This shuttling is not altered by either the phosphatase (calcineurin) or the kinases (GSK, PKA, and CK1) that strongly influence NFATc1 nuclear translocation in response to electrical stimulation. Electrical stimulation with patterns typical of slow-twitch muscle (Hennig and Lomo, 1985) caused a net cytoplasmic-to-nuclear translocation of both NFATc1-GFP and NFATc3-mRFP. Translocation of NFATc1-GFP and NFATc3-monomeric red fluorescent protein (mRFP) resulting from electrical stimulation was completely blocked by the calcineurin inhibitor cyclosporin A (CsA). Therefore, both calcineurin-dependent and -independent translocation pathways determine the intracellular distribution of NFAT proteins in cultured skeletal muscle fibers.
MATERIALS AND METHODS
Construction of Recombinant Adenoviruses
The expression plasmid of NFATc1 cDNA (NFAT2 isoform α, 716 residues; Rao et al., 1997) was a gift from Dr. Gerald R. Crabtree (Howard Hughes Medical Institute, Stanford, CA). The construction of recombinant adenoviruses of NFATc1-GFP and FLAG-NFATc1 were described in detail previously (Liu et al., 2001). The recombinant adenovirus of human NFATc3-mRFP was based on NFAT4 isoform x (Rao et al., 1997) and was kindly provided by Dr. Luis F. Santana (University of Washington, Seattle, WA). Monomeric red fluorescent protein 1 (mRFP1; Campbell et al., 2002) in which the stop codon was removed was cloned in frame at the N terminus of human NFATc3 (GenBank accession no. NM_173165). These viral constructs were confirmed by sequencing the viral DNA. A recombinant adenovirus encoding untagged human NFATc3 was generously provided by Dr. Jeffery D. Molkentin (Children's Hospital Medical Center, Cincinnati, OH).
Chemicals and Reagents
Leptomycin B was purchased from LC laboratories (Woburn, MA). Cyclosporin A, KT5720, alsterpaullone, SP600125, and SB 202190 were obtained from Sigma-Aldrich (St. Louis, MO). CKI-7 was purchased from Seikagaku America (Falmouth, MA).
FDB Fiber Preparation and Infection with Recombinant Adenoviruses
Single muscle fibers were enzymatically dissociated from FDB muscles of 4- to 5-wk-old CD-1 mice and cultured as described previously (Liu et al., 1997). Isolated fibers were cultured on laminin-coated glass coverslips, each glued over a 10-mm-diameter hole through the center of a plastic Petri dish. Fibers were cultured in minimal essential medium (MEM) containing 10% fetal bovine serum (FBS) and 50 μg/ml gentamicin sulfate in 5% CO2 (37°C). Infection of muscle fiber cultures by recombinant adenoviruses was carried out ∼20 h after the fibers were plated. Before infection, the FDB cultures were rinsed twice with MEM without serum. The recombinant adenoviruses were added to the culture dishes with MEM without serum. The cultures were kept in the 5% CO2 incubator for 1 h, and the medium was then changed to MEM with FBS and gentamicin for continuous culture (Liu et al., 2001).
Microscopy, Image Acquisition, and Fiber Treatment
Approximately 48 h after viral infection, culture medium was changed to Ringer's solution (135 mM NaCl, 4 mM KCl, 1 mM MgCl2, 10 mM HEPES, 10 mM glucose, and 1.8 mM CaCl2, pH 7.4). The culture chamber was mounted on an Olympus IX70 inverted microscope equipped with an Olympus FluoView 500 laser scanning confocal imaging system, using an excitation wavelength of 488 or 543 nm. Fibers were viewed with an Olympus 60×/1.2 numerical aperture water immersion objective and scanned at 3.0× zoom using constant laser power and gain. Each confocal fluorescence image represents an individual optical section. For treatment with chemical reagents, fibers were rinsed with Ringer's solution and were maintained and imaged at room temperature (24 °C) every 10 min. For electrical stimulation, two platinum electrodes connected to a stimulator were placed into the fiber culture chamber to give field stimulation. An appropriate protocol for stimulation was used to successfully induce NFATc1-GFP nuclear translocation, as described in our previous report (Liu et al., 2001). Briefly, fibers were stimulated with 5-s trains of 10-Hz stimuli once every 50 s. The duration of the individual stimulating pulses was 1 ms. The stimulation voltage was adjusted to give microscopically observed fiber contraction in all cases. Most fibers remained attached to the laminin-coated coverslip throughout the period of fiber stimulation, and only such fibers were used to obtain the data reported here. Confocal images were taken at regular intervals before, during, and after stimulation and/or application of chemical reagents.
Analysis of Translocation of NFAT Proteins in Living Fibers
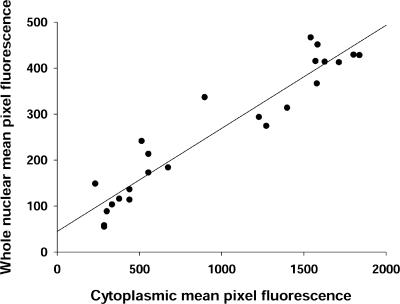
The average fluorescence of pixels for both cytoplasm and nucleus within user-specified areas of interest (AOI) in each image were quantitated using software custom-written in the IDL programming language (Research Systems, Boulder, CO). For cytoplasm, all the fluorescence values for the AOI at each time point were divided (normalized) by the time 0 value. The resulting data gives the relative value of fluorescence of each individual AOI at different time points normalized to the time 0 value in the same AOI. For nucleus, the mean pixel fluorescence values for the AOI at each time point were divided (normalized) by the mean pixel fluorescence values in the cytoplasmic AOI at the corresponding time point in the same fiber. This method of normalization by cytoplasmic fluorescence, which is used throughout all the experimental groups, is based on the observation that after infection for 2 d, the expression level of NFATc1-GFP in whole nuclei in resting muscle fibers is proportional to the fluorescence level in the cytoplasm of the same fiber (correlation factor r2 equal to 0.90; Figure 1). Thus, to avoid the variations caused by the difference in expression level, the expression level of NFATc1-GFP in whole nuclei was normalized by the fluorescence intensity of the corresponding cytoplasm of the same fiber in all the experiments. Results are expressed as the mean ± SEM. The rate (percentage of mean cytoplasmic pixel fluorescence per minute) of change in fluorescence was calculated from the slope of the linear fit through the normalized fluorescence data for each nuclear or each cytoplasmic AOI.
Figure 1.
NFATc1-GFP expression in resting fibers. The expression level of NFATc1-GFP in whole nuclei of untreated muscle fibers was proportional to its level in a large area of cytoplasm (r2 = 0.90).
Immunocytochemistry
Forty-eight hours after infection with adenovirus encoding either FLAG-NFATc1 or untagged NFATc3, cultured FDB muscle fibers were treated with 40 nM leptomycin B for 1 h in Ringer's solution at room temperature. Muscle fibers were fixed with 4% paraformaldehyde at room temperature for 10 min, permeabilized with 0.2% Triton X-100 in phosphate-buffered saline for 20 min. Nonspecific binding sites were blocked by incubation with 5% normal goat serum. Subsequently, the fibers were incubated for 48 h at 4°C with either mouse monoclonal antibody against NFATc1 (1:200; Affinity Bioreagents, Golden, CO) or rabbit polyclonal antibody against NFATc3 (Santa Cruz Biotechnology, Santa Cruz, CA) followed by overnight incubation with Cy5-conjugated goat anti-mouse or fluorescein isothiocyanate-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA), respectively. The fluorescence of the stained fibers was visualized using the confocal laser scanning imaging system described above.
Nuclear Fluorescence Photobleaching
Nuclear fluorescence photobleaching experiments were carried out on an Olympus FluoView 500 confocal microscope. The GFP moiety was excited at 488 nm, and emission was detected at above 505 nm. After recording a prebleach image, a rectangular region, slightly larger than the nucleus and covering the entire nucleus, was scanned/photobleached with maximum laser power. Subsequently, images were captured at lower laser power.
RESULTS
Shuttling of NFATc1 in Resting Muscle Fibers
Previous studies showed that wild-type NFATc2 expressed in HeLa or BHK cells is predominantly cytoplasmic but that treatment with the CRM1 nuclear export inhibitor leptomycin B alone increased the nuclear content of NFATc2, suggesting that NFATc2 shuttles between the cytoplasm and nucleus in these unstimulated cells (Okamura et al., 2000; Terui et al., 2004). Whether nucleocytoplasmic shuttling of NFAT proteins occurs in skeletal muscle is not known. To address this issue, we used primary adult FDB muscle fiber cultures and adenovirus encoding NFATc1, fused to the fluorescent protein GFP.
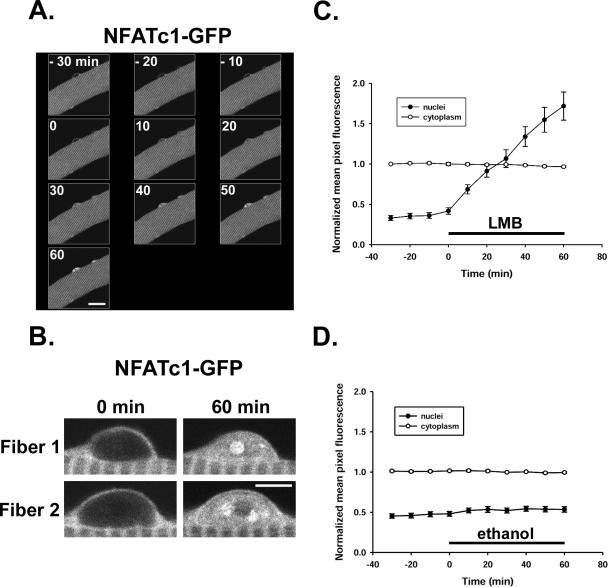
NFATc1-GFP fusion protein was present predominantly in the cytoplasm in a sarcomeric pattern in fully differentiated adult FDB skeletal muscle fibers in culture after transduction with adenovirus and expression for 2 d (Figure 2A; Liu et al., 2001). However, upon treatment with 40 nM leptomycin B, NFATc1-GFP continuously increased in the nuclei (Figure 2A), indicating that NFATc1 is cycling in resting fibers and that the nuclear export of NFATc1 in resting fibers is CRM1 dependent. The nuclear NFATc1-GFP translocated in response to leptomycin B formed foci or NFAT intranuclear bodies, shown in the enlarged pictures of two nuclei from two different fibers (Figure 2B).
Figure 2.
NFATc1-GFP nuclear accumulation during leptomycin B treatment. (A) A fiber expressing NFATc1-GFP is shown in Ringer's solution at room temperature 30 min before treatment (–30 to –10), at the start of 40 nM LMB treatment (0), and during the treatment for 60 min (0–60). After 1-h incubation with LMB, there is a significant increase of fluorescence in all nuclei, and most of the nuclei show NFATc1-GFP nuclear body formation. (B) Enlarged images of two nuclei from two different fibers before and after 60-min incubation with LMB, showing the NFATc1 nuclear bodies formed during LMB treatment. (C) Treatment with 40 nM LMB (dissolved in ethanol) resulted in a time-dependent net accumulation of NFATc1-GFP in the nuclei of cultured resting mouse FDB muscle fibers (24 nuclei from 21 fibers; same nuclei as Figure 1). (D) No effect on nuclear fluorescence of NFATc1-GFP was found with the same amount of solvent (ethanol; 7 nuclei from 6 fibers). In both groups, the cytoplasmic fluorescence remained constant during the experiment (C and D). The average fluorescence intensity per pixel over whole nuclei (closed circles) or over the cytoplasm (open circles) was quantitated and normalized to cytoplasmic mean pixel fluorescence (see Materials and Methods). Bars, 20 μm (A) and 5 μm (B).
The nuclear NFATc1-GFP fluorescence was enhanced after exposure to leptomycin B for 10 min and continuously increased over a 60-min experimental period, whereas the cytoplasmic fluorescence signal was stable (Figure 2C), consistent with a large pool of cytoplasmic NFATc1-GFP compared with that entering the nucleus. On treatment with leptomycin B, NFATc1-GFP translocated into the nucleus at a constant rate of 2.03 ± 0.15% of mean cytoplasmic pixel fluorescence per minute (Figure 2C). Assuming that leptomycin B completely inhibited the efflux of NFATc1 from fiber nuclei, these results reveal that the unidirectional influx rate of NFATc1 into nuclei is 2.03% of mean cytoplasmic pixel fluorescence per minute. If influx was not altered by leptomycin B, then under resting conditions, before addition of leptomycin B and in the absence of muscle activity to activate protein kinases or protein phosphatases, there was balanced unidirectional nuclear influx and efflux of NFATc1 at this rate to give zero net flux of NFATc1 (and thus no change in nuclear NFATc1 with time). Nuclear NFATc1 was constant in resting fibers under control conditions when muscle fibers were exposed to solvent ethanol (Figure 2D). These results strongly suggest that instead of locating statically in the cytoplasm, NFATc1-GFP shuttled between cytoplasm and nucleus under resting conditions and that the export pathway is CRM1 dependent. Additionally, these results also indicate that the export of NFATc1-GFP is very efficient in resting fibers under control conditions, keeping this transcription factor from accumulating in the nuclei and maintaining it primarily in the cytoplasm in unstimulated muscle fibers.
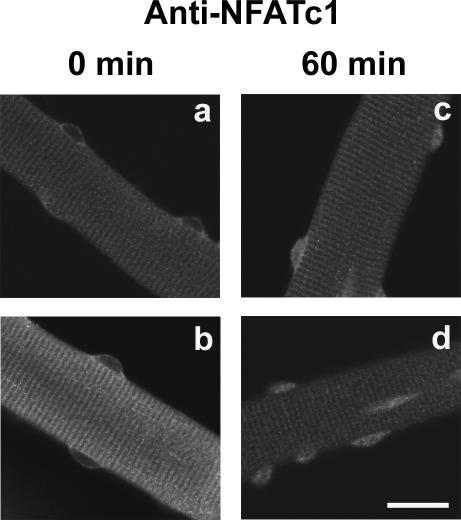
To rule out possible modification of NFAT behavior by the GFP moiety, we also expressed a non-GFP-tagged NFATc1 construct (FLAG-NFATc1) in muscle fibers. Staining with anti-NFATc1 antibody indicated that non-GFP-tagged NFATc1 also moved into the nucleus in resting fibers in the presence of leptomycin B (Figure 3).
Figure 3.
Nuclear entry of non-GFP-tagged NFATc1. Anti-NFATc1 antibody staining of four different muscle fibers expressing recombinant non-GFP-tagged NFATc1, fibers a and b before and fibers c and d after 60-min treatment with LMB. Bar, 20 μm.
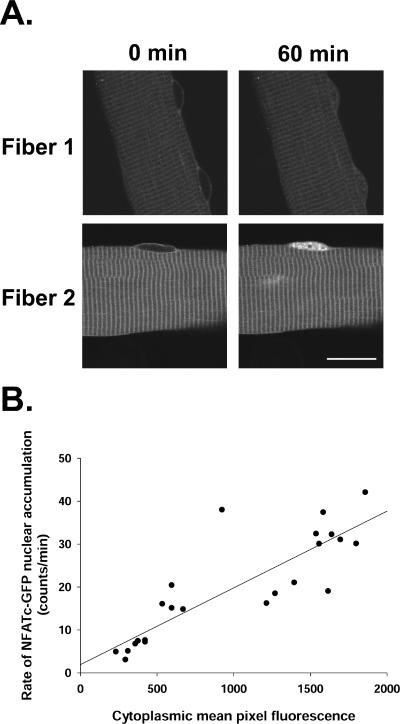
Expression Level Influences the Rate of NFATc1 Resting Cycling
During exposure to leptomycin B, we noticed that the rate of NFATc1-GFP nuclear accumulation varied according to the protein expression level in the cytoplasm in the same fiber (Figure 4A). To examine whether there is a correlation between the rates of resting cycling of NFATc1-GFP and the protein expression level in cytoplasm, we analyzed 21 different muscle fibers (including 24 nuclei) from four separate experiments. Our results showed that the rate of NFATc1 unidirectional efflux (and influx) in resting fibers, as calculated from the uptake rate of resting fibers exposed to 40 nM leptomycin B was largely proportional to its expression level in the cytoplasm of the same fiber (correlation factor r2 equal to 0.73) (Figure 4B). This indicates that the influx rate of NFATc1-GFP into nuclei of resting muscle fibers was influenced by its concentration in the cytosol. The linear relationship between influx rate and cytosolic NFATc1 level indicates that the NFATc1 uptake system was not saturated even in the presence of the high level of expressed NFATc1-GFP.
Figure 4.
The rate of nuclear entry of NFATc1-GFP in resting fibers depends on its level of expression in the cytoplasm. (A) Images of two fibers expressing NFATc1-GFP at the start of 40 nM LMB treatment (0 min) and after treatment for 60 min. LMB treatment resulted in nuclear accumulation of NFATc1-GFP in both fibers. However, the level of the NFATc1-GFP nuclear accumulation of fiber 2, which had higher cytoplasmic fluorescence, was much higher than that of fiber 1, which had lower cytoplasmic fluorescence. (B) The rate of nuclear entry of NFATc1-GFP in resting fibers exposed to LMB is close to proportional to its expression level in the cytoplasm of the same fiber (r2 = 0.73; same fibers as Figures 1 and 2C). Bar, 20 μm.
NFATc1 Resting Cycling in the Presence of Protein Kinase Inhibitors
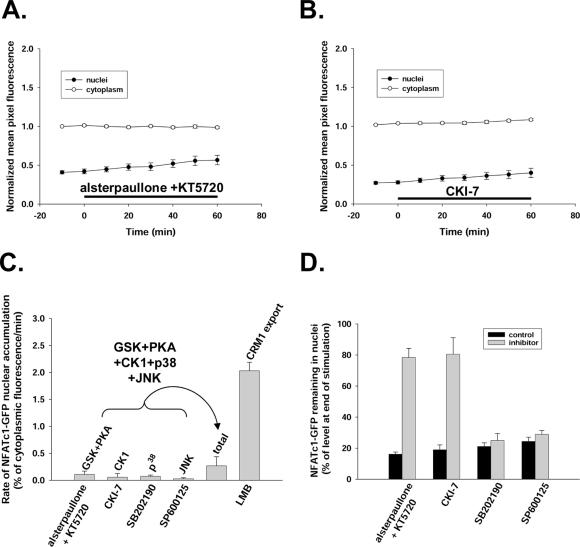
If dephosphorylated nuclear NFATc1 leaves the nucleus only after rephosphorylation, then inhibition of the kinase(s) responsible for NFAT nuclear phosphorylation should have similar effects as blocking NFAT efflux by leptomycin B. We therefore tested the role of protein kinases in NFATc1-GFP shuttling in resting fibers. Simultaneous application of inhibitors of GSK (20 μM alsterpaullone) and PKA (5 μM KT5720) for 60 min did not appreciably increase the rate of nuclear accumulation of NFATc1-GFP (rate 0.11 ± 0.06% of mean cytoplasmic pixel fluorescence per minute; Figure 5A). Inhibitors of CK1 (900 μM CKI-7; Figure 5B), p38 (20 μM SB202190), or JNK (30 μM SP600125) had no significant effects on NFATc1-GFP nuclear accumulation, with respective NFATc1-GFP mean net import rates of 0.057 ± 0.066, 0.074 ± 0.022, or 0.027 ± 0.02% of mean cytoplasmic pixel fluorescence per minute averaged over a 60-min period (Figure 5C). The total sum of the rates of NFATc1-GFP nuclear accumulation under each of these five kinase inhibitors (0.268 ± 0.169% of mean cytoplasmic pixel fluorescence per minute) was only ∼13% of that under the treatment of leptomycin B (2.03 ± 0.15% of mean cytoplasmic pixel fluorescence per minute; Figure 5C).
Figure 5.
No significant effects of protein kinase inhibitors on the rate of nuclear entry of NFATc1-GFP in resting fibers. (A and B) Neither simultaneous inhibition of GSK with 20 μM alsterpaullone and PKA with 5 μM KT5720 (A) nor inhibition of CK1 with 900 μM CKI-7 (B) for 60 min significantly increased nuclear NFATc1-GFP. (C) Rates of NFATc1-GFP nuclear accumulation in muscle fibers during exposure to different kinase inhibitors or to 40 nM LMB. Total, the sum of the rates of NFATc1-GFP nuclear accumulation under each of the five kinase inhibitors: alsterpaullone and KT5720, CKI-7, SB202190, and SP600125. (D) Percentage of the NFATc1-GFP nuclear fluorescence increase because of 60 min of electrical stimulation still remaining in the nucleus 90 min after cessation of stimulation in the absence (control) or presence of various kinase inhibitors. For A and B and for each bar graph in C and D, at least five nuclei from five different fibers were monitored.
We tested for the intranuclear effectiveness of the inhibitors used in Figure 5C by examining their effect on the decay of the nuclear NFATc1-GFP accumulated during 1 h of electrical stimulation. At the end of 1 h of electrical stimulation, 1 μM cyclosporin A was added to all fibers to halt calcineurin-dependent dephosphorylation of NFAT, and the decrease of nuclear NFATc1-GFP was monitored in the presence of the same concentration of kinase inhibitors as in Figure 5C or in solvent control. In the presence of both PKA and GSK inhibitors or the casein kinase inhibitor, the percentage of accumulated nuclear NFATc1-GFP remaining in the nucleus 90 min after cessation of stimulation was significantly increased (Figure 5D), indicating that the corresponding kinases were inhibited in muscle fiber nuclei. Without inhibition, these kinases contributed to the removal of accumulated nuclear NFAT, presumably by NFAT rephosphorylation after calcineurin-dependent dephosphorylation and nuclear entry during electrical stimulation. In contrast, the inhibitors of p38 and JNK had no significant effect on the decay of accumulated nuclear NFAT after termination of fiber electrical stimulation (Figure 5D). Thus, p38 and JNK were either not involved in removal of nuclear NFATc1 after electrical stimulation or were not inhibited under the conditions of our study. However, antibody staining for phospho-cAMP response element-binding protein was suppressed by the p38 inhibitor SB202190 (our unpublished data), indicating that this inhibitor was effective in the nuclei of adult mouse skeletal muscle fibers, as shown previously for rat fibers (Geiger et al., 2005). Thus, p38 does not seem to be involved in removing NFATc1 from nuclei either after electrical stimulation or during NFATc1 shuttling in resting fibers. Although a similar concentration of the JNK inhibitor SP600125 as used here has been shown to be effective in rat smooth muscle nuclei (Zhai et al., 2004), we have not established its effectiveness in mouse skeletal muscle fiber nuclei. Finally, the multiple kinase inhibitor staurosporine at 1 μM caused only slight increase of nuclear NFATc1-GFP accumulation in unstimulated fibers (rate 0.298 ± 0.08% of mean cytoplasmic pixel fluorescence per minute), ∼14% of that during exposure to leptomycin B. We have previously shown that staurosporine is effective in muscle fiber nuclei because 1 μM staurosporine causes histone deacetylase 4 (HDAC4) nuclear accumulation in resting fibers, presumably by blocking HDAC4 nuclear exit mediated by intranuclear phosphorylation (Liu et al., 2005). Together, these results show that the resting nucleocytoplasmic cycling of NFATc1-GFP in cultured adult mouse FDB skeletal muscle fibers was only slightly modulated by inhibition of a number of protein kinases within the nucleus, suggesting that these kinases are not involved in the mechanism underlying NFATc1 nuclear export in unstimulated fibers.
Inhibition of Calcineurin Does Not Block the Resting Cycling of NFATc1
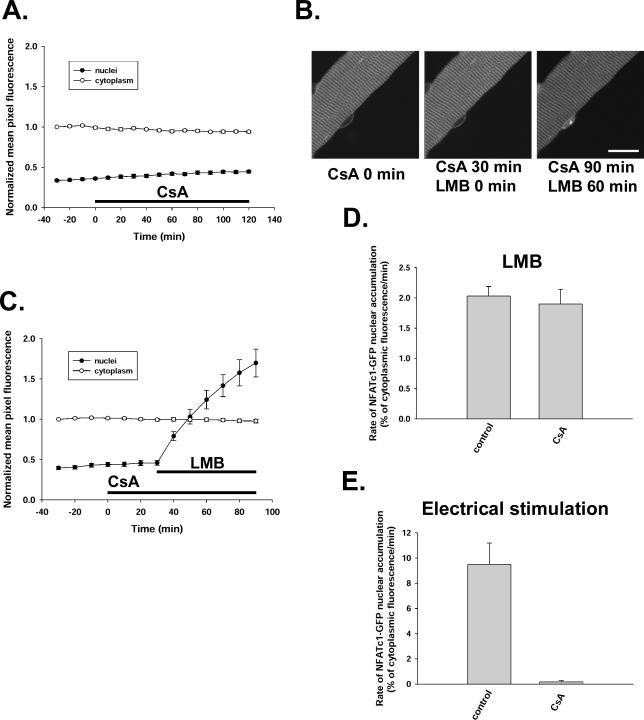
Calcineurin, which is activated by signals that increase intracellular calcium, can dephosphorylate cytoplasmic NFATc1 and induce this transcription factor to translocate into nuclei in FDB fibers (Liu et al., 2001). This translocation of NFATc1 can be completely blocked by treatment with the specific inhibitor of calcineurin activity cyclosporin A in stimulated muscle fibers (Liu et al., 2001). However, cyclosporin A did not change the subcellular localization of NFATc1-GFP under resting conditions in cultured fibers (Figure 6A). Exposure to 1 μM cyclosporin A for 2 h had no effect on either the cytoplasmic or nuclear fluorescence level of NFATc1-GFP. To further investigate the role of calcineurin in the resting cycling of NFATc1, cultured FDB muscle fibers were preincubated with 1 μM cyclosporin A for 30 min before leptomycin B treatment and then continuously exposed to cyclosporin A during leptomycin B treatment. To our surprise, cyclosporin A could not block the leptomycin B-induced nuclear fluorescence accumulation and formation of nuclear bodies of NFATc1-GFP in cultured resting mouse FDB muscle fibers (Figure 6, B and C). On treatment with leptomycin B alone, NFATc1-GFP translocated into the nucleus at a constant rate of 2.03 ± 0.15% of mean cytoplasmic pixel fluorescence per minute (Figures 2C and 6D). During continuous treatment of fibers with 1 μM cyclosporin A starting 30 min before exposure to leptomycin B, the rate constant of NFATc1 nuclear translocation caused by leptomycin B was 1.90 ± 0.24% of mean cytoplasmic pixel fluorescence per minute, which is not significantly different from leptomycin B treatment alone (Figure 6D).
Figure 6.
Inhibition of calcineurin by cyclosporin A had no effect on resting cycling of NFATc1-GFP in cultured adult mouse FDB muscle fibers. (A) CsA (1 μM) treatment for 2 h had no effect on the subcellular localization of NFATc1-GFP (6 nuclei from 6 fibers). (B) A fiber expressing NFATc1-GFP is shown at the start treatment of 1 μM CsA (0 min), after 30 min of CsA (the start of 40 nM LMB), and after 90 min of CsA (and 60 min of LMB) treatment, showing that in the presence of CsA, LMB still caused nuclear accumulation as well as nuclear body formation of NFATc1-GFP. (C) Time course of cytoplasmic-to-nuclear translocation of NFATc1-GFP during LMB treatment after 30-min preincubation with 1 μM CsA to inhibit calcineurin activity (11 nuclei from 11 fibers). (D) The rates of NFATc1-GFP nuclear accumulation in the presence of 40 nM LMB with (CsA; same fibers as Figure 6C) or without (control; same fibers as Figures 1 and 2C) 1 μM CsA. (E) The rates of NFATc1-GFP nuclear translocation during electrical stimulation with (CsA; 5 nuclei from 5 fibers) or without (control; 14 nuclei from 13 fibers) 1 μM CsA. Bar, 20 μm.
It is well documented that CsA is a potent inhibitor of calcineurin activity when added to cells or when added as CsA–cyclophilin complexes to purified calcineurin in vitro (Fruman et al., 1992; Liu et al., 1992). In the present study, to test the effectiveness of this drug, we compared electrical stimulation-induced nuclear translocation of NFATc1-GFP with or without cyclosporin A. As shown previously (Liu et al., 2001), electrical stimulation with an activity pattern of slow-twitch muscle (5-s trains of 10-Hz stimuli, every 50 s) resulted in marked translocation of NFATc1-GFP from cytoplasm to nucleus. In agreement with our previous findings, prior treatment of the muscle fibers with 1 μM cyclosporin A for 30 min completely prevented the electrical stimulation induced nuclear translocation of NFATc1-GFP (Figure 6E), demonstrating the efficiency of this inhibitor. Thus, the inability of CsA to block import in resting fibers, together with its efficiency in inhibiting electrical stimulation-induced import of NFATc1-GFP from cytoplasm to nucleus, suggests that the mechanisms underlying the nuclear entry of NFATc1-GFP are different under resting and activated conditions. Although the electrical stimulation-induced NFATc1 nuclear translocation is dependent on calcium activation of calcineurin, the resting cycling of NFATc1-GFP was independent of dephosphorylation by calcineurin and mainly influenced by its concentration in the cytosol.
NFATc1, but Not NFATc3, Exhibits Resting Cycling
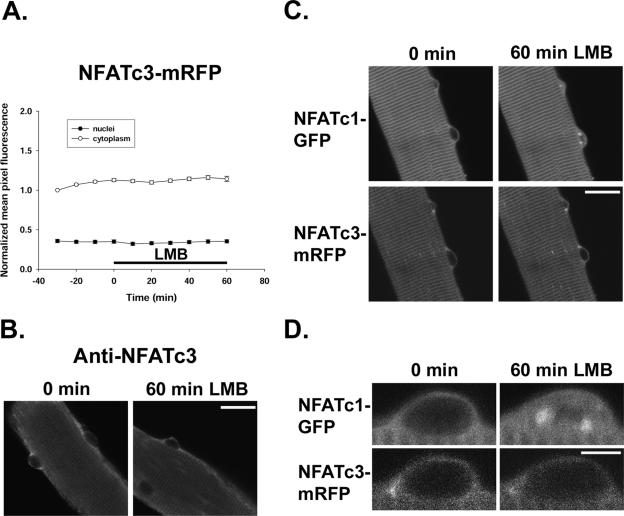
Both NFATc1 and NFATc3 exhibit high levels of endogenous expression at mRNA level in skeletal muscle relative to other tissues and may be involved in muscle differentiation (Hoey et al., 1995; Delling et al., 2000). To investigate the specificity of the resting cycling for NFATc1 in cultured adult muscle fibers, we examined NFATc3 movements in adult skeletal muscle fibers. In fibers expressing NFATc3-mRFP, the NFATc3-mRFP fluorescence in both cytoplasm and nuclei stayed constant during 60-min exposure to leptomycin B (Figure 7A). Expressed untagged NFATc3 also did not move into nuclei in the presence of leptomycin B (Figure 7B), indicating that the mRFP moiety did not cause failure of nuclear entry of NFATc3. In fibers coexpressing NFATc1-GFP and NFATc3-mRFP, both proteins were colocalized at the sarcomeric z-lines and absent from nuclei under resting control conditions (Figure 7C). On addition of 40 nM leptomycin B, sufficient to block the interaction of nuclear exporter CRM1 with the NES regions of any protein, NFATc1-GFP showed a time-dependent nuclear fluorescence accumulation and formation of nuclear NFAT bodies in muscle fibers. In contrast, no nuclear accumulation of NFATc3-mRFP was found in the same fibers (Figure 7C). The nuclear accumulation of NFATc1, but not NFATc3, promoted by leptomycin B is clearly shown in the enlarged pictures in Figure 7D. These data indicate that the resting nucleocytoplasmic shuttling of NFATc1 via a leptomycin B-sensitive export system is isoform specific in fully differentiated adult FDB skeletal muscle fibers. However, we have not ruled out the possibility of NFATc3 shuttling via a nonleptomycin B-sensitive nuclear efflux pathway.
Figure 7.
Resting cycling of NFATc1-GFP, but not NFATc3-mRFP, in cultured adult mouse FDB skeletal muscle fibers. (A) LMB (40 nM) did not promote redistribution of NFATc3-mRFP in resting fibers (10 nuclei from 9 fibers). (B) Anti-NFATc3 antibody staining confirmed that fibers expressing untagged NFATc3 did not accumulate NFATc3 in nuclei after 1 h in the presence of 40 nM LMB. (C) Images of a muscle fiber coinfected by NFATc1-GFP and NFATc3-mRFP adenoviruses before (0 min) and after 60 min of 40 nM LMB treatment. LMB caused nuclear accumulation of NFATc1-GFP, but not NFATc3-mRFP. (D) Enlarged images of a nucleus from the same fiber as in C before (0 min) and after 60-min incubation with LMB. Bars, 20 μm (B and C) and 5 μm (D).
Activity-dependent Translocation of NFATc1 and NFATc3
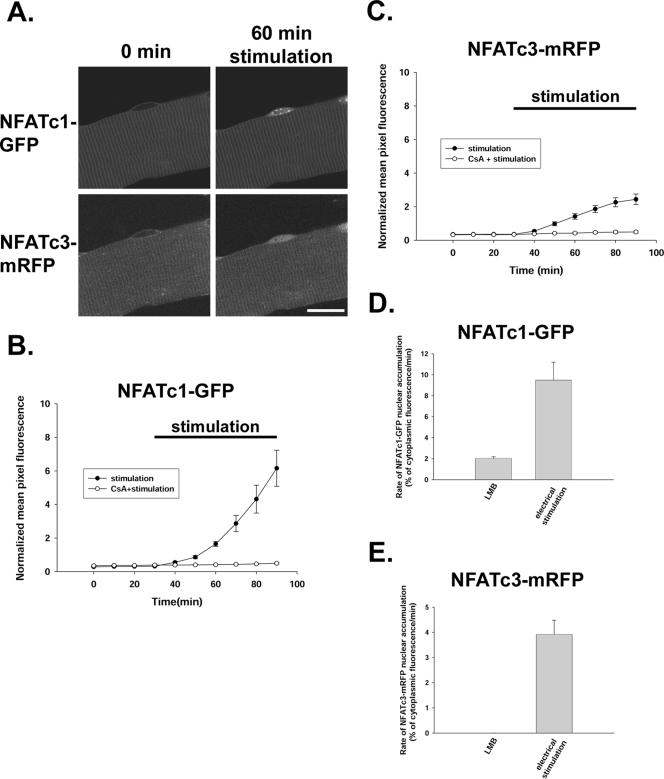
We next investigated translocation of NFATc1-GFP and NFATc3-mRFP from cytoplasm to nucleus in response to electrical stimulation patterns mimicking the physiological activity pattern of slow skeletal muscle. Muscle fibers were coinfected with NFATc1-GFP and NFATc3-mRFP adenoviruses and 2 d later, fibers expressing both proteins were stimulated to produce action potentials using 5-s trains of 10-Hz stimuli once every 50 s. Field stimulation with this protocol results in visible twitches throughout the period of stimulation in all fibers used for analysis. Figure 8A presents images of the same fiber before stimulation and 60 min after the start of 10-Hz stimulation. This electrical stimulation caused a translocation of NFATc1-GFP into nucleus (Figure 8A), in agreement with our previous findings (Liu et al., 2001). In contrast to the lack of effect of leptomycin B on the distribution of NFATc3-mRFP (Figure 7), electrical stimulation did give rise to a redistribution of NFATc3-mRFP from the cytoplasm to the nucleus, resulting in a nuclear accumulation of NFATc3-mRFP fluorescence (Figure 8A).
Figure 8.
Electrical stimulation induced translocation of both NFATc1-GFP and NFATc3-mRFP from cytosol to nucleus. (A) Images of one muscle fiber infected by both NFATc1-GFP adenovirus and NFATc3-mRFP adenovirus before (0 min) and 60 min after start of electrical stimulation, which induced a time-dependent increase in nuclear fluorescence of both NFATc1-GFP and NFATc3-mRFP. (B and C) The time course of NFATc1-GFP (B, same fibers as Figure 6E) and NFATc3-mRFP (C; 11 nuclei from 8 fibers for stimulation alone and 8 nuclei from 8 fibers for stimulation with CsA) nuclear translocation during stimulation, both of which were completely blocked by preincubation with 1 μM CsA. (D) The rate of NFATc1-GFP nuclear accumulation was ∼4.6 times enhanced with electrical stimulation (same fibers as Figures 6E and 8B) compared with LMB treatment (same fibers as Figures 1 and 2C). (E) The rate of NFATc3-mRFP nuclear accumulation was changed from zero in resting fibers exposed to LMB (same fibers as Figure 7A) to 3.16% of mean cytoplasmic pixel fluorescence per minute with electrical stimulation (same fibers as Figure 8C). Bar, 20 μm.
Compared with the rate of NFATc1-GFP translocation into the nucleus in resting fibers as determined from the accumulation rate in response to leptomycin B (2.03 ± 0.15% of mean cytoplasmic pixel fluorescence per minute), electrical stimulation increased the rate of NFATc1 nuclear uptake by >4.6 times (9.49 ± 1.71% of mean cytoplasmic pixel fluorescence per minute; Figure 8, B and D). The effects of leptomycin B and electrical stimulation on the rate of NFATc1 nuclear entry were additive (our unpublished data). The relative effect of electrical stimulation was even more striking for NFATc3-mRFP, with no accumulation in response to leptomycin B under resting conditions, changing to a net nuclear accumulation rate of 3.16 ± 0.84% of mean cytoplasmic pixel fluorescence per minute during stimulation (Figure 8, C and E). The nuclear translocation of NFATc1-GFP or NFATc3-mRFP resulting from electrical stimulation was completely blocked by calcineurin activity inhibitor cyclosporin A (Figure 8, B and C). Together, these results indicate a much higher rate of nuclear translocation of NFAT proteins during electrical stimulation than in the unstimulated resting fiber.
NFATc1 Nuclear Bodies
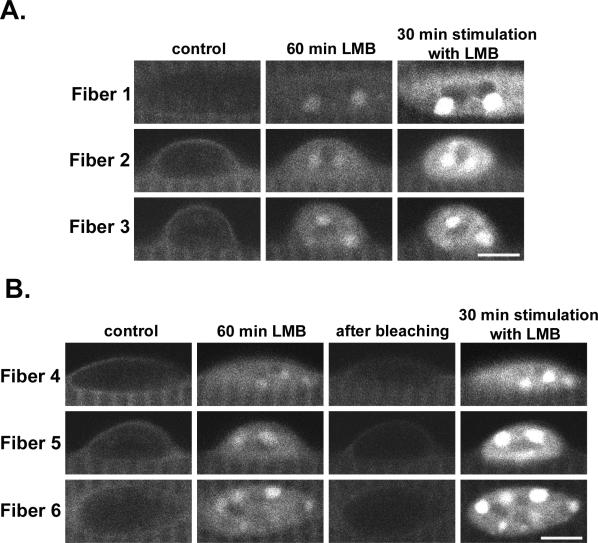
In agreement with our previous report (Liu et al., 2001), during electrical stimulation NFATc1 translocated into skeletal muscle fiber nuclei and formed distinct foci, termed “NFATc1 bodies” (Liu et al., 2005b) because of their similarity to other previously described nuclear bodies (Zimber et al., 2004). Because leptomycin B treatment also promoted formation of NFATc1 bodies in nuclei of muscle fibers (Figure 2), we investigated whether the NFATc1 bodies formed in response to these two different procedures exhibited the same intranuclear pattern. Fibers expressing NFATc1-GFP were first exposed to 40 nM leptomycin B for 60 min. Then, in the continued presence of leptomycin B, the fibers were either immediately electrically stimulated for 30 min (Figure 9A) or were photobleached followed by electrical stimulation for 30 min (Figure 9B). On 60-min leptomycin B treatment, weak but clearly visible NFATc1 bodies were formed in the nuclei (Figure 9, A and B). Further incubation in leptomycin B together with electrical stimulation for 30 min under both conditions profoundly increased the fluorescence intensity of the nuclei and of the NFATc1 bodies. However, the pattern of intranuclear distribution of NFATc1 fluorescence after electrical stimulation was the same as that in leptomycin B treatment alone before stimulation. These results demonstrate that the NFATc1 dephosphorylated by activated calcineurin during electrical stimulation enters the nucleus and locates in the same intranuclear bodies as visualized after calcineurin-independent NFATc1 entry because of exposure to leptomycin B in unstimulated fibers.
Figure 9.
The same NFATc1 nuclear body organization was observed during leptomycin B treatment and during electrical stimulation. (A) Images of three nuclei from three different muscle fibers (fiber 1–3) expressing NFATc1-GFP and imaged at the start of 40 nM LMB treatment (control), after 60 min of LMB, and after 30 min additional LMB treatment together with electrical stimulation. (B) Images of three nuclei from three other different muscle fibers (fiber 4–6) expressing NFATc1-GFP and imaged at the start of 40 nM LMB treatment (control), after 60 min of LMB, immediately after photobleaching the nuclei, and after 30 min additional LMB treatment together with electrical stimulation. Under both conditions, LMB caused nuclear accumulation and nuclear body formation of NFATc1-GFP, which was maintained in the same pattern, but with markedly increased fluorescence intensity after repetitive electrical stimulation. Bar, 5 μm.
DISCUSSION
Both NFATc1 and NFATc3 transcripts have been shown to be relatively highly expressed in skeletal muscle (Hoey et al., 1995). During muscle-development, only a particular NFAT isoform undergoes calcium-induced cytoplasm-to nuclear translocation at specific stages of muscle differentiation, although three NFAT isoforms (NFATc1, NFATc2, and NFATc3) are all expressed in the cytoplasm of muscle cells at different stages of myogenesis (Abbott et al., 1998). In predifferentiated human myoblasts or in C2C12 myoblasts, activated calcineurin caused NFATc3, but not NFATc1, translocation into nucleus (Abbott et al., 1998; Delling et al., 2000). In multinucleated myotubes, NFATc1, but not NFATc3, responds to a thapsigargin-induced elevation of cytosolic calcium by translocating to nuclei from cytoplasm (Abbott et al., 1998). Thus, there is specificity in the cellular handling of NFATc1 and NFATc3.
Here, we found that during exposure to the CRM1 nuclear export inhibitor leptomycin B, NFATc1-GFP continuously accumulated in the nucleus where it formed distinct foci, whereas NFATc3-mRFP maintained its cytoplasmic localization without entering into the nucleus. However, similar to NFATc1-GFP, NFATc3-mRFP did move into the nucleus during slow fiber type electrical stimulation. These results demonstrate that NFATc1-GFP, but not NFATc3-mRFP, has the ability to move into skeletal muscle nuclei in the absence of stimulation. The differential ability of expressed NFAT isoforms to undergo nuclear translocation in resting fibers indicates specificity of handling of different members of the NFAT family in adult muscle fibers. At present, it is unclear what molecular basis underlies the different behavior of NFAT isoforms and what functional role NFATc1 plays in shuttling in resting muscle fibers. The NFATc1 splice variant used here (human NFATc1α), which corresponds to the sole NFATc1 isoform expressed in mice (Pan et al., 1997; 4.5-kb mRNA, encoding a predicted protein having 87% amino acid identity with human NFATc1α), but not the NFATc3 (NFATc3x), has a truncated C-terminal domain (Rao et al., 1997). Furthermore, NFATc1 and NFATc3 differ in the location of their NLS-masking sequences (Beals et al., 1997; Zhu et al., 1998). These differences, as well as other differences, could underlie the ability of NFATc1, but not NFATc3, to enter nuclei and undergo nucleocytoplasmic shuttling in resting skeletal muscle fibers. NFATc3 also does not exhibit cycling in resting vascular smooth muscle (Gomez et al., 2003).
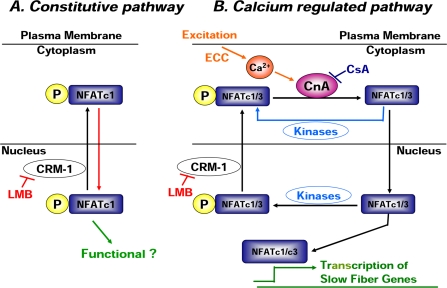
The protein kinases GSK (Beals et al., 1997b), PKA (Sheridan et al., 2002), CK1 (Zhu et al., 1998; Okamura et al., 2004), p38 MAPK (Braz et al., 2003), and JNK (Dong et al., 1998; Liang et al., 2003) have each been proposed to influence NFAT subcellular localization during cell activation. Unexpectedly, we found that inhibitors of these kinases had minimal effect on NFATc1 nuclear distribution in resting muscle fibers, even though the GSK and PKA or CK1 inhibition did retard the movement of NFATc1 out of nuclei after fiber stimulation. Thus, we hypothesize that there is a constitutive shuttling of NFATc1 in resting muscle fibers and that NFATc1 entry via this pathway might be independent of phosphorylation and dephosphorylation (Figure 10A, red arrow). However, at this time, we cannot distinguish the phosphorylation status of the NFATc1-GFP entering the nucleus in resting muscle fibers.
Figure 10.
A model of two independent NFAT translocation pathways in cultured adult mouse skeletal muscle fibers. (A) Constitutive pathway: phosphorylated NFATc1 enters the nucleus (red arrow) and constitutively shuttles between cytoplasm and nucleus in cultured FDB muscle fibers under resting conditions. The export during this resting cycling is CRM-1 dependent, and this export of phosphorylated NFATc1 in resting cells is highly efficient and prevents nuclear accumulation. The effectiveness of phosphorylated nuclear NFATc1 in transcriptional activation is not established. Alternatively, dephosphorylation through a CsA-insensitive phosphatase might expose the NLS of NFATc1 in resting fibers and thereby cause its nuclear translocation, with return to the cytoplasm after rephosphorylation by kinases other than GSK, PKA, CK1 or p38 (our unpublished data) to allow NFATc1 shuttling in resting fibers. (B) Calcium-regulated pathway: calcium-mediated activation of calcineurin during muscle activity induces the dephosphorylation and nuclear translocation of NFATc1 and NFATc3. The dephosphorylated nuclear NFATc1 and NFATc3 have a high DNA binding affinity and thus can bind to the promoter of target genes and induce slow muscle fiber gene transcription. The dephosphorylated NFATc1 and NFATc3 can also be rephosphorylated by protein kinases and be relocated to the cytoplasm.
The hypothesis that NFATc1 can be imported into nucleus without Ca2+-activated dephosphorylation was further supported with data obtained by inhibiting the phosphatase calcineurin. Our results obtained using the calcineurin inhibitor cyclosporin A supported the idea that a calcium-calcineurin–independent signaling pathway is responsible for the nucleocytoplasmic shuttling of NFATc1-GFP in resting skeletal muscle fibers (Figure 10A, constitutive pathway). NFATc1 is a physiological substrate of calcineurin, which is in turn an immediate target of cyclosporin A. The activation of calcineurin is totally dependent on the elevation of intracellular calcium. In agreement with our previous findings (Liu et al., 2001), we showed here that slow fiber type stimulation (5-s trains of 10-Hz stimuli, every 50 s) resulted in translocation of NFATc1-GFP from cytoplasm to nucleus and that this activity-dependent translocation can be entirely blocked by cyclosporin A (Figure 10B, calcium-regulated pathway). This type of electrical stimulation has been shown to cause transiently elevated intracellular calcium concentration in muscle fibers (Liu et al., 2005). However, cyclosporin A had no effect on the subcellular distribution of NFATc1-GFP in resting fibers, nor did it change the rate of resting NFATc1-GFP nucleocytoplasmic shuttling.
One possible explanation of our findings on muscle fibers is that without activation of calcineurin, the fully phosphorylated NFATc1-GFP was still capable of entering the fiber nucleus (Figure 10A), as suggested previously for NFATc2, which was slowly imported into nuclei in resting HeLa cells (Okamura et al., 2000). This slow import in HeLa cells was not because of dephosporylation by basally active calcineurin, because it was not blocked by cyclosporin A. Moreover, when the calcineurin docking site of NFATc2 was mutated, although this mutant protein was highly resistant to calcineurin-mediated dephosphorylation and did not undergo detectable nuclear translocation in ionomycin-treated cells, it was still slowly imported into the nucleus in the unstimulated HeLa cells (Okamura et al., 2000). If the phosphorylated form of NFATc1 does enter the nucleus in resting adult muscle fibers, then phosphorylated NFATc1 is accumulated in the same nuclear bodies that contain the dephosphorylated NFATc1 that enters during electrical stimulation.
The question then arises as to how NFATc1 (78 kDa) or NFATc1-GFP (105 kDa) can cross the nuclear envelope and move into nucleus without dephosphorylation, because generally only molecules of <30 kDa can freely traverse the nuclear pore complex by passive diffusion (Paine et al., 1975). A direct interaction with nuclear pore structure proteins nucleoporins Nup153 and Nup214 has been proposed to translocate inactive Stat1 (signal transducers and activators of transcription factors; Mr > 87 kDa) from cytoplasm into nucleus in unstimulated cells (Marg et al., 2004). Whether the activity-independent nuclear import of NFATc1-GFP in resting muscle fibers requires specific interactions with the nuclear pore complex or other intermediary carriers provides an interesting question for future study. In constrast, it is equally possible that NFATc1-GFP is dephosphorylated to a certain extent by other phosphatases in unstimulated fibers, which partially exposes its NLS domain and consequently renders it competent to be imported into the nucleus. This alternative possibility for NFAT cycling in resting fibers requires calcineurin-independent dephosphorylation of NFATc1 in cytoplasm, and intranuclear rephosphorylation by kinases other than GSK, PKA, CK1, and p38. In an in vitro study, Beals et al. (1997a) reported that amino acids 196–304 of NFATc1 when expressed in bacteria as a glutathione S-transferase fusion protein could be dephosphorylated by protein phosphatase 1. However, the catalytic subunit of protein phosphatase 1 shows relatively low substrate specificity in vitro.
The functional significance of NFATc1 shuttling in resting muscle fibers is an unanswered question. Shuttling of phosphorylated NFATc1 would contribute to the amount of phosphorylated NFATc1 resident in nucleus. Phosphorylated nuclear NFATc1 may bind with low affinity to NFAT-responsive elements in genes and thereby modulate gene expression. Alternatively, some nuclear phosphorylated NFATc1 may be dephosphorylated by intranuclear phosphatases during muscle activity and thereby be retained in nucleus with full transcriptional activity. Intranuclear phosphorylated NFATc1 would thus provide a potentially rapidly activated pool of dephosphorylated NFATc1 in the nucleus.
In conclusion, our results indicate that in cultured adult skeletal muscle fibers, NFATc1 moves from the cytoplasm into the nucleus both under resting and stimulated conditions, but via distinct mechanisms in resting and active muscle fibers. Stimulation greatly accelerates the rate of influx of NFATc1 and NFATc3 into the nucleus.
Acknowledgments
We thank Dr. Gerald R. Crabtree for NFATc1 cDNA, Dr. Luis F. Santana for adenovirus encoding NFATc3-mRFP, Dr. Jeffery D. Molkentin for adenovirus encoding untagged NFATc3, Steve Techman for participation in generation of Figure 5, and Carrie Wagner for technical assistance. This work was supported by National Institutes of Health Grant R01-NS33578 from the National Institute of Neurological Disorders and Stroke. T. S. and A. H. were supported by National Institutes of Health Training Grant T32-AR-07592 to the Interdisciplinary Program in Muscle Biology, University of Maryland School of Medicine.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-08-0780) on January 25, 2006.
Abbreviations used: CK1, casein kinase 1; CsA, cyclosporin A; FDB, flexor digitorum brevis; GFP, green fluorescence protein; GSK, glycogen synthase kinase-3; HDAC4, histone deacetylase 4; JNK, c-Jun NH2-terminal kinase; LMB, leptomycin B; mRFP, monomeric red fluorescent protein; NFAT, nuclear factor of activated T-cells; PKA, protein kinase A; p38, p38 MAPK.
References
- Abbott, K. L., Friday, B. B., Thaloor, D., Murphy, T. J., and Pavlath, G. K. (1998). Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells. Mol. Biol. Cell 9, 2905–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beals, C. R., Clipstone, N. A., Ho, S. N., and Crabtree, G. R. (1997a). Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev. 11, 824–834. [DOI] [PubMed] [Google Scholar]
- Beals, C. R., Sheridan, C. M., Turck, C. W., Gardner, P., and Crabtree, G. R. (1997b). Nuclear export of NFATc enhanced by glycogen synthase kinase-3. Science 275, 1930–1934. [DOI] [PubMed] [Google Scholar]
- Braz, J. C., et al. (2003). Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J. Clin. Investig. 111, 1475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, R. E., Tour, O., Palmer, A. E., Steinbach, P. A., Baird, G. S., Zacharias, D. A., and Tsien, R. Y. (2002). A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 7877–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin, E. R., Olson, E. N., Richardson, J. A., Yang, Q., Humphries, C., Shelton, J. M., Wu, H., Zhu, W., Bassel-Duby, R., and Williams, R. S. (1998). A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 12, 2499–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delling, U., Tureckova, J., Lim, H. W., De Windt, L. J., Rotwein, P., and Molkentin, J. D. (2000). A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol. Cell. Biol. 20, 6600–6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, C., Yang, D. D., Wysk, M., Ahitmarsh, R. J., Davis, R. J., and Flavell, R. A. (1998). Defective T cell differentiation in the absence of JNK1. Science 282, 2092–2095. [DOI] [PubMed] [Google Scholar]
- Fruman, D. A., Klee, C. B., Bierer, B. E., and Burakoff, S. J. (1992). Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. Proc. Natl. Acad. Sci. USA 89, 3686–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, M., Asano, S., Nakamura, T., Adachi, M., Yoshida, M., Yanagida, M., and Nishida, E. (1997). CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390, 308–311. [DOI] [PubMed] [Google Scholar]
- Geiger, P. C., Wright, D. C., Han, D., and Holloszy, J. O. (2005). Activation of p38 MAP kinase enhances sensitivity of muscle glucose transport to insulin. Am. J. Physiol. 288, E782–E788. [DOI] [PubMed] [Google Scholar]
- Gomez, M. F., Gonzalez Bosc, L. V., Stevenson, A. S., Wilkerson, M. K., Hill-Eubanks, D. C., and Nelson, M. T. (2003). Constitutively elevated nuclear export activity opposes Ca2+-dependent NFATc3 nuclear accumulation in vascular smooth muscle. J. Biol. Chem. 278, 46847–46853. [DOI] [PubMed] [Google Scholar]
- Hennig, R., and Lomo, T. (1985). Firing patterns of motor units in normal rats. Nature 314, 164–166. [DOI] [PubMed] [Google Scholar]
- Hoey, T., Sun, Y. L., Williamson, K., and Xu, X. (1995). Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity 2, 461–472. [DOI] [PubMed] [Google Scholar]
- Hogan, P. G., Chen, L., Nardone, J., and Rao, A. (2003). Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17, 2205–2232. [DOI] [PubMed] [Google Scholar]
- Kehlenbach, R. H., Dickmanna, A., and Gerace, L. (1998). A role for RanBP1 in the release of CRM1 from nuclear pore complex in a terminal step of nuclear export. J. Cell Biol. 141, 863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, Q., Bueno, O. F., Wilkins, B. J., Kuan, C. Y., Xia, Y., and Molkentin, J. D. (2003). c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. EMBO J. 22, 5079–5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Albers, M. W., Wandless, T. J., Luan, S., Alberg, D. G., Belshaw, P. J., Cohen, P., MacKintosh, C., Klee, C. B., and Schreiber, S. L. (1992). Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry 31, 3896–3901. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Carroll, S. L., Klein, M. G., and Schneider, M. F. (1997). Calcium transients and calcium homeostasis in adult mouse fast-twitch skeletal muscle fibers in culture. Am. J. Physiol. 272, C1919–C1927. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Cseresnyes, Z., Randall, W. R., and Schneider, M. F. (2001). Activity-dependent nuclear translocation and intranuclear distribution of NFATc in adult skeletal muscle fibers. J. Cell Biol. 155, 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., Randall, W. R., and Schneider, M. F. (2005). Activity-dependent and -independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J. Cell Biol. 168, 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., and Schneider, M. F. (1998). Fiber type-specific gene expression activated by chronic electrical stimulation of adult mouse skeletal muscle fibers in culture. J. Physiol. 512, 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., Shen, T., Randall, W. R., and Schneider, M. F. (2005b). Signaling pathways in activity-dependent fiber type plasticity in adult skeletal muscle. J. Muscle Res. Cell Motil. 26, 13–21. [DOI] [PubMed] [Google Scholar]
- Macian, F. (2005). NFAT proteins: key regulators of T-cell development and function. Nat. Rev. 5, 472–484. [DOI] [PubMed] [Google Scholar]
- Marg, A., Shan, Y., Meyer, T., Meissner, T., Brandenburg, M., and Vinkemeier, U. (2004). Nucleocytoplasmic shuttling by nucleoporins Nup153 and Nup214 and CRM1-dependent nuclear export control the subcellular distribution of latent Stat1. J. Cell Biol. 165, 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa, H., Woo, S. K., Dahl, S. C., Handler, J. S., and Kwon, H. M. (1999). Tonicity-responsive enhancer binding protein, a Rel-like protein that stimulates transcription in response to hypertonicity. Proc. Natl. Acad. Sci. USA 96, 2538–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura, H., Aramburu, J., Garcia-Rodriguez, C., Viola, J. P., Raghavan, A., Tahiliani, M., Zhang, X., Qin, J., Hogan, P. G., and Rao, A. (2000). Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol. Cell 6, 539–550. [DOI] [PubMed] [Google Scholar]
- Okamura, H., Garcia-Rodriguez, C., Martinson, H., Qin, J., Virshup, D. M., and Rao, A. (2004). A conserved docking motif of CK1 binding controls the nuclear localization of NFAT1. Mol. Cell. Biol. 24, 4184–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine, P. L., Moore, L. C., and Horowitz, S. B. (1975). Nuclear envelope permeability. Nature 254, 109–114. [DOI] [PubMed] [Google Scholar]
- Pan, S., Koyano-Nakagawa, N., Tsuruta, L., Amasaki, Y., Yokota, T., Mori, S., Arai, N., and Arai, K. (1997). Molecular cloning and functional characterization of murine cDNA encoding transcription factor NFATc. Biochem. Biophys. Res. Commun. 240, 314–323. [DOI] [PubMed] [Google Scholar]
- Rao, A., Luo, C., and Hogan, P. G. (1997). Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15, 707–747. [DOI] [PubMed] [Google Scholar]
- Shaw, J. P., Utz, P. J., Durand, D. B., Toole, J. J., Emmel, E. A., and Crabtree, G. R. (1988). Identification of a putative regulator of early T cell activation genes. Science 241, 202–205. [DOI] [PubMed] [Google Scholar]
- Sheridan, C. M., Heist, E. K., Beals, C. R., Crabtree, G. R., and Gardner, P. (2002). Protein kinase A negatively modulates the nuclear accumulation of NF-ATc1 by priming for subsequent phosphorylation by glycogen synthase kinase-3. J. Biol. Chem. 277, 48664–48676. [DOI] [PubMed] [Google Scholar]
- Terui, Y., Saad, N., Jia, S., McKeon, F., and Yuan, J. (2004). Dual role of sumoylation in the nuclear localization and transcriptional activation of NFAT1. J. Biol. Chem. 279, 28257–28265. [DOI] [PubMed] [Google Scholar]
- Zhai, W., Eynott, P. R., Oltmanns, U., Leung, S. Y., and Chung, K. F. (2004). Mitogen-activated protein kinase signaling pathways in IL-1β-dependent rat airway smooth muscle proliferation. Br. J. Pharmacol. 143, 1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J., and McKeon, F. (1999). NF-AT activation requires suppression of Crm1-dependent export by calcineurin. Nature 398, 256–260. [DOI] [PubMed] [Google Scholar]
- Zhu, J., Shibasaki, F., Price, R., Guillemot, J. C., Yano, T., Dötsch, V., Wagner, G., Ferrara, P., and McKeon, F. (1998). Intramolecular masking of nuclear import signal on NF-AT4 by casein kinase I and MEKK1. Cell 93, 851–861. [DOI] [PubMed] [Google Scholar]
- Zimber, A., Nguyen, Q. D., and Gespach, C. (2004). Nuclear bodies and compartments: functional roles and cellular signaling in health and disease. Cell Signal 16, 1085–1104. [DOI] [PubMed] [Google Scholar]