Abstract
We present evidence that LIM kinases can control cell adhesion and compaction in human epidermis. LIMK2 is expressed in the epidermal basal layer and signals downstream of the GTPase Rac1 to promote extracellular matrix adhesion and inhibit terminal differentiation. Conversely, LIMK1 is expressed in the upper granular layers and phosphorylates and inhibits cofilin. Expression of LIMK1 is lost in psoriatic lesions and other skin disorders characterized by lack of cell compaction in the differentiating cell layers. In psoriatic lesions down-regulation of LIMK1 correlates with up-regulation of Myc. Expression of constitutively active cofilin or Myc in reconstituted human epidermis blocks cell compaction. Overexpression of LIMK1 leads to down-regulation of Myc, whereas inhibition of Rho kinase, an upstream activator of LIMK1, stimulates Myc expression. Inhibition of Myc by LIMK1 is via inhibition of Stat3 phosphorylation, because constitutively active cofilin or inhibition of Rho kinase results in Stat3 phosphorylation and increased Myc levels, whereas dominant negative Stat3 abolishes the effect. In conclusion, we have uncovered a novel antagonistic relationship between the LIMK1/phosphocofilin and Myc/Stat3 pathways in the differentiating layers of human epidermis and propose that down-regulation of LIMK1 contributes to one of the pathological features of psoriatic epidermal lesions.
INTRODUCTION
Human interfollicular epidermis is a stratified epithelium that is water impermeable and forms a protective barrier against external insults (Freedberg et al., 2003). In normal undamaged epidermis proliferation is largely confined to the basal layer of keratinocytes attached to the underlying basement membrane, and there is a balance between production of new cells in the basal layer and loss of terminally differentiated cells from the outermost layers. As cells move through the suprabasal layers toward the surface of the skin, they undergo a complex series of changes in gene expression and morphology, culminating in destruction of the nucleus and assembly of a layer of cross-linked proteins and lipids known as the cornified envelope. The viable cell layers immediately underneath the cornified layers (subcorneal layers) are known as the granular layers because of the presence of cytoplasmic keratohyalin granules. One feature of cells in the granular layer is that, by an unknown mechanism, they are compacted (flattened) relative to cells in the layers below.
Psoriasis is a common inflammatory skin disorder in which epidermal homeostasis is disturbed. Hallmarks of psoriatic epidermis are hyperproliferation of basal and suprabasal keratinocytes, failure of the outermost viable cells to form keratohyalin granules or undergo compaction, and retention of nuclei in the cornified layers (Lever and Lever, 1990). Although dysfunction of the immune system is known to be an important factor in the pathogenesis of psoriasis (Bowcock and Krueger, 2005), there is also strong evidence that keratinocytes contribute to the disease. Changes in psoriatic keratinocytes include perturbation of intracellular signaling cascades involving integrins (Carroll et al., 1995), TGFβ (Li et al., 2004), VEGF-A (Kunstfeld et al., 2004), epidermal growth factor (EGF; Suzuki et al., 2002), amphiregulin (Cook et al., 1997), Erk MAP kinases (Haase et al., 2001; Takahashi et al., 2002a), the JAK-Stat pathway (Sano et al., 2005), and the transcription factors c-jun and jun-B (Zenz et al., 2005). In these studies attention has focused on keratinocyte hyperproliferation and communication with the immune system, but not on the mechanism by which the granular layer is altered. Indeed in transgenic mouse models it is clear that loss of keratohyalin granules and cell compaction are under separate control from keratinocyte hyperproliferation (Hobbs et al., 2004).
In considering mechanisms that could potentially control epidermal cell compaction, LIM kinases (LIMK) are attractive candidates. LIMKs are serine-threonine–specific kinases that are involved in the organization of the actin cytoskeleton (Arber et al., 1998; Yang et al., 1998). LIMKs phosphorylate and inactivate the actin-severing protein cofilin, thus promoting actin polymerization (Arber et al., 1998; Yang et al., 1998), and can also coordinate microtubule stability and actin polymerization (Gorovoy et al., 2005). In addition, LIMKs can translocate to the nucleus where they induce cyclin D1 expression and stimulate proliferation (Roovers et al., 2003). There are two LIMK subtypes, LIM kinase 1 (LIMK1) and LIM kinase 2 (LIMK2), that have the same enzymatic activity (Ikebe et al., 1997). In addition, two splice variants of LIMK2 are expressed, one of which has two complete LIM domains (LIMK2a) and one of which has 1.5 LIM domains (LIMK2b).
LIM kinases are differentially regulated by Rho GTPases (Yang et al., 1998; Edwards et al., 1999; Maekawa et al., 1999). Downstream of RhoA and RhoC, LIMKs are phosphorylated and activated by Rho kinases (ROCK) to promote actin polymerization during formation of linear actin protrusions (Etienne-Manneville and Hall, 2002). On the other hand, Rac GTPases activate LIMKs via p21-activated kinases (PAK) to induce branching of the actin cytoskeleton (Misra et al., 2005). In addition, LIM kinase and cofilin have been reported to have actin-independent effects and to act as modifiers of several intracellular signaling pathways. For instance, nonphosphorylated cofilin localizes to mitochondria during staurosporine-induced apoptosis (Chua et al., 2003).
In this report, we have investigated the functions of the LIMK-cofilin pathway in human epidermis. We present evidence that LIMK2 is expressed in the epidermal basal layer and acts downstream of Rac1 to promote extracellular matrix adhesion and suppress terminal differentiation. Conversely, LIMK1 is expressed in the upper granular layer, and its activity in inhibiting cofilin may be important for granular cell compaction. We show that LIMK1 negatively regulates Myc and Stat3 and that this pathway is blocked in psoriatic lesions.
MATERIALS AND METHODS
Antibodies and Reagents
The following antibodies were used. Mouse anti-Rac1 and rabbit anti-phospho-EGFR (Y1086) were purchased from Upstate Biotechnology (Lake Placid, NY). Mouse anti-RhoA, rabbit anti-LIMK2, mouse anti-Stat3, mouse anti-phospho-Stat3 (Y705), rabbit anti-SOCS3, rabbit anti-ERK2, and rabbit anti-EGFR were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-cofilin, rabbit anti-phospho-cofilin (S3), rabbit anti-LIMK1, rabbit anti-phospho-LIMK1 (T508)/LIMK2 (T505), rabbit anti-phospho EGFR (Y845), and mouse anti-phospho-ERK were obtained from Cell Signaling (Beverly, MA). Mouse anti-β-tubulin was purchased from Sigma (St. Louis, MO).
ROCK inhibitor Y-27632 (Calbiochem, La Jolla, CA), Src Inhibitor I (Calbiochem), JAK2 inhibitor AG490 (Sigma), cytochalasin D and jasplakinolide (Calbiochem) were used at the concentrations indicated.
Expression Vectors
cDNAs for cofilin and LIM kinase were obtained by RT-PCR using RNA extracted from primary human keratinocytes and cloned into the retroviral vector pBabePuro (Gandarillas and Watt, 1997). S3A-cofilin and LIMKKD mutants were generated by site-directed mutagenesis using the QuickChange II XL mutagenesis kit, as described by the manufacturer (Stratagene, La Jolla, CA). The expression vector for dominant negative Stat3 (ΔC) IRES-EGFP was kindly provided by Dr. Miyajima (Chida et al., 1999).
Nineteen base pairs of sequence (366–384 of NM 005507) were used for the cofilin shRNA. Briefly, 5′-gatccccgaagaacatcatcctggagttcaagagactccaggatgatgttcttctttttggaaa-3′ and 5′-agcttttccaaaaagaagaacatcatcctggagtctcttgaactccaggatgatgttcttcggg-3′ were annealed, phosphorylated, and inserted into the BglII and HindIII sites of pRetroSuperPuro vector (Brummelkamp et al., 2002). The pBabePuro MycER and eGFP-Rac1QL vectors have been described previously (Gandarillas and Watt, 1997; Benitah et al., 2005). Retroviral vectors were packaged by a two-stage procedure involving transient transfection of ecotropic Phoenix cells and stable infection of amphotropic AM12 cells (Benitah et al., 2005).
Cell Culture and Retroviral Infection
Primary human keratinocytes were isolated from neonatal foreskin and cocultured with mitomycin C–treated J2-3T3 cells in FAD medium (1 part Ham's F12 medium, 3 parts DMEM, 1.8 × 10-4 M adenine) supplemented with 10% fetal calf serum, 0.5 μg/ml hydrocortisone, 5 μg/ml insulin, 10-10 M cholera toxin, and 10 ng/ml EGF, as described previously (Gandarillas and Watt, 1997). J2-3T3 cells were cultured in DMEM containing 10% donor calf serum. For experiments in which keratinocytes were grown under serum-free conditions, keratinocyte-SFM (Invitrogen, Carlsbad, CA) was used. For retroviral infection, primary human keratinocytes were cocultured with amphotropic AM12 retroviral packaging cell lines as previously described (Benitah et al., 2005).
Cell Spreading Assay
Spreading assays were performed as previously described (Frye et al., 2003) with minor modifications. Briefly, 10-mm diameter glass coverslips were coated with 10 μg/ml type I collagen (BD Bioscience, San Diego, CA). Keratinocytes were harvested with trypsin and EDTA and seeded at 103 cells per coverslip for 10 or 30 min at 37°C. The coverslips were washed to remove nonadherent cells, then fixed with 4% paraformaldehyde, permeabilized with 3% Triton X-100, and stained with Alexa-488–conjugated phalloidin. The total number of adherent cells and the percentage of spread cells were determined.
Clonogenicity Assay
Keratinocytes, 103 or 5 × 103, were plated per 35-mm dish on a feeder layer of J2–3T3. Fourteen days later the cultures were fixed in 4% formaldehyde for 10 min and stained with 1% Rhodanile Blue. Any group of two or more cells was scored as a colony. Colony forming efficiency was calculated as the percentage of plated cells that formed colonies. All experiments were performed in triplicate and more than 100 colonies were scored per experimental condition.
Reconstitution of Human Epidermis on De-epidermised Dermis and in Nude Mice
Reconstituted human epidermis was prepared as described previously (Gandarillas and Watt, 1997). Briefly, adult human breast skin from mastectomy operations was heated at 56°C for 30 min and the epidermis was peeled away from the underlying dermis. De-epidermised dermis was cut into 1.0-cm2 squares and subjected to 10 freeze/thaw cycles in liquid nitrogen in order to kill any remaining cells. Retrovirally infected primary human keratinocytes, 2 × 105, were seeded per piece of de-epidermised dermis (DED) and grown at the air/liquid interface for 10–14 d. DED cultures were embedded in paraffin after fixation in 4% paraformaldehyde.
To reconstitute human epidermis in nude mice, the method described by Morris et al. (2004) was used with slight modifications. Briefly, 5 × 105 neonatal mouse skin fibroblasts and 5 × 105 human keratinocytes infected with pBabe or S3A-cofilin were suspended in DMEM medium with 10% fetal bovine serum and injected subcutaneously into nude mice. Three weeks after injection, the epidermal cysts that had formed were collected, fixed in 4% paraformaldehyde, and embedded in paraffin for histological analysis.
Immunostaining
Samples of normal and diseased skin were obtained with informed consent and local ethical approval and generously provided by Dr. Iizuka (Asahikawa Medical College, Japan). Paraffin embedded sections were de-waxed and subjected to antigen retrieval by incubation in 10 mM sodium citrate buffer (pH 6.0) at 100°C for 10 min. Sections were then blocked with 10% BSA in phosphate-buffered saline (PBS) for 1 h and incubated with primary antibodies diluted in 1% BSA in PBS overnight at 4°C. Alexa-488– or 594–conjugated anti-mouse or anti-rabbit antibodies (Molecular Probes, Eugene, OR) were used to detect primary antibodies. Nuclei were counterstained with DAPI or Hoechst dyes (Molecular Probes).
Western Blotting
Cells were washed twice with ice-cold PBS and lysed in ice-cold RIPA buffer containing a protease inhibitor cocktail (Roche, Indianapolis, IN). Protein concentrations were determined with the BCA protein assay kit (Pierce, Rockford, IL). Proteins (20–50 μg per lane) were separated by SDS-PAGE and transferred to Hybond-P Nitrocellulose membranes (Amersham Bioscience, Piscataway, NJ). Blotted membranes were blocked with Tris-buffered saline containing 0.1% Tween-20 and 5% skimmed milk and then incubated with primary antibodies overnight at 4°C. Proteins were visualized with anti-mouse or anti-rabbit IgG horseradish-peroxidase–linked antibodies (Amersham Bioscience) for 1 h, followed by chemiluminescence detection (ECL, Amersham Bioscience).
RT-PCR
Ten micrograms of total RNA extracted from primary human keratinocytes using TriReagent (Invitrogen) were reverse transcribed using the Thermoscript System (Invitrogen). Subsequently, the same amount of first strand cDNA was amplified after 30 cycles using the following forward primers: 5′-tcctcctccccatttccg-3′ for amplification of the LIMK2a variant, or 5′-cagaggtgccgggagccc-3′ for the LIMK2b variant. The same reverse primer was used for both LIMK2 variants (5′-cagaactccccaaacttcc-3′). 5′-ctggtgctccatgaggagacac-3′ and 5′-tccaagacgttgtgtgttcgc-3′ were used for detection of c-Myc transcripts, and 5′-gccatcctaaaagccacccc-3′ and 5′-cacccactcccagggagacc-3′ primers were used to detect β-actin.
RESULTS
Expression of LIMK1, LIMK2, and Cofilin in Human Epidermis and Cultured Keratinocytes
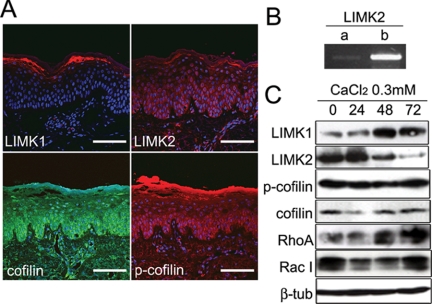
Immunofluorescence staining of sections of normal human epidermis showed high expression of LIMK1 in the granular layers (Figure 1A). In contrast, LIMK2 was predominantly expressed in the basal and lower spinous (i.e., the first suprabasal) layers (Figure 1A). Cofilin was expressed throughout the epidermis, although it was most abundant in the basal and lower spinous layers. The inactive, phosphorylated form of cofilin (pS3) was expressed in the sites of LIMK2 and LIMK1 expression, namely basal/lower spinous and granular layers, respectively. However, expression was stronger in the basal and lower spinous layers than in the granular layer (p-cofilin; Figure 1A). RT-PCR analysis of primary human keratinocytes revealed that the predominant epidermal isoform of LIMK2 is LIMK2b (Figure 1B).
Figure 1.
LIM kinase and cofilin expression in normal epidermis and cultured keratinocytes. (A) Normal human epidermis immunostained for LIMK1, LIMK2, cofilin, and phosphorylated cofilin with DAPI nuclear counterstain. Scale bars, 100 μm. (B) RT-PCR of primary human keratinocytes with primers specific for LIMK2a and LIMK2b. (C) Western blots of cultured human keratinocytes grown in low calcium medium (0) or transferred to medium containing 0.3 mM calcium ions for the number of hours shown. β-Tubulin was used as a loading control.
We next examined whether there was differential expression of LIMK1 and LIMK2 in cultured primary human keratinocytes. We performed Western blotting on keratinocytes that had been maintained in medium containing a low concentration of calcium ions or transferred to medium containing 0.3 mM calcium for up to 72 h to induce stratification and accumulation of terminally differentiated keratinocytes (Hennings et al., 1980; Watt and Green, 1982). Expression of LIMK1 increased during calcium-induced stratification, whereas LIMK2 levels decreased (Figure 1C). Cofilin and phosphocofilin levels were unaffected by addition of calcium (Figure 1C). We conclude that the patterns of expression of LIM kinases in culture mimic those observed in vivo.
Because LIM kinases are regulated by the Rho family of GTPases (Etienne-Manneville and Hall, 2002), we also examined expression of RhoA and Rac1. The level of RhoA increased during calcium induced stratification, whereas Rac1 had a similar bimodal pattern of expression to LIM kinases (Figure 1C).
Loss of LIMK1 in Psoriatic Epidermis and Other Epidermal Disorders with Aberrant Cell Compaction
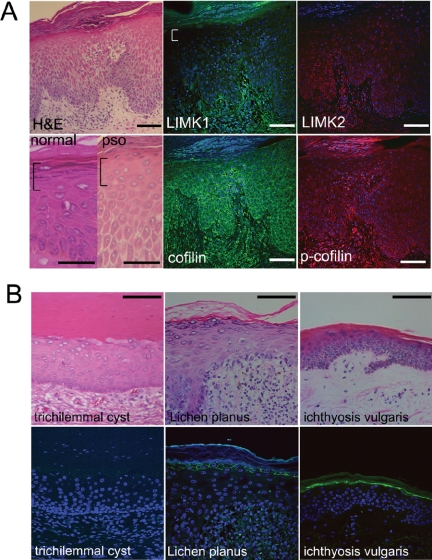
Because LIMK1 is expressed in the flattened granular cells of normal epidermis, we examined whether expression was altered in psoriatic lesions. Unaffected skin from psoriasis patients showed patterns of LIMK1 and cofilin expression similar to normal epidermis (unpublished data). However, LIMK1 expression was markedly reduced in psoriatic lesions (Figure 2A, white bracket). In contrast to LIMK1, expression of LIMK2 and cofilin was unaltered (Figure 2A). Phosphocofilin was expressed normally in the basal and lower spinous layers of psoriatic epidermis, but showed decreased expression in the subcorneal layers, correlating with the loss of LIMK1 (Figure 2A).
Figure 2.
Loss of LIMK1 in skin disorders with a failure of subcorneal cell compaction. (A) Histological appearance of normal and psoriatic (H&E, pso) epidermis stained with hematoxylin and eosin (left-hand panels) and immunostaining of psoriatic epidermis with the antibodies shown. Brackets denote region of cell compaction in normal epidermis and equivalent region in psoriatic epidermis. (B) Other skin diseases with granular layer abnormalities, stained with hematoxylin and eosin (top panels) or immunostained for LIMK1 (bottom panels). (A and B) Immunostained sections were counterstained with DAPI in order to visualize cell nuclei. Scale bars, (A) 50 μm, (B) 100 μm.
We next examined whether expression of LIMK1 was reduced in other skin disorders with known granular layer abnormalities. The epidermis that forms the wall of a trichilemmal cyst lacks proper cell compaction and granular layer formation (Lever and Lever, 1990) and showed complete loss of LIMK1 (Figure 2B). In Lichen planus there is hyper-granulosis, an increased number of granular layers, with normal cell compaction (Lever and Lever, 1990); as expected, LIMK1 expression was retained (Figure 2B). Ichthyosis vulgaris lesions lack keratohyalin granules, but still undergo compaction of the subcorneal layers (Lever and Lever, 1990; Figure 2B). Expression of LIMK1 was maintained in Ichthyosis vulgaris, indicating that loss of LIMK1 correlates with a failure of cell compaction, rather than loss of a granular layer (Figure 2B). These results led us to hypothesize that LIMK1-induced actin polymerization via inhibition of cofilin activity might mediate cell flattening in the subcorneal layers, and thus play a role in epidermal barrier formation.
LIMK2 Is Involved in Adhesion and Spreading of Basal Undifferentiated Keratinocytes
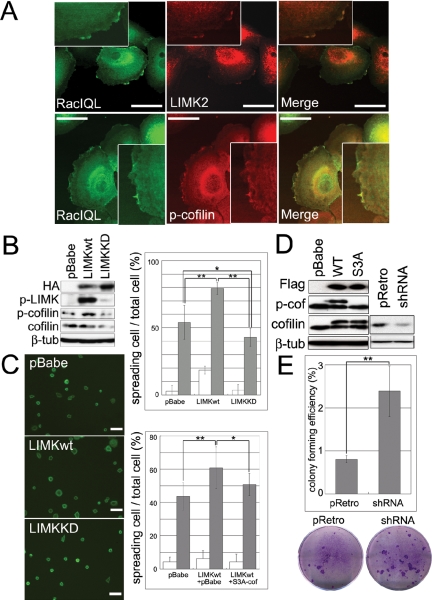
We have previously shown that the small GTPase Rac1 is required to maintain basal keratinocytes in an undifferentiated state (Benitah et al., 2005). Rac1 activates LIMKs (Yang et al., 1998) and LIMK2, like Rac1, is expressed in the basal layer of human epidermis (Figure 1A; Benitah et al., 2005). In addition, when human keratinocytes were transduced with a retroviral vector encoding activated Rac1 tagged with eGFP (eGFP-Rac1QL), Rac1 colocalized with endogenous LIMK2 and phosphocofilin in ruffled membranes (Figure 3A). This led us to investigate whether the LIMK pathway had any effect on keratinocyte adhesion and differentiation.
Figure 3.
LIMK and cofilin regulate keratinocyte spreading and clonal growth. (A) Keratinocytes were infected with eGFP-Rac1 (Rac1QL), and the subcellular localizations of Rac1, LIMK2 and p-cofilin were compared by double-label immunofluorescence. Scale bars, 50 μm. Insets show cell periphery at higher magnification. (B) Western blotting of keratinocytes infected with control retroviral vector (pBabe), HA-tagged wild-type LIMK (LIMKwt), or HA-tagged kinase dead LIMK (LIMKKD). (C) Keratinocytes were plated on type I collagen for 10 min (white bars; Alexa 488-phalloidin labeling; scale bars, 100 μm) or 30 min (gray bars). Top graph shows ratio of spread cells to total adherent cells infected with pBabe, LIMKwt, and LIMKKD. Bottom graph shows ratio of spread cells to total adherent cells infected with pBabe alone, LIMKwt and pBabe, or LIMKwt and S3A-cofilin. More than 10 microscopic fields were analyzed per data set. Mean ± SEM is shown. * p < 0.05, ** p < 0.01. (D) Western blots of primary keratinocytes infected with control vector (pBabe), Flag-tagged wild-type (WT) or constitutively active (S3A) cofilin, shRNA control vector (pRetro) or shRNA for cofilin (shRNA) Upper and lower bands detected with anti-cofilin antibody are Flag-tagged and endogenous cofilin, respectively. (E) Effect of cofilin RNAi on colony forming efficiency of primary human keratinocytes. Representative culture dishes are shown. Data are mean ± SEM (** p < 0.01).
Although LIMK1 and LIMK2 are expressed in different epidermal layers, they have the same enzymatic activity and thus overexpression of LIMK1 in basal keratinocytes has the same biological effect as activating LIMK2 (Arber et al., 1998; Yang et al., 1998; Sumi et al., 1999). Primary human keratinocytes were transduced with retroviral vectors encoding HA-tagged wild-type LIMK1 (LIMKwt), an HA-tagged kinase dead mutant (LIMKKD), in which threonine 508 was mutated to alanine, or empty vector alone (pBabe). Expression of the LIMK constructs was verified by Western blotting (Figure 3B). Ectopic LIMKwt, but not LIMKKD, was phosphorylated, and therefore active, and led to an increase in the phosphorylation of endogenous cofilin (Figure 3B).
When keratinocytes transduced with empty vector were plated on type I collagen, fewer than 10% had begun to spread by 10 min, but by 30 min 40–50% had started spreading (Figure 3C). Keratinocytes that overexpressed LIMKwt had an accelerated rate of spreading at both time points, whereas LIMKKD reduced cell spreading slightly (Figure 3C). LIMK-induced spreading was at least partially dependent on inhibition of cofilin, because introduction of constitutively active S3A-cofilin reduced the effect of LIMKwt on keratinocyte spreading (Figure 3C). Expression of S3A-cofilin or wild-type cofilin could be distinguished from endogenous cofilin by Western blotting, because the Flag tagged constructs ran at a higher molecular mass (Figure 3D). While wild-type and endogenous cofilin were phosphorylated, S3A cofilin was not (Figure 3D).
To determine whether the effects of the LIMK/cofilin pathway on keratinocyte spreading correlated with an effect on clonal growth, we reduced endogenous cofilin expression by ∼50% using a retroviral vector encoding cofilin RNAi (Figure 3D). RNAi-induced diminution of cofilin expression resulted in a threefold increase in colony forming efficiency compared with control cells (Figure 3E). Taken together, the data suggest that one mechanism by which Rac1 prevents keratinocyte differentiation and promotes clonal growth (Benitah et al., 2005) is by activating LIMK2 and thereby inhibiting cofilin.
LIMK1 and Cofilin Promote Compaction of Cells in the Subcorneal Layers
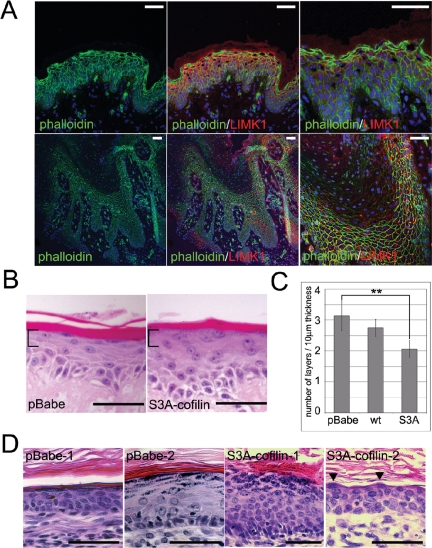
We next examined the role of LIMK1 and cofilin in epidermal cell compaction. Phalloidin staining of normal human epidermis showed that the LIMK1-positive cell layers contained abundant polymerized actin (Figure 4A). In contrast, the uncompacted subcorneal layers of psoriatic epidermis, which lacked LIMK1, showed a reduction in actin polymerization (Figure 4A).
Figure 4.
Failure of cell compaction correlates with reduced LIMK1, reduced actin polymerization and increased cofilin activation. (A) Sections of normal (top panels) and psoriatic (bottom panels) epidermis were labeled with Alexa 488–conjugated phalloidin (green), anti-LIMK1 antibody (red), and DAPI nuclear counterstain. (B–D) Keratinocytes transduced with empty retroviral vector (pBabe), wild-type cofilin (wt), and constitutively active cofilin (S3A-cofilin) were cultured on DED (B and C) or injected subcutaneously into nude mice and allowed to form subcutaneous epidermal cysts (D). (C) Quantitation of number of cell layers per 10 μm of subcorneal region (corresponding to brackets in B); ** p < 0.01. In D the results of two independent experiments (1, 2) are shown. Scale bars, 50 μm.
To determine whether repression of cofilin by LIMK might be involved in cell compaction, we reconstituted human epidermis in culture by growing cells at the air-medium interface on DED (Gandarillas and Watt, 1997; Haase et al., 2001). As shown in Figure 4B, keratinocytes transduced with empty vector generated an epidermis with a flattened subcorneal layer, containing keratohyalin granules. In contrast, keratinocytes transduced with S3A-cofilin failed to undergo cell compaction, although they did form keratohyalin granules and anucleate cornified layers (Figure 4B, brackets). The compaction of the subcorneal layers of S3A-cofilin DED cultures was reduced by ∼30% when compared with control keratinocytes (Figure 4C). The inhibition of compaction was dependent on active, nonphosphorylated cofilin, because introduction of wild-type cofilin had a very modest effect (Figure 4C).
As a test of whether constitutively active cofilin could also block cell compaction in vivo, we injected primary human keratinocytes, in combination with neonatal mouse dermal fibroblasts, subcutaneously into nude mice. As shown in Figure 4D, expression of S3A-cofilin significantly impaired cell compaction in the subcorneal layers, irrespective of the overall thickness of the epidermis or total number of granular layers. Our results therefore suggest that inhibition of cofilin by LIMK1 is necessary for adequate cell compaction in the granular layer of the epidermis and that overactivation of cofilin leads to a lack of cell compaction, reminiscent of psoriatic epidermis.
Antagonistic Relationship between LIMK and Myc in Regulating Cell Compaction
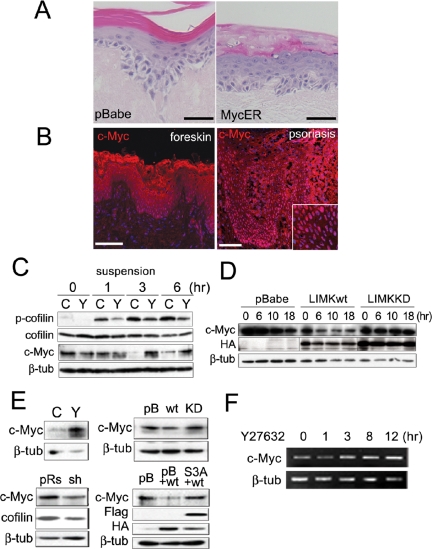
We have previously reported that Rac1 negatively regulates Myc via PAK2 phosphorylation (Benitah et al., 2005) and that expression of a 4-hydroxy-tamoxifen (4OHT) inducible form of Myc (MycER) stimulates keratinocyte differentiation in vitro and in vivo (Gandarillas and Watt, 1997; Arnold and Watt, 2001). We noted that when MycER-transduced keratinocytes were cultured on DED, the subcorneal layers did not undergo compaction (Gandarillas and Watt, 1997; Figure 5A) and that Myc was highly expressed in the suprabasal layers of psoriatic epidermis (Figure 5B; Tadini et al., 1989; Elder et al., 1990). This led us to investigate whether there was any relationship between LIMK1 and Myc in regulating cell compaction in the subcorneal epidermal layers.
Figure 5.
Relationship between LIMK, cofilin, Myc, and cell compaction. (A) Keratinocytes transduced with empty retroviral vector (pBabe) or MycER were cultured on DED in the presence of 4OHT and stained with hematoxylin and eosin. (B) Immunostaining for Myc (red; DAPI nuclear counterstain in blue) in normal neonatal foreskin and adult psoriatic lesion. Inset shows higher magnification view. Scale bars, (A and B) 50 μm. (C–E) Western blots of adherent keratinocytes (E) or keratinocytes held in suspension for the number of hours shown (C and D). Blots were probed with antibodies to the proteins shown. β-Tubulin (β-tub) served as a loading control. Cells were either not transduced with retroviral vectors (C and E, top left-hand panel) or transduced with empty retroviral vector (pBabe, pB; pRs) or vectors encoding LIMKwt (wt), LIMKKD (KD), cofilin RNAi (sh), or constitutively active cofilin (S3A). Cells in C and E (top left hand panel) were untreated (C) or treated with Y27632 (Y). (F) RT-PCR of mRNA from keratinocytes treated with Y27632 for the number or hours shown.
When primary human keratinocytes are placed in suspension, terminal differentiation is induced within 24 h: markers such as involucrin and cornifin are up-regulated and there is a corresponding reduction in Myc expression (Gandarillas and Watt, 1995; Figure 5C). We found that down-regulation of Myc in suspension was inhibited by the ROCK inhibitor, Y27632 (Figure 5C, c-Myc panel). Down-regulation of Myc in suspension was correlated with an increase in the level of phosphocofilin, which was also inhibited by Y27632 (Figure 5C, top panel). Ectopic expression of LIMK1wt reduced Myc levels, whereas LIMKKD prevented the decrease in Myc during suspension induced differentiation (Figure 5D).
The effects of ROCK, LIMK1 and cofilin on expression of Myc were also observed in adherent primary keratinocytes. Treatment of adherent cells with the ROCK inhibitor led to a robust increase in Myc expression (Figure 5E, top left panel). Accordingly, LIMKwt reduced, whereas LIMKKD enhanced, Myc expression (Figure 5E, top right panel). This effect was dependent on cofilin, because cofilin RNAi reduced Myc expression, whereas introduction of S3A-cofilin prevented LIMKwt-mediated down-regulation of Myc (Figure 5E, bottom panels). RT-PCR analysis of keratinocytes treated with Y27632 showed that the increase in Myc protein correlated with an increase in Myc mRNA (Figure 5F). This suggests that regulation of Myc expression by the ROCK/LIMK1/cofilin pathway occurs, at least in part, at the level of transcription, although effects on Myc protein stability cannot be ruled out (Figure 5F).
Taken together, these results demonstrate that one mechanism by which the ROCK/LIMK1/cofilin pathway promotes cell compaction in the granular layer is by inhibiting the expression of Myc.
Inhibition of Myc Expression by LIMK1 Is via Inhibition of Stat3 Phosphorylation
Recently, Sano et al. (2005) have demonstrated a role for Stat3 in psoriasis. Psoriatic keratinocytes have high levels of active, tyrosine-phosphorylated Stat3, and overexpression of Stat3 in transgenic mouse epidermis leads to the development of psoriatic lesions (Sano et al., 2005). Stat3 is a transcription factor that acts downstream of several growth factors and cytokines to regulate cell proliferation, motility, differentiation, and survival (Yu and Jove, 2004). Myc is a direct target of Stat3 (Bowman et al., 2001), and Stat3-mediated transcriptional activation of some genes depends on a functional interaction with Myc (Barre et al., 2005). We therefore sought to determine whether there was any relationship between LIMK1 and Stat3 in regulating cell compaction in normal and psoriatic epidermis.
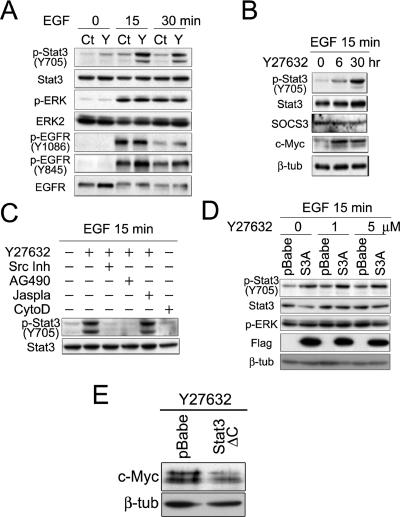
Treatment of primary human keratinocytes with the known Stat3 activator EGF induced an increase in phospho-Y705-Stat3 (Figure 6A, top panel). The ROCK inhibitor Y27632 increased basal levels of active pY-Stat3 to the same extent as EGF and synergized with EGF to induce stronger activation of Stat3 than by EGF or Y27632 alone (Figure 6A, p-Stat3 Y705 panel). In contrast, inhibition of ROCK did not affect EGF-induced ERK MAPK phosphorylation (Figure 6A, p-ERK and ERK2 panels), nor phosphorylation of EGFR on tyrosine 1086, which is responsible for the activation of the Ras/MAPK and PI3K/Akt pathways via Grb2 and Gab1 (Batzer et al., 1994; Rodrigues et al., 2000; Figure 6A). Treatment of primary keratinocytes with Y27632 did result in an increase in EGFR phosphorylation on tyrosine 845, which is required for stimulation of the Src/Stat3 pathway (Sato et al., 1995; Figure 6A).
Figure 6.
The ROCK/LIMK/p-cofilin pathway inhibits c-Myc expression by down-regulating Stat3 tyrosine phosphorylation. (A) Growth factor–starved keratinocytes were treated with 100 ng/ml EGF for the number of minutes shown. Keratinocytes were untreated (Ct) or treated with 15 μM Y27632 (Y) 24 h before addition of EGF. (B) Keratinocytes cultured in complete medium were pretreated with Y27632 (15 μM) for the times shown and then harvested after treatment with EGF for 15 min. (C) Keratinocytes were pretreated with 15 μM Y27632, 100 μM AG490, 10 μM of Src inhibitor I (Src Inh), 300 nM Jasplakinolide (Jaspla), or 3 μM cytochalasin D (CytoD) for 2 h before EGF administration. (D) Growth factor–starved keratinocytes infected with empty vector (pBabe) or Flag-tagged S3A-cofilin constructs (S3A) were treated with 100 ng/ml EGF after pretreatment with the concentrations of Y27632 shown. (E) Keratinocytes infected with pBabe or dominant negative Stat3(ΔC) were treated with 15 μM Y27632 for 24 h before lysis. (A–E) Lysates were subjected to Western blotting with the antibodies shown. In some cases β-tubulin (β-tub) served as a loading control.
We next investigated the mechanism whereby the ROCK pathway regulated Stat3 activity. Up-regulation of p-Stat3 upon ROCK inhibition was not due to changes in the expression of SOCS, a Stat3-specific inhibitor (Figure 6B; Wormald and Hilton, 2003). Up-regulation of Stat3 activity after Y27632 treatment occurred as early as 6 h, a time at which an increase of Myc expression was observed (Figure 6B, Myc panel). Src and JAK2 kinases lie downstream of receptor tyrosine kinases to directly bind and activate Stat proteins (Levy and Darnell, 2002). Treatment of keratinocytes with the ROCK inhibitor increased tyrosine phosphorylation of Stat3 and cotreatment with Src inhibitor I, or AG490, a JAK2 inhibitor, completely abolished this effect (Figure 6C). Thus, negative regulation of Stat3 by ROCK/LIMK depends on the activities of Src and JAK2 kinases.
The effects of LIMK/cofilin on Stat3 activation were not dependent on actin dynamics. Treatment with an inhibitor of actin depolymerization, jasplakinolide, did not prevent the effect of Y27632 treatment (Figure 6C, jaspla). In addition, treatment with the actin depolymerizing agent cytochalasin D (CytoD) was not sufficient to induce phosphorylation of Stat3 (Figure 6C, CytoD). These results suggest that cofilin has actin organization–independent effects on keratinocyte gene expression (Figure 6D).
Dominant active mutant cofilin (S3A) synergized with Y27632 to increase Stat3 tyrosine phosphorylation (Figure 6D). Up-regulation of Myc expression upon treatment of cells with Y27632 depended on the activity of Stat3, because a dominant negative mutant of Stat3 completely abolished this effect (Figure 6E). Thus, taken together, our results suggest that the ROCK/LIMK1/p-cofilin pathway promotes subcorneal keratinocyte compaction by preventing phosphorylation of Stat3 and up-regulation of Myc.
DISCUSSION
We have found that LIMK1 and LIMK2b have complementary patterns of expression in normal human epidermis, with LIMK2b expressed in the basal and lower spinous layers and LIMK1 in the granular layers. Our studies suggest that LIMK2b contributes to promoting extracellular matrix adhesion of basal keratinocytes, whereas LIMK1 may mediate granular cell compaction by inhibiting cofilin. Although LIMK1 and LIMK2 single knock-out mice are not reported to have any epidermal abnormalities (Meng et al., 2002; Takahashi et al., 2002b), the consequences of losing the functions we have identified might be too subtle to attract attention. For example, the effect of overexpressing the β1 integrin subunit in the basal layer of transgenic mouse epidermis becomes manifest only when adhesion is disrupted by activation of c-Myc (Gebhardt et al., 2006).
LIM kinases are known to be activated by Rac GTPases via PAK (Misra et al., 2005). Rac1 is highly expressed in the epidermal basal layer (Benitah et al., 2005) and we found that LIMK2b colocalized with activated Rac1 in cultured keratinocytes. LIMK promoted keratinocyte adhesion, an effect that was dependent on inhibition of cofilin. Partial down-regulation of cofilin using RNAi increased the colony forming ability of cultured keratinocytes.
Keratinocyte adhesion to extracellular matrix has long been known to suppress terminal differentiation in culture, and epidermal stem cells are more adhesive than transit amplifying cells (Watt, 2002). High levels of integrin expression and Erk MAPK activation were previously shown to promote keratinocyte adhesion and suppress differentiation (Zhu et al., 1999). In contrast, Myc reduces adhesion and promotes terminal differentiation (Gandarillas and Watt, 1997), in part by directly repressing expression of genes encoding integrins and components of the actin cytoskeleton (Frye et al., 2003; Gebhardt et al., 2006). Activation of Rac promotes clonal growth of keratinocytes, down-regulating Myc via PAK2-mediated phosphorylation (Benitah et al., 2005). We have now defined an additional mechanism by which Rac1 can promote adhesion and suppress differentiation, namely via LIMK2 and suppression of cofilin, which can also impact on Myc expression.
We found that loss of expression of LIMK1 in the subcorneal layers correlated with a failure of cell compaction in psoriasis and other epidermal disorders. Cell compaction is under different control from keratohyalin granule formation; for example, in Ichthyosis vulgaris cell compaction and LIMK1 expression are normal, yet the epidermis lacks a granular layer. In normal epidermis desquamation is a highly ordered process, with continuous shedding of individual cornified cells (Goldschmidt and Kligman, 1967; Milstone, 2004; Lippens et al., 2005). The lack of cell compaction that occurs in the suprabasal layers of psoriatic epidermis may contribute to formation of psoriatic scales, accumulated cornified layers that are periodically shed en masse.
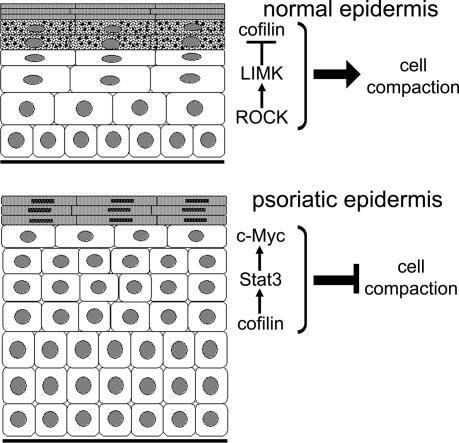
Keratinocytes expressing a dominant active cofilin mutant failed to undergo subcorneal layer compaction in epidermis reconstituted in vivo and in culture, even though keratohyalin granule formation occurred normally. Our observations lead us to propose the model shown in Figure 7, in which expression of LIMK1 in the granular layers mediates cell compaction by negatively regulating cofilin. LIMK is activated by ROCK, and previous studies have shown a requirement for ROCK in formation of a polarized actin cytoskeleton within the epidermis (Vaezi et al., 2002). In addition, ROCK activation in keratinocytes stimulates expression of genes associated with terminal differentiation (McMullan et al., 2003). Because LIMK1 is implicated in coordinating the dynamics of microtubules and actin (Gorovoy et al., 2005), it is possible that microtubules also play a role in granular cell compaction.
Figure 7.
Schematic representation of the role of the ROCK/LIMK/p-cofilin pathway in controlling subcorneal cell compaction in normal and psoriatic epidermis. In normal epidermis LIMK1 is up-regulated in the subcorneal layers, thereby stimulating cell compaction by phosphorylating and inactivating cofilin. In psoriatic epidermis LIMK1 is not up-regulated and as a result cofilin is active. Cofilin activation leads to Stat3 phosphorylation and upregulation of Myc, thereby blocking cell compaction.
In psoriatic epidermis there is loss of LIMK1 and increased expression of Myc and phophoStat3 (Figures 2 and 5; Tadini et al., 1989; Elder et al., 1990). We propose that subcorneal cell compaction is inhibited because in the absence of LIMK1 cofilin positively regulates Stat3 and Myc (Figure 7). Keratinocytes that overexpress dominant active cofilin or Myc fail to form a compacted granular layer, either in vivo or in culture (Figures 4 and 5; Gandarillas and Watt, 1997; Pelengaris et al., 1999). The findings that downstream of ROCK LIMK negatively regulates Myc activity by inhibiting cofilin, and Rac1 negatively regulates Myc via PAK2-mediated phosphorylation (Benitah et al., 2005; Huang et al., 2004) suggest that Rho GTPases may be a general mechanism that by which Myc levels are controlled.
Stat3 is up-regulated in psoriatic lesions and contributes to epidermal hyperproliferation and inflammation (Sano et al., 2005). Potential signals that could activate Stat3 are IL-6, IFN-γ, and EGF, all of which are up-regulated in psoriatic skin (Nickoloff and Nestle, 2004). Our data suggest that EGF is involved in the cofilin pathway. We have found that inhibition of ROCK or expression of dominant active cofilin synergizes with EGF to promote Stat3 activation in a JAK2- and Src-dependent manner. Inhibition of ROCK increased the level of phosphorylated EGFR on tyrosine 845, which is the tyrosine responsible for Src activation (Sato et al., 1995).
In conclusion, we have described a novel relationship between LIMK1, Myc, and Stat3 that controls subcorneal cell compaction in normal and diseased epidermis. Future studies will be necessary to test whether this pathway is a useful target for new antipsoriatic treatments.
Acknowledgments
We are grateful to everyone who gave us advice and reagents. M.H. was on leave from Asahikawa Medical College. S.A.B. is the recipient of European Molecular Biology Organization and European Union Marie Curie Fellowships. This work was supported by Cancer Research UK.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-12-1173) on February 8, 2006.
References
- Arber, S., Barbayannis, F. A., Hanser, H., Schneider, C., Stanyon, C. A., Bernard, O., and Caroni, P. (1998). Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393, 805-809. [DOI] [PubMed] [Google Scholar]
- Arnold, I., and Watt, F. M. (2001). c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr. Biol. 11, 558-568. [DOI] [PubMed] [Google Scholar]
- Barre, B., Vigneron, A., and Coqueret, O. (2005). The STAT3 transcription factor is a target for the Myc and riboblastoma proteins on the Cdc25A promoter. J. Biol. Chem. 280, 15673-15681. [DOI] [PubMed] [Google Scholar]
- Batzer, A. G., Rotin, D., Urena, J. M., Skolnik, E. Y., and Schlessinger, J. (1994). Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol. Cell. Biol. 14, 5192-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitah, S. A., Frye, M., Glogauer, M., and Watt, F. M. (2005). Stem cell depletion through epidermal deletion of Rac1. Science 309, 933-935. [DOI] [PubMed] [Google Scholar]
- Bowcock, A. M., and Krueger, J. G. (2005). Getting under the skin: the immunogenetics of psoriasis. Nat. Rev. Immunol. 5, 699-711. [DOI] [PubMed] [Google Scholar]
- Bowman, T., Broome, M. A., Sinibaldi, D., Wharton, W., Pledger, W. J., Sedivy, J. M., Irby, R., Yeatman, T., Courtneidge, S. A., and Jove, R. (2001). Stat3-mediated Myc expression is required for Src transformation and platelet-derived growth factor-induced mitogenesis. Proc. Natl. Acad. Sci. USA 98, 7319-7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp, T. R., Bernards, R., and Agami, R. (2002). A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550-553. [DOI] [PubMed] [Google Scholar]
- Carroll, J. M., Romero, M. R., and Watt, F. M. (1995). Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell 83, 957-968. [DOI] [PubMed] [Google Scholar]
- Chida, D., Miura, O., Yoshimura, A., and Miyajima, A. (1999). Role of cytokine signaling molecules in erythroid differentiation of mouse fetal liver hematopoietic cells: functional analysis of signaling molecules by retrovirus-mediated expression. Blood 93, 1567-1578. [PubMed] [Google Scholar]
- Chua, B. T., Volbracht, C., Tan, K. O., Li, R., Yu, V. C., and Li, P. (2003). Mitochondrial translocation of cofilin is an early step in apoptosis induction. Nat. Cell Biol. 5, 1083-1089. [DOI] [PubMed] [Google Scholar]
- Cook, P. W., Piepkorn, M., Clegg, C. H., Plowman, G. D., DeMay, J. M., Brown, J. R., and Pittelkow, M. R. (1997). Transgenic expression of the human amphiregulin gene induces a psoriasis-like phenotype. J. Clin. Invest. 100, 2286-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, D. C., Sanders, L. C., Bokoch, G. M., and Gill, G. N. (1999). Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell Biol. 1, 253-259. [DOI] [PubMed] [Google Scholar]
- Elder, J. T., Tavakkol, A., Klein, S. B., Zeigler, M. E., Wicha, M., and Voorhees, J. J. (1990). Protooncogene expression in normal and psoriatic skin. J. Invest. Dermatol. 94, 19-25. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and Hall, A. (2002). Rho GTPases in cell biology. Nature 420, 629-635. [DOI] [PubMed] [Google Scholar]
- Freedberg, I. M., Eisen, A. Z., Wolff, K., Austen, K. F., Goldsmith, L. A. and Katz, S. I. (2003). Dermatology in General Medicine, New York: McGraw-Hill, 107 pp.
- Frye, M., Gardner, C., Li, E. R., Arnold, I., and Watt, F. M. (2003). Evidence that Myc activation depletes the epidermal stem cell compartment by modulating adhesive interactions with the local microenvironment. Development 130, 2793-2808. [DOI] [PubMed] [Google Scholar]
- Gandarillas, A., and Watt, F. M. (1995). Changes in expression of members of the fos and jun families and myc network during terminal differentiation of human keratinocytes. Oncogene 11, 1403-1407. [PubMed] [Google Scholar]
- Gandarillas, A., and Watt, F. M. (1997). c-Myc promotes differentiation of human epidermal stem cells. Genes Dev. 11, 2869-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt, A., Frye, M., Herold, S., Benitah, S. A., Braun, K., Samans, B., Watt, F. M., Elsasser, H. P., and Eilers, M. (2006). Myc regulates keratinocyte adhesion and differentiation via complex formation with Miz1. J. Cell Biol. 172, 139-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt, H., and Kligman, A. M. (1967). Desquamation of the human horny layer. Arch. Dermatol. 95, 583-586. [PubMed] [Google Scholar]
- Gorovoy, M., Niu, J., Bernard, O., Profirovic, J., Minshall, R., Neamu, R., and Voyno-Yasenetskaya, T. (2005). LIM kinase 1 coordinates microtubule stability and actin polymerization in human endothelial cells. J. Biol. Chem. 280, 26533-26542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase, I., Hobbs, R. M., Romero, M. R., Broad, S., and Watt, F. M. (2001). A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J. Clin. Invest. 108, 527-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings, H., Michael, D., Cheng, C., Steinert, P., Holbrook, K., and Yuspa, S. H. (1980). Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell 19, 245-254. [DOI] [PubMed] [Google Scholar]
- Hobbs, R. M., Silva-Vargas, V., Groves, R., and Watt, F. M. (2004). Expression of activated MEK1 in differentiating epidermal cells is sufficient to generate hyperproliferative and inflammatory skin lesions. J. Invest. Dermatol. 123, 503-515. [DOI] [PubMed] [Google Scholar]
- Huang, Z., Traugh, J. A., and Bishop, J. M. (2004). Negative control of the Myc protein by the stress-responsive kinase Pak2. Mol. Cell. Biol. 24, 1582-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebe, C., Ohashi, K., Fujimori, T., Bernard, O., Noda, T., Robertson, E. J., and Mizuno, K. (1997). Mouse LIM-kinase 2 gene: cDNA cloning, genomic organization, and tissue-specific expression of two alternatively initiated transcripts. Genomics 46, 504-508. [DOI] [PubMed] [Google Scholar]
- Kunstfeld, R. et al. (2004). Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood 104, 1048-1057. [DOI] [PubMed] [Google Scholar]
- Lever, W. F. and Lever, G. S. (1990). Histopathology of the Skin, Philadelphia: Lippincott.
- Levy, D. E., and Darnell, J. E., Jr. (2002). Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 3, 651-662. [DOI] [PubMed] [Google Scholar]
- Li, A. G., Wang, D., Feng, X. H., and Wang, X. J. (2004). Latent TGFbeta1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. EMBO J. 23, 1770-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippens, S., Denecker, G., Ovaere, P., Vandenabeele, P., and Declercq, W. (2005). Death penalty for keratinocytes: apoptosis versus cornification. Cell Death Differ. 12(Suppl 2), 1497-1508. [DOI] [PubMed] [Google Scholar]
- Maekawa, M., Ishizaki, T., Boku, S., Watanabe, N., Fujita, A., Iwamatsu, A., Obinata, T., Ohashi, K., Mizuno, K., and Narumiya, S. (1999). Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285, 895-898. [DOI] [PubMed] [Google Scholar]
- McMullan, R., Lax, S., Robertson, V. H., Radford, D. J., Broad, S., Watt, F. M., Rowles, A., Croft, D. R., Olson, M. F., and Hotchin, N. A. (2003). Keratinocyte differentiation is regulated by the Rho and ROCK signaling pathway. Curr. Biol. 13, 2185-2189. [DOI] [PubMed] [Google Scholar]
- Meng, Y. et al. (2002). Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron 35, 121-133. [DOI] [PubMed] [Google Scholar]
- Milstone, L. M. (2004). Epidermal desquamation. J. Dermatol. Sci. 36, 131-140. [DOI] [PubMed] [Google Scholar]
- Misra, U. K., Deedwania, R., and Pizzo, S. V. (2005). Binding of activated alpha2-macroglobulin to its cell surface receptor GRP78 in 1-LN prostate cancer cells regulates PAK-2-dependent activation of LIMK. J. Biol. Chem. 280, 26278-26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, R. J., Liu, Y., Marles, L., Yang, Z., Trempus, C., Li, S., Lin, J. S., Sawicki, J. A., and Cotsarelis, G. (2004). Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 22, 411-417. [DOI] [PubMed] [Google Scholar]
- Nickoloff, B. J., and Nestle, F. O. (2004). Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J. Clin. Invest. 113, 1664-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelengaris, S., Littlewood, T., Khan, M., Elia, G., and Evan, G. (1999). Reversible activation of c-Myc in skin: induction of a complex neoplastic phenotype by a single oncogenic lesion. Mol. Cell 3, 565-577. [DOI] [PubMed] [Google Scholar]
- Rodrigues, G. A., Falasca, M., Zhang, Z., Ong, S. H., and Schlessinger, J. (2000). A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol. Cell. Biol. 20, 1448-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roovers, K., Klein, E. A., Castagnino, P., and Assoian, R. K. (2003). Nuclear translocation of LIM kinase mediates Rho-Rho kinase regulation of cyclin D1 expression. Dev. Cell 5, 273-284. [DOI] [PubMed] [Google Scholar]
- Sano, S., Chan, K. S., Carbajal, S., Clifford, J., Peavey, M., Kiguchi, K., Itami, S., Nickoloff, B. J., and DiGiovanni, J. (2005). Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat. Med. 11, 43-49. [DOI] [PubMed] [Google Scholar]
- Sato, K., Sato, A., Aoto, M., and Fukami, Y. (1995). c-Src phosphorylates epidermal growth factor receptor on tyrosine 845. Biochem. Biophys. Res. Commun. 215, 1078-1087. [DOI] [PubMed] [Google Scholar]
- Sumi, T., Matsumoto, K., Takai, Y., and Nakamura, T. (1999). Cofilin phosphorylation and actin cytoskeletal dynamics regulated by rho- and Cdc42-activated LIM-kinase 2. J. Cell Biol. 147, 1519-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, Y. et al. (2002). Targeted disruption of LIG-1 gene results in psoriasiform epidermal hyperplasia. FEBS Lett. 521, 67-71. [DOI] [PubMed] [Google Scholar]
- Tadini, G., Cerri, A., Crosti, L., Cattoretti, G., and Berti, E. (1989). P53 and oncogenes expression in psoriasis. Acta Derm. Venereol. Suppl. (Stockh). 146, 33-35. [PubMed] [Google Scholar]
- Takahashi, H., Ibe, M., Nakamura, S., Ishida-Yamamoto, A., Hashimoto, Y., and Iizuka, H. (2002a). Extracellular regulated kinase and c-Jun N-terminal kinase are activated in psoriatic involved epidermis. J. Dermatol. Sci. 30, 94-99. [DOI] [PubMed] [Google Scholar]
- Takahashi, H., Koshimizu, U., Miyazaki, J., and Nakamura, T. (2002b). Impaired spermatogenic ability of testicular germ cells in mice deficient in the LIM-kinase 2 gene. Dev. Biol. 241, 259-272. [DOI] [PubMed] [Google Scholar]
- Vaezi, A., Bauer, C., Vasioukhin, V., and Fuchs, E. (2002). Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev. Cell 3, 367-381. [DOI] [PubMed] [Google Scholar]
- Watt, F. M. (2002). Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 21, 3919-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt, F. M., and Green, H. (1982). Stratification and terminal differentiation of cultured epidermal cells. Nature 295, 434-436. [DOI] [PubMed] [Google Scholar]
- Wormald, S., and Hilton, D. J. (2003). Inhibitors of cytokine signal transduction. J. Biol. Chem. 279, 821-824. [DOI] [PubMed] [Google Scholar]
- Yang, N., Higuchi, O., Ohashi, K., Nagata, K., Wada, A., Kangawa, K., Nishida, E., and Mizuno, K. (1998). Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 393, 809-812. [DOI] [PubMed] [Google Scholar]
- Yu, H., and Jove, R. (2004). The STATs of cancer—new molecular targets come of age. Nat. Rev. Cancer 4, 97-105. [DOI] [PubMed] [Google Scholar]
- Zenz, R., Eferl, R., Kenner, L., Florin, L., Hummerich, L., Mehic, D., Scheuch, H., Angel, P., Tschachler, E., and Wagner, E. F. (2005). Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature 437, 369-375. [DOI] [PubMed] [Google Scholar]
- Zhu, A. J., Haase, I., and Watt, F. M. (1999). Signaling via beta1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc. Natl. Acad. Sci. USA 96, 6728-6733. [DOI] [PMC free article] [PubMed] [Google Scholar]