Abstract
Glutamate-mediated damage to oligodendrocytes contributes to mental or physical impairment in periventricular leukomalacia (pre- or perinatal white matter injury leading to cerebral palsy), spinal cord injury, multiple sclerosis and stroke1–4. Unlike neurons5, white matter oligodendrocytes reportedly lack NMDA receptors6,7 and it is believed that glutamate damages oligodendrocytes, especially their precursor cells, by acting only on calcium-permeable AMPA/kainate receptors1–4 or by reversing cystine-glutamate exchange and depriving cells of antioxidant protection8. We now show that precursor, immature and mature oligodendrocytes in the white matter of the cerebellum and corpus callosum exhibit NMDA evoked currents, mediated by receptors which are blocked only weakly by Mg2+, which may contain NR1, NR2C and NR3 subunits. NMDA receptors are present in the myelinating processes of oligodendrocytes, where the small intracellular space could lead to a large rise of intracellular ion concentration in response to NMDA receptor activation. Simulating ischaemia led to an inward current developing in oligodendrocytes, which was partly mediated by NMDA receptors. These results point to NMDA receptors of unusual subunit composition as a novel therapeutic target for preventing white matter damage in a range of diseases.
Blocking AMPA/kainate receptors attenuates white matter injury in animal models of hypoxia/ischaemia9,10, spinal cord injury11,12 and multiple sclerosis13,14. Neverthless, NMDA receptors were found in some cultured oligodendrocyte precursors15 and in spinal grey (but not white) matter oligodendrocytes16, and NMDA receptor blockers slow the loss of white matter action potentials10 and reduce white matter damage in ischaemia17 and in a model of multiple sclerosis18. We have, therefore, re-examined the involvement of NMDA receptors in the physiology and pathology of oligodendrocytes.
Whole-cell clamped precursor, immature and mature oligodendrocytes in the white matter could be distinguished by their morphology and antibody labelling7,19 (Fig. 1a–c). Precursor cells (Fig. 1a) had short processes not aligned with nearby axons, were labelled by antibodies to NG2 proteoglycan (for the specificity of this, see Methods), and often exhibited spontaneous synaptic currents (as in grey matter oligodendrocytes20) which were blocked by TTX (Fig. 1d). Immature cells (Fig. 1b) had some processes aligned with adjacent axons, and were labelled by antibody to O4 lipid sulfatide. Mature cells (Fig. 1c) had most processes aligned with axons, and were labelled by antibody to myelin basic protein (MBP). Oligodendrocyte membrane resistance decreased with maturity (Fig. 1e). The resting potential was approximately −60mV (−57.5±2.9mV in 37 mature cells: without shunting by the electrode seal it would be ~4mV more negative).
Figure 1. Glutamate-evoked current in oligodendrocytes.
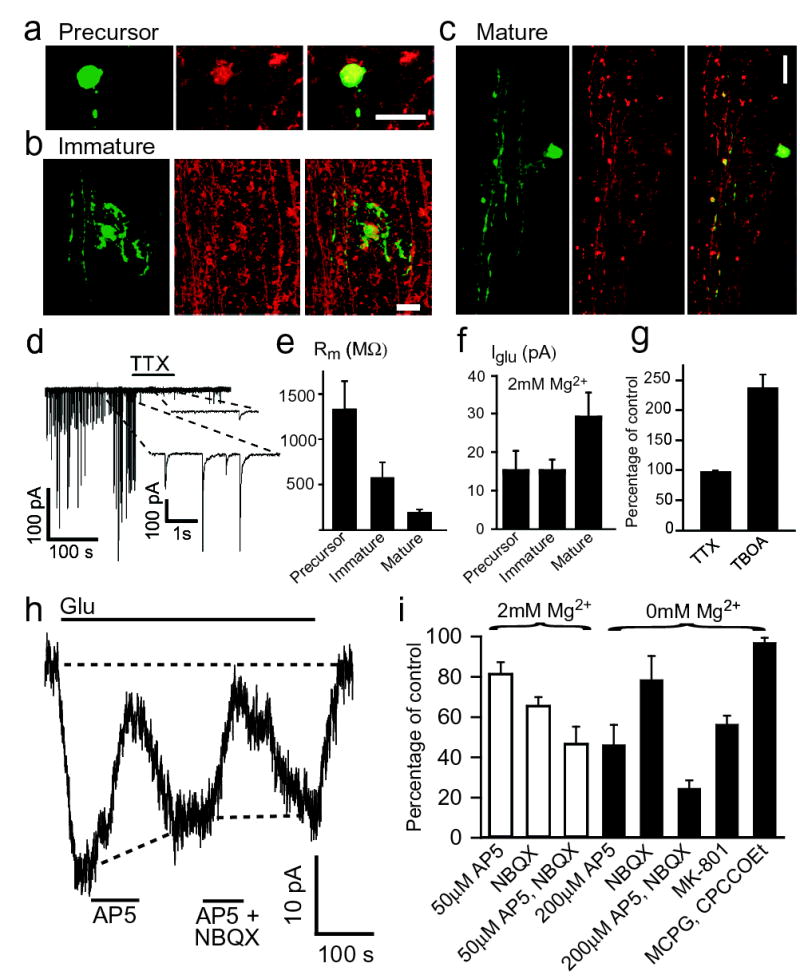
a-c Lucifer (green) and antibody (red) to (a) NG2, (b) O4, and (c) MBP (colocalization in overlay, yellow). Scale 20μm. d TTX blocks synaptic currents in precursor. e Membrane resistance (±s.e.m.), p<0.001 comparing precursors with immature or mature). f Glutamate (100μM) evoked current (p=0.11 and 0.14 comparing 33 mature with 22 immature or 22 precursors). g Effect of TTX (n=5) and TBOA (n=4). h Effect of 200μM D-AP5 (blocks NMDA responses by ~78%: Supplementary Material) and 25μM NBQX (blocks AMPA responses >99%) in 0mM Mg. i Current remaining in 2mM Mg2+ (n=7) in 50μM AP5 (p=0.02, blocks NMDA receptors by ~39%), 25μM NBQX (p=0.0003) and AP5+NBQX (p=0.0008), and in 0 Mg2+ (n=4-6) in 200μM AP5 (p=0.01), 25μM NBQX (p=0.16), AP5+NBQX (p=0.0003), 10μM MK-801 (p=0.002) and 1mM MCPG plus 200μM CPCCOEt (mGluR blockers, p=0.25). All 2mM Mg2+ (except h,i), −63mV, 24°C.
Precursor, immature and mature cerebellar white matter oligodendrocytes responded to glutamate (100μM) with an inward current at −63mV, which was unaffected by 1μM TTX (p=0.4; Fig. 1f–h, Supplementary Fig. 1), showing that axonal action potentials generated by glutamate depolarizing neurons did not contribute to the current (e.g. by releasing K+). The current was potentiated when the local glutamate concentration was raised by blocking glutamate transporters with TBOA (p=0.016; Fig. 1g, Supplementary Fig 1). D-AP5, MK-801 and NBQX all reduced the glutamate-evoked current (Fig. 1h,i), suggesting the presence of both NMDA and AMPA/kainate receptors.
NMDA (60μM) evoked an inward current in corpus callosum (Fig. 2a–c) and cerebellum (Fig. 2d,e) oligodendrocytes, which was comparable in size (at −63mV in 0mM Mg2+) to the current produced by AMPA (20μM) or kainate (30μM), and was larger than that produced by the metabotropic glutamate receptor agonist (1S,3R)-ACPD (100μM). In cerebellar oligodendrocytes the NMDA-evoked current was largest in mature cells (Fig. 2f, p=0.042 compared with precursors) although the increased membrane area of mature cells may imply a lower current density. The NMDA-evoked current ran down slightly with time (Fig. 2g), so the current in drugs was quantified relative to the average of preceding and following control responses. It was blocked by D-AP5 (Fig. 2h,j) and unaffected by TTX, NBQX, strychnine or bicuculline (Fig. 2i,j), and not significantly affected (p=0.072) when glycine (100μM, with strychnine 5μM) was present, implying that the NMDA receptors’ glycine-binding sites are well activated by endogenous glycine or D-serine. Changing from Mg2+-free superfusion solution to solution containing 2mM Mg2+ decreased the NMDA-evoked current at −63mV 3 to 5-fold, independent of developmental age (Fig. 2k,l). This decrease is much less than is found for most neurons21, or for most cloned NMDA receptors22, which show a 60-fold reduction for receptors comprising NR1 and NR2A or 2B subunits and a 20-fold reduction for receptors comprising NR1 and NR2C or 2D subunits, but is comparable to that seen for cloned receptors comprising NR1, NR2A and NR3A subunits23.
Figure 2. Oligodendrocyte NMDA receptors show weak Mg2+-block.
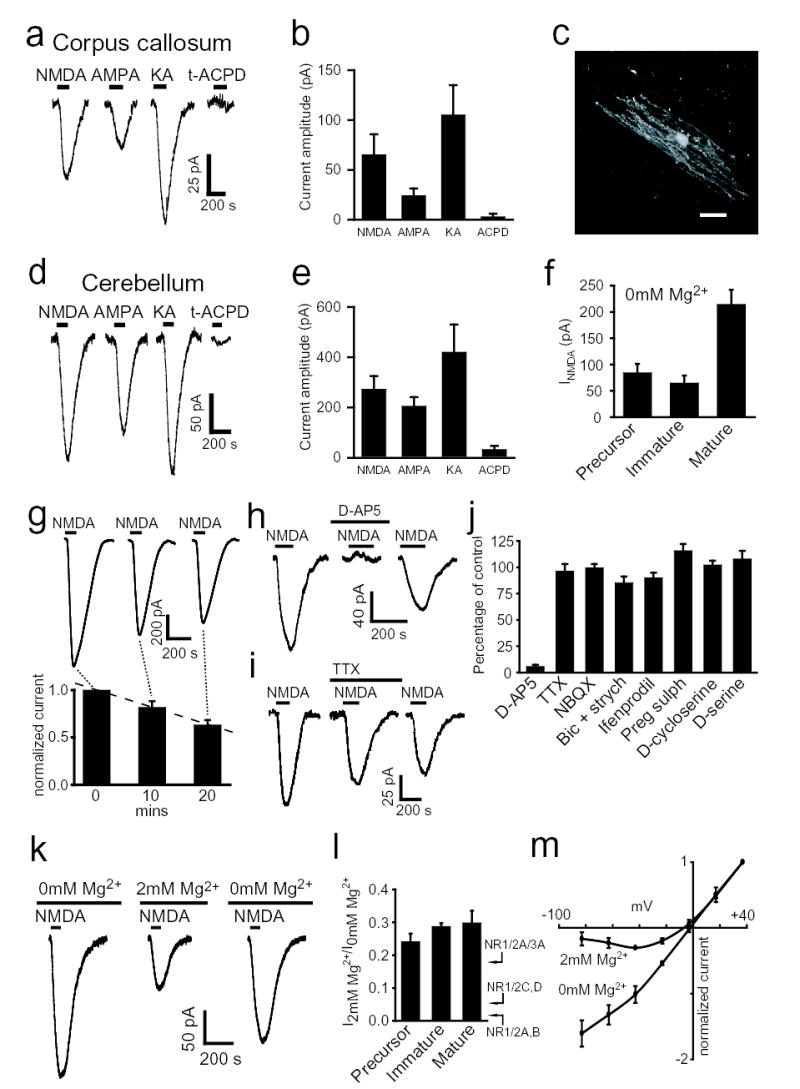
a Response of mature corpus callosum oligodendrocyte to 60μM NMDA, 20μM AMPA, 30μM kainate (KA) and 100μM trans-ACPD (0mM Mg2+). b Peak current in a for NMDA (n=22 cells), AMPA (n=12), KA (n=10) and ACPD (n=5). c Mature Lucifer-filled oligodendrocyte in corpus callosum (scale 20μm). d Responses of mature cerebellar oligodendrocyte in 0mM Mg2+. e Current in d for NMDA (n=26), AMPA (n=23), KA (n=5) and ACPD (n=16). f NMDA-evoked current in cerebellar cells (p=0.059 and 0.042 comparing 79 mature with 19 immature or 26 precursors). g NMDA responses rundown linearly with time (dashed line) during repeated (1/10 mins) applications: top, specimen cell; bottom, normalised data (n=10). h Effect of D-AP5 (50μM). i Effect of TTX (1μM). j Effect of 50μM D-AP5 (n=16, p=10−18), 1μM TTX (n=5, p=0.64), 25μM NBQX (n=4, p=0.96), 5μM strychnine plus 20μM bicuculline (n=16, p=0.14), ifenprodil (10μM, n=3, p=0.18), pregnenolone sulphate (100μM, n=4, p=0.085), D-cycloserine (1mM, n=3, p=0.6) and D-serine (100μM, n=5, p=0.34) on NMDA-evoked current at −63mV. k NMDA response in 0mM, 2mM and 0mM Mg2+ again. l Fraction of NMDA-evoked current remaining in 2mM Mg2+, in 4 precursor, 6 immature and 9 mature cells (insignificantly different, p=0.51). Arrows: values22,23 for NR1 with NR2C or 2D, 2A or 2B, or 2A and 3A. m Normalized I-V relation for NMDA-evoked current in two precursors in 0mM and three different precursors in 2mM Mg2+. Cerebellum except a-c; 0 Mg2+ (except k-m); 100μM glycine and 5μM strychnine (except D-cycloserine, D-serine in j); 60μM NMDA; 24°C, −63mV.
Ifenprodil (10μM), which blocks NR2B-containing receptors, had no effect on the NMDA-evoked current (Fig. 2j). Neither pregnenolone sulphate (100μM, which potentiates NR1/NR2A and NR1/NR2B receptors by 60–82% but inhibits NR1/NR2C and NR1/NR2D receptors by 32%), nor D-cycloserine (1mM, with no added glycine, which potentiates NR1/NR2C receptors but inhibits NR1/NR2A and NR1/NR2B receptors), nor D-serine (100μM, with no glycine, which potentiates receptors lacking NR3 subunits but inhibits NR3-containing receptors) affected the current (Fig. 2j). This suggests the presence of a subunit (perhaps NR3) which suppresses modulatory actions on NR2 subunits, or the presence of a combination of subunits that these agents modulate in opposite directions (e.g. NR1/NR2A/NR2C or NR1/NR2/NR3), or a mix of receptors with different subunit combinations. Although the subunit composition currently remains uncertain, it allows these receptors to generate a significant current even at the resting potential in physiological [Mg2+].
In precursor oligodendrocytes the NMDA-evoked current reversed around 0mV (Fig 2m), and in 2mM Mg2+ the I-V relation showed a region of negative slope which was similar to, but less marked than, that produced by Mg2+-block of neuronal NMDA receptor channels. In mature oligodendrocytes the I-V relation often failed to reverse at positive potentials, which may reflect the NMDA receptors being electrotonically distant from the soma in the cell processes (see below), or NMDA receptor activation leading to Na+ entry and block of a K+ current in the cell24.
Antibodies to NMDA receptor subunits labelled the myelinating processes, and some cell bodies, of oligodendrocytes in the cerebellar white matter. Labelling for NR2C (Fig. 3a,b) was abolished by omitting the primary antibody, or by preabsorption with the peptide to which it was raised (Supplementary Fig. 2). Labelling was also seen for NR1 subunits, which colocalized with MBP in mature cells (data not shown), and NR3 subunits (Fig 3c,d, Supplementary Fig 2). Weaker labelling was seen for NR2A, NR2B and NR2D, and was abolished by omitting the primary antibody (NR2A, NR2B and NR2D) or by peptide absorption (NR2D). Double labelling showed colocalization of NR3 and NR2C subunits, and of NR1 and NR2C subunits in oligodendrocyte processes (Supplementary Figs. 3 & 4). These data, the lack of ifenprodil block, and the weak Mg2+-block of the NMDA-evoked current, suggest that oligodendrocyte NMDA receptors contain at least NR1, NR2C and NR3 subunits.
Figure 3. Oligodendrocyte NMDA receptors.
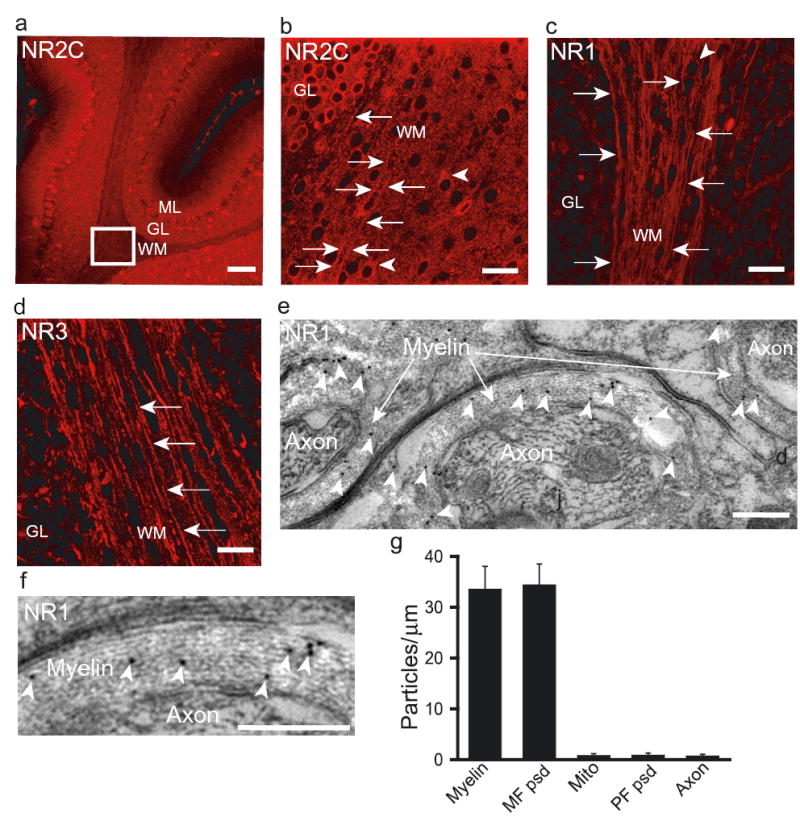
a Cerebellar cortex labelled for NR2C (WM, white matter, GL granular layer, ML molecular layer). b Enlarged box in a. Arrows: oligodendrocyte process; arrow heads: oligodendrocyte soma. c Oligodendrocyte processes labelling with Chemicon NR1 antibody (see also Supplementary Fig. 6). d NR3 labelling. e Immunogold (black dots, arrowheads, Wenthold NR1 antibody) labelling cerebellar myelin. f Enlarged view of myelin. g Gold particle density over cerebellar myelin (17 sheaths), mossy fibre synapse (MF psd, n=20), parallel fibre-Purkinje synapse (PF psd, n=26), mitochondria in MF terminal (Mito, n=30), and axon cytoplasm (Axon, n=17). Scale bars: a 100μm, b-d 20μm, e-f 0.25μm.
Post-embedding electron microscopic immunochemistry showed that NR1 subunits were present in the myelinating processes of adult cerebellar oligodendrocytes (Fig. 3e,f, Supplementary Fig. 5), in the outer- and innermost membranes and also within the myelin (perhaps remaining from earlier in development). By quantifying the immunogold particles in the myelin and in the postsynaptic densities of mossy fibre-granule cell (Supplementary Fig. 5) and parallel fibre-Purkinje cell synapses, we found that the density of NMDA receptors throughout the myelin is as high as at the mossy fibre to granule cell synapse (Fig. 3g). The density of particles in the outer membrane of the myelin (where the receptors may sense glutamate released from surrounding cells) tended (p=0.064) to be larger than that in the innermost membrane (where the receptors may sense glutamate released from the axon), but both were similar to the average density measured over all of the myelin (36.4±8.3, 17.7±4.3 and 33.6±4.5 particles/μm2 respectively).
To examine the role of oligodendrocyte NMDA receptors in pathological situations, we simulated25 the energy deprivation which contributes to periventricular leukomalacia, or occurs after ischaemia in spinal cord injury. Simulated ischaemia solution evoked a slowly developing inward current in precursor and mature oligodendrocytes (Fig. 4, peak inward current at −63mV after ~7 mins was 307±83 and 280±48 pA in 7 precursor and 8 mature cells in 2mM Mg2+). This current was generated partly by a rise of extracellular [glutamate], since it was reduced by D-AP5 and NBQX (Fig. 4a). In some precursors (Fig. 4a,c) the inward current early in ischaemia included an increased frequency of synaptic current-like events (Fig. 1d), presumably reflecting exocytotic transmitter release20 triggered by action potentials or a rise of axonal [Ca2+]i. The relative contribution of NMDA and AMPA receptors to the glutamate-mediated current was analysed in 0mM Mg2+ to detect NMDA receptor currents more accurately (in 2mM Mg2+ the NMDA component at −63mV would be 4-fold smaller (Fig. 2l), but would be increased in unclamped cells by the ischaemia-evoked current depolarizing cells and reducing NMDA receptor Mg2+-block). The NMDA receptor mediated current in precursors was on average 50% larger than that mediated by AMPA/kainate receptors (Fig 4b–d), and in some cells comprised almost all the current (Fig. 4c). In mature oligodendrocytes the fraction of the inward current that was generated by glutamate was smaller than in precursors (Fig. 4d), suggesting that (since mature cells can generate larger glutamate and NMDA-evoked currents: Figs. 1f, 2f) less glutamate release occurs around mature cells in ischaemia. Further work is needed to establish the origin of the part of the inward current that is not generated by glutamate release.
Figure 4. Ischaemia activates NMDA receptors.
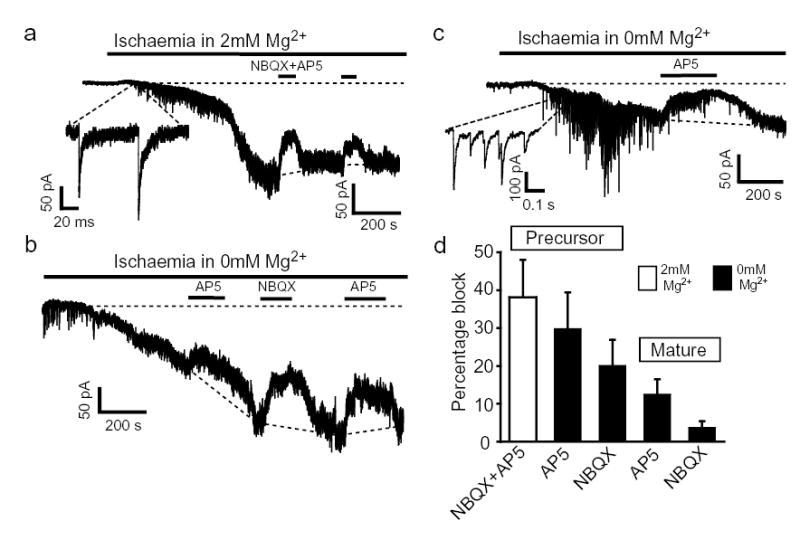
a Ischaemia-evoked current in precursor (2mM Mg2+) blocked by NBQX (25μM) plus D-AP5 (50μM) Inset: ischaemia-induced synaptic-like currents. b Precursor response to ischaemia (0mM Mg2+), blocked by D-AP5, NBQX. c Precursor response (0mM Mg2+) showing transient increase in synaptic currents, and most inward current blocked by D-AP5. d Fractional block of inward current: in 2mM Mg2+ by D-AP5 plus NBQX in 7 precursors, and in 0mM Mg2+ by D-AP5 or NBQX in precursors (9 for D-AP5, 7 for NBQX) and mature cells (9 for D-AP5, 10 for NBQX). P=0.098 comparing D-AP5 in precursors and mature cells; P=0.019 comparing NBQX in precursors and mature cells. All cerebellum, −63mV, 33°C.
We have shown that oligodendrocytes express functional NMDA receptors. The NMDA-evoked currents that we observe are unlikely to be produced by an agent released secondarily from neurons because they are unaffected by TTX, or by block of AMPA/KA, mGluR, GABAA, or glycine receptors (Figs. 2j, 1i) and oligodendrocytes show no current in response to application of noradrenaline, dopamine, histamine, 5-HT, ATP, adenosine or ACh (data not shown). Antibody labelling (Fig. 3) and the voltage-dependence of the current (Fig 2m) show that oligodendrocytes themselves express NMDA receptors. Previous work which failed to detect NMDA receptor currents in oligodendrocytes was partly on cultured cells6, which may down-regulate their NMDA receptor expression. Our data (Fig. 2a) contradict the absence of NMDA responses reported in corpus callosum oligodendrocytes7. Since oligodendrocyte NMDA receptor currents occur both in cerebellum and corpus callosum (Fig. 2), they may represent a general property of white matter oligodendrocytes. Our electrophysiological recordings are from precursor, immature and mature oligodendrocytes in P7-P14 rats, and remain to be extended to the adult, but NMDA receptor subunits are also present in adult oligodendrocytes (Fig. 3e).
Oligodendrocyte NMDA receptors are likely to play a role in controlling oligodendrocyte development and myelination26, and in damaging these cells in pathological conditions. They show only weak Mg2+-block at the cells’ resting potential (Fig. 2l)16, and mediate part of the inward current generated in oligodendrocytes in response to simulation of the energy deprivation which occurs in periventricular leukomalacia, in stroke, and after ischaemia in spinal cord injury. Notably, they are present in the oligodendrocytes’ myelinating processes, where the intracellular volume is small and receptor-mediated ion influx may produce a large intracellular concentration rise and osmotic water flux which could disrupt myelination. The higher glutamate affinity of NMDA receptors, relative to AMPA receptors, makes them more likely to be activated in neurodegenerative disorders which involve a prolonged but small rise of extracellular glutamate concentration, as may occur in multiple sclerosis. Thus, oligodendrocyte NMDA receptors could contribute to causing the white matter damage which occurs when the extracellular [glutamate] is raised in periventricular leukomalacia, spinal cord injury, multiple sclerosis and stroke1–4. Indeed, in the optic nerve, activation of NMDA receptors on oligodendrocyte processes, when glutamate is released during ischaemia, leads to the disintegration of those processes27. The unusual subunit combination of these receptors, which may contain NR1, NR2C and NR3 subunits, suggests they may be a useful therapeutic target in a variety of brain disorders.
Methods
Brain slices
Cerebellar or forebrain slices (225μm thick) were prepared from P7-P14 rats in solution containing 1mM Na-kynurenate to block glutamate receptors. Cerebellum myelinates relatively late, facilitating investigation of different developmental stages, and corpus callosum is an area which is thinned in severe periventricular leukomalacia. Slices were superfused at 33±1°C with bicarbonate-buffered solution (for ischaemia) or at 24±1°C with HEPES-buffered solution (for non-ischaemia experiments), containing (mM) 126 NaCl, 24 NaHCO3, 1 NaH2PO4, 2.5 KCl, 2 MgCl2, 2.5 CaCl2, 10 glucose, 0.1 glycine (activates the NMDA receptor glycine site), strychnine 0.005 (blocks glycine receptors), bubbled with 95% O2/5% CO2, pH 7.4; or 144 NaCl, 2.5 KCl, 2 MgCl2, 10 HEPES, 1 NaH2PO4, 2.5 CaCl2, 10 glucose, 0.1 glycine, strychnine 0.005, pH set to 7.4 with NaOH. Omitting glycine or adding D-serine did not affect the NMDA receptor current (see text). To simulate ischaemia we replaced external O2 by N2, and external glucose by 7mM sucrose, added 2mM iodoacetate to block glycolysis, and added 100μM rotenone or 25μM antimycin to block oxidative phosphorylation25. Without iodoacetate and rotenone/antimycin, it took ~3-fold longer for the ischaemia-evoked inward current to develop, probably because in an open chamber O2 can diffuse to the slice allowing glycogen metabolism in mitochondria for longer than would occur in vivo25.
Recording & cell identification
White matter cells (avoiding cerebellar nuclei) were whole-cell clamped with pipettes containing a K+-based solution (for glutamate application) comprising (mM) 130 KCl, 4 NaCl, 0.5 CaCl2, 10 HEPES, 10 EGTA, 2 MgATP, 0.5 Na2GTP, K-Lucifer yellow 2, pH set to 7.2 with KOH, or a Cs+-based solution (for NMDA application and ischaemia), comprising (mM) 130 CsCl, 4 NaCl, 0.5 CaCl2, 10 HEPES, 10 BAPTA, 2 MgATP, 0.5 Na2GTP, K-Lucifer yellow 2, pH set to 7.2 with CsOH. Series resistance was 8-20MΩ, before 60% compensation. Cells were identified from post-recording dye-fill morphology and antibody labelling. NG2 antibody labelled 15 out of 15 tested cells with precursor morphology (we take NG2 labelling to indicate oligodendrocyte precursors20, however some NG2 cells may be a glial class distinct from oligodendrocytes28, or (in grey matter) neuronal precursors29; demonstrating that oligodendrocytes express NMDA receptors does not depend on recording precursors, since NMDA receptor currents were also seen in immature and mature cells). O4 antibody labelled 6 out of 6 cells with immature morphology. MBP antibody labelled all 17 cells tested with mature morphology. Antibody to the oligodendrocyte transcription factor Olig2 labelled all 10 cells tested (7 precursors, 1 immature and 2 mature cells). Oligodendrocytes could be distinguished from astrocytes, which showed gap junction coupling, revealed by Lucifer yellow spreading to other cells, and GFAP labelling (5 out of 5 cells). Electrode junction potentials were compensated. I-V relations were from responses to 200 msec voltage steps.
Immunocytochemistry
Antibody labelling and post-embedding electron microscopic immunocytochemistry are described in Supplementary Material.
Statistics
Data are mean ± s.e.m. P values are from Student’s 2-tailed t-tests, except for multiple comparisons done with one-way Anova and Tukey post hoc test.
Supplementary Material
Acknowledgments
We thank D. Rowitch, C.D. Stiles & J. Alberta for Olig2 antibody, F.A. Stephenson, R.J. Wenthold & O.P. Ottersen for NR1 antibody, and A. Gibb, K. Jessen, R. Mirsky, W. Richardson, D. Rossi, J. Rothman, A. Silver & J. Storm-Mathisen for advice. Supported by the Wellcome Trust, EU, Norwegian Research Council & a Wolfson-Royal Society Award. R. Káradóttir was in the 4 year PhD Programme in Neuroscience at UCL.
References
- 1.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Stys PK. White matter injury mechanisms. Curr Mol Med. 2004;4:113–130. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- 3.Matute C, Alberdi E, Domercq M, Perez-Cerda F, Perez-Samartin A, Sanchez-Gomez MV. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci. 2001;24:224–230. doi: 10.1016/s0166-2236(00)01746-x. [DOI] [PubMed] [Google Scholar]
- 4.Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab. 2003;23:263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- 5.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 6.Patneau DK, Wright PW, Winters C, Mayer ML, Gallo V. Glial cells of the oligodendrocyte lineage express both kainate- and AMPA-preferring subtypes of glutamate receptor. Neuron. 1994;12:357–371. doi: 10.1016/0896-6273(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 7.Berger T, Walz W, Schnitzer J, Kettenmann H. GABA- and glutamate-activated currents in glial cells of the mouse corpus callosum slice. J Neurosci Res. 1992;31:21–27. doi: 10.1002/jnr.490310104. [DOI] [PubMed] [Google Scholar]
- 8.Oka A, Belliveau MJ, Rosenberg PA, Volpe JJ. Vulnerability of oligodendroglia to glutamate: pharmacology, mechanisms, and prevention. J Neurosci. 1993;13:1441–1453. doi: 10.1523/JNEUROSCI.13-04-01441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX attenuates excitotoxic injury in developing white matter. J Neurosci. 2000;20:9235–9241. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tekkök SB, Goldberg MP. AMPA/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci. 2001;21:4237–4248. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal SK, Fehlings MG. Role of NMDA and non-NMDA ionotropic glutamate receptors in traumatic spinal cord axonal injury. J Neurosci. 1997;17:1055–1063. doi: 10.1523/JNEUROSCI.17-03-01055.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wrathall JR, Teng YD, Marriott R. Delayed antagonism of AMPA/kainate receptors reduces long-term functional deficits resulting from spinal cord trauma. Exp Neurol. 1997;145:565–573. doi: 10.1006/exnr.1997.6506. [DOI] [PubMed] [Google Scholar]
- 13.Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nature Medicine. 2000;6:67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- 14.Smith T, Groom A, Zhu B, Turski L. Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nature Medicine. 2000;6:62–66. doi: 10.1038/71548. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Pralong WF, Schulz MF, Rougon G, Aubry JM, Pagliusi S, Robert A, Kiss JZ. Functional N-methyl-D-aspartate receptors in O-2A glial precursor cells: a critical role in regulating polysialic acid-neural cell adhesion molecule expression and cell migration. J Cell Biol. 1996;135:1565–1581. doi: 10.1083/jcb.135.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziak D, Chvatal A, Sykova E. Glutamate, kainate and NMDA-evoked membrane currents in identified glial cells in rat spinal cord slice. Physiol Res. 1998;47:365–375. [PubMed] [Google Scholar]
- 17.Schäbitz WR, Li F, Fisher M. The N-methyl-D-aspartate antagonist CNS 1102 protects cerebral gray and white matter from ischemic injury following temporary focal ischemia in rats. Stroke. 2000;31:1709–1714. doi: 10.1161/01.str.31.7.1709. [DOI] [PubMed] [Google Scholar]
- 18.Wallström E, Diener P, Ljungdahl A, Khademi M, Nilsson CG, Olsson T. Memantine abrogates neurological deficits, but not CNS inflammation, in Lewis rat experimental autoimmune encephalomyelitis. J Neurol Sci. 1996;137:89–96. doi: 10.1016/0022-510x(95)00339-4. [DOI] [PubMed] [Google Scholar]
- 19.Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergles DE, Roberts JDB, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 21.Kirson ED, Schirra C, Konnerth A, Yaari Y. Early postnatal switch in magnesium sensitivity of NMDA receptors in rat CA1 pyramidal cells. J Physiol. 1999;521:99–111. doi: 10.1111/j.1469-7793.1999.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuner T, Schoepfer R. Multiple structural elements determine subunit specificity of Mg2+ block in NMDA receptor channels. J Neurosci. 1996;16:3549–3558. doi: 10.1523/JNEUROSCI.16-11-03549.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasaki YF, et al. Characterization and comparison of the NR3A subunit of the NMDA receptor in recombinant systems and primary cortical neurons. J Neurophysiol. 2002;87:2052–2063. doi: 10.1152/jn.00531.2001. [DOI] [PubMed] [Google Scholar]
- 24.Borges K, Kettenmann H. Blockade of K+ channels induced by AMPA/kainate receptor activation in mouse oligodendrocyte precursor cells is mediated by Na+ entry. J Neurosci Res. 1995;42:579–593. doi: 10.1002/jnr.490420416. [DOI] [PubMed] [Google Scholar]
- 25.Allen NJ, Káradóttir R, Attwell D. A preferential role for glycolysis in preventing the anoxic depolarization of rat hippocampal area CA1 pyramidal cells. J Neurosci. 2005;25:848–859. doi: 10.1523/JNEUROSCI.4157-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan X, Eisen AM, McBain CJ, Gallo V. A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development. 1998;125:2901–2914. doi: 10.1242/dev.125.15.2901. [DOI] [PubMed] [Google Scholar]
- 27.Salter, M. & Fern, R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. (2005). Submitted. [DOI] [PubMed]
- 28.Berry M, Hubbard P, Butt AM. Cytology and lineage of NG2-positive glia. J Neurocytol. 2002;31:457–467. doi: 10.1023/a:1025735513560. [DOI] [PubMed] [Google Scholar]
- 29.Chittajulu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 2004;561:109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


