Abstract
The recent discovery of apparent fossils of embryos contemporaneous with the earliest animal remains may provide vital insights into the metazoan radiation. However, although the putative fossil remains are similar to modern marine animal embryos or larvae, their simple geometric forms also resemble other organic and inorganic structures. The potential for fossilization of animals at such developmental stages and the taphonomic processes that might affect preservation before mineralization have not been examined. Here, we report experimental taphonomy of marine embryos and larvae similar in size and inferred cleavage mode to presumptive fossil embryos. Under conditions that prevent autolysis, embryos within the fertilization envelope can be preserved with good morphology for sufficiently long periods for mineralization to occur. The reported fossil record exhibits size bias, but we show that embryo size is unlikely to be a major factor in preservation. Under some conditions of death, fossilized remains will not accurately reflect the cell structure of the living organism. Although embryos within the fertilization envelope have high preservation potential, primary larvae have negligible preservation potential. Thus the paleo-embryological record may have strong biases on developmental stages preserved. Our data provide a predictive basis for interpreting the fossil record to unravel the evolution of ontogeny in the origin of metazoans.
Keywords: embryo taphonomy, fossil record, metazoan origins, developmental evolution
Discovery of microfossils interpreted as metazoan embryos and larvae in rocks of Ediacaran and Cambrian age putatively coeval with the metazoan radiation (1–11) has the promise to provide direct insight into developmental mode in animal evolution. In contrast to the record for living metazoans, the Ediacaran and Cambrian record of fossilized embryos has been interpreted to represent only direct developing lecithotrophic forms. However, the available fossil record is biased and in different ways in different deposits (6, 12, 13). Putative fossil cleavage-stage embryos from the Ediacaran Doushantuo Formation are large in comparison with many modern embryos. Discussion to date has centered on the difficulty of distinguishing fossils of cleaving embryos from fossils of other multicellular forms, such as algae (5). Moreover, the validity of some putative fossils, particularly those interpreted as larval forms, has been questioned (2, 3, 5, 14). Whether primary larvae can be preserved as fossils has not been addressed.
Previous experimental studies of soft tissue mineralization showed that after introduction of an anaerobic bacterial community and sea floor sediment to shrimp carcasses, oxygen levels fell, sulfide levels rose, pH levels fell, and phosphatization of muscle tissue occurred within a month (15). A link was made between anaerobic decay and mineralization. Where oxygen is available, calcium carbonate deposition dominates, whereas in a closed system, calcium phosphate deposition can replicate soft tissues within 2 weeks (16, 17). Similar studies of embryos were not as successful at preserving cell structure. Experimental mineralization of lobster eggs in the presence of anaerobic sediments resulted in calcium carbonate deposition on the tough external egg envelope within 15–36 days, but no preservation or mineralization of the embryos within was observed (18).
Anyone who works with marine embryos would consider preservation for sufficient time for mineralization via phosphatization unlikely, given the seeming fragility of such embryos. Freshly killed marine embryos in normal seawater decompose within a few hours. We carried out taphonomy experiments designed to uncover the impact of the mode of death and postdeath environment on the preservational potential of marine embryos and larvae.
The presumptive fossil cleavage embryos described to date are large in size (≈500 μm) and exhibit a covering that resembles a fertilization envelope and closely packed equal-sized cells resembling a pattern of cell division in which blastomere size decreases with increasing cell number, typical of cell division without cell growth in early embryos. We used the lecithotrophic Australian sea urchin Heliocidaris erythrogramma as a model in our decay experiments because its embryos exhibit a comparable suite of features (19, 20). Mineralization was not studied, but conditions consistent with phosphatization (15–18) were investigated. We also studied the effects of preservation conditions on small, planktotrophic sea urchin embryos and larvae to determine whether size of embryos is a determining factor in preservability. If it was, the bias toward reports of large-sized embryos in the Precambrian and Cambrian record might reflect a preservation bias and not contemporaneous embryological diversity. Our results show that under some experimental circumstances compatible with natural conditions maintenance of marine embryos for time periods compatible with the authigenic replication of soft tissue can potentially occur, but for only a limited set of developmental stages.
Results
Effect of Death and Postdeath Conditions on Embryo Morphology.
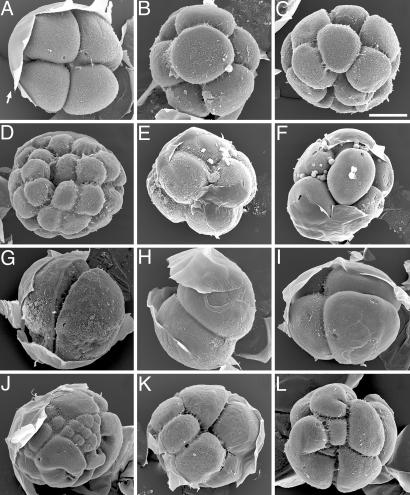
We compared normal cleavage stage H. erythrogramma embryos (Fig. 1A–D) with embryos subjected to various experimental treatments (Fig. 1 E–L). Scanning electron microscopic images of H. erythrogramma embryos in Fig. 1 bear a striking resemblance in general appearance and size to putative fossil cleavage embryos described from Ediacaran and Cambrian rocks (1, 6, 13, 14).
Fig. 1.
Cleavage-stage H. erythrogramma embryos under various experimental conditions. Fertilization envelopes (arrow in A) were manually peeled back after processing for scanning electron microscopy. (A–D) Normal embryos at the 4-cell stage (A), 8-cell stage (B), 16-cell stage (C), and 32-cell stage (D). (E and F) Embryos from a culture placed in seawater containing 100 mM β-ME at the eight-cell stage. Embryos arrested at time of treatment and fixed after 12 days. (G–J) Embryos killed at mid-2- to 4-cell stage by various treatments and fixed after 8.5 h. Untreated controls had progressed to late blastula stage. (G) Embryo from a culture placed in 50% seawater. Embryos arrested at time of treatment; blastomeres expanded to fill the fertilization envelope. (H–J) Embryos placed in seawater containing 0.1% ammonium. Some embryos arrested immediately (H and I), and some underwent aberrant cleavage before arresting (J). (K) Embryo placed at 2-cell stage in N2-saturated seawater and fixed after 2 h. Cleavage continued during partial anoxia, but some blastomeres arrested or slowed cleavage rate. Untreated controls had progressed to the 16- to 32-cell stage. (L) Embryo fertilized under polyspermic conditions and fixed at 2.25 h (normal embryos would be 4- to 8-cell stage). (Scale bar: 100 μm.)
The rate of killing was important. Embryos killed rapidly (Fig. 1 E–I) retained blastomere numbers and arrangements of the time of death. Rapid death allowed retention of normal morphology, except if by lowered salinity, which affected cell shape. In hypotonic seawater, two-cell embryos retained uniform blastomere configuration, but the cells swelled to fill the fertilization envelope, producing a distinct morphology that if fossilized might be interpreted as an embryo of a different taxon (Fig. 1G). When embryos died slowly, individual blastomeres displayed differential cleavage patterns and timing of arrest, yielding abnormal, nonuniform morphologies (Fig. 1 J and K). The aberrant cleavage patterns resulting from slow death resembled those in polyspermic embryos (Fig. 1L).
Extended Preservation of Cleavage-Stage Embryos in Strong Reducing Conditions.
Because in situ mineralization is associated with anoxic reducing environments (15–18), we examined H. erythrogramma embryos placed into comparably strong reducing conditions, either directly or after they had been killed by other treatments. In a reducing environment, cleavage-stage embryos exhibited striking potential for extended preservation with normal morphology (Figs. 1 E and F and 2A–C).
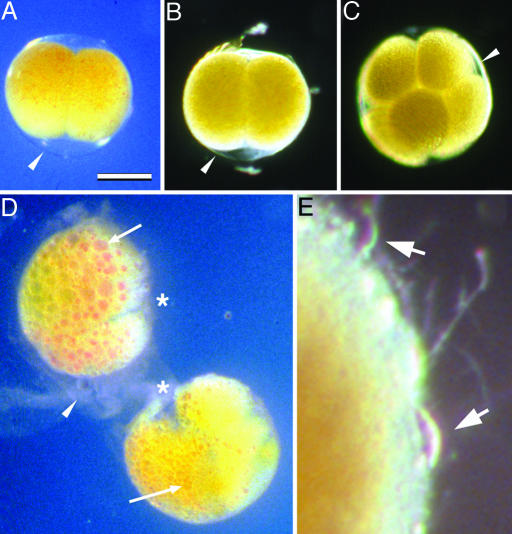
Fig. 2.
Cleavage-stage H. erythrogramma embryos under preserving and nonpreserving conditions. (A and B) Embryos killed at the two-cell stage by placing them in seawater containing 1% ammonium for 10 min, then transferring them to seawater containing 100 mM β-ME. They were photographed after 2 (A) or 10 (B) days. (C) Embryo killed at the eight-cell stage by transfer into seawater containing 100 mM β-ME, photographed after 12 days. (D) Embryos from the same two-cell stage culture as in A and B but returned to normal seawater after killing, photographed after 2 days. Embryos have undergone autolysis: cytoplasmic lipid and pigment have coalesced (arrows); cleavage furrows have degraded (asterisks); and fertilization envelopes are disintegrating (arrowhead). Autolysis is further advanced in the top embryo than in the bottom embryo. In the bottom embryo, the process is further advanced in the left-hand blastomere (arrow) than in the right-hand blastomere. (E) Decaying surface of an embryo from the set shown in A and B, returned to normal seawater after 4 days in reducing conditions, photographed 7 days later (total 11 days postdeath). Onset and progress of decay is slower than autolysis in embryos never exposed to reducing conditions. The fertilization envelope degrades and the cytoplasm of the embryo is then exposed to external decay processes, including attack by protists (arrows). (Scale bar: 200 μm for A–D; 32 μm for E.)
Examination of killed embryos returned to normal seawater showed that the inactivation of proteins that occurs under reducing conditions is a necessary correlate of preservation. For cleavage-stage embryos, the presence of the fertilization envelope provides a protective barrier both from physical damage and external decay processes, including bacterial action and protist predation. Within an intact fertilization envelope, the pericellular space provides an apparently sterile medium surrounding the embryo (21, 22). Nonetheless, in normal seawater, dead embryos rapidly degrade through the process of autolysis. This internal cell destruction is caused by the action of endogenous proteases and other lytic enzymes, resulting in loss of cell boundaries, swelling, fusion of lipid droplets, and finally disintegration (Fig. 2D). Autolysis of dead cleavage-stage embryos was well underway by 18 h postdeath. To be fossilized with good morphology, embryos would need to encounter conditions that block autolysis, such as a reducing environment, very soon after death.
Autolysis was prevented experimentally in the reducing conditions produced by the addition of 100 mM β-mercaptoethanol (β-ME) to normal seawater (Figs. 1 E and F and 2 A–C). In this environment, protein disulfide bonds were reduced and enzymes were inactivated; embryos were still intact 3 weeks after their killing, when the experiment was concluded. If embryos were returned from reducing conditions to normal seawater, fertilization envelopes degenerated, and the embryos were subject to normal decay processes, including bacterial action and attack by hypotrichous ciliates (Fig. 2E).
Because of the extreme toxicity and difficulty of working with H2S, we used seawater containing 100 mM β-ME as a stand-in for the high H2S levels that may be present under anoxic conditions on the sea floor. Concentrations of H2S up to 30–100 mM have been reported from some modern marine environments (23, 24). At least some deposits containing Ediacaran and Cambrian fossil embryos are rich in pyrite, including the Doushantuo phosphorite, indicating substantial H2S levels at the time of fossilization (10, 25). H2S has similar reducing potential as thiols (26–29), and at the reported concentrations should effectively reduce protein S-S bonds in embryos that fall into such a sea-floor environment. The key is an environment in which autolytic processes are inactivated.
Role of Embryo Size.
The preponderance of reported large-sized embryos (10, 11, 30) in fossil fauna raised the possibility that large size might be a determinant in survival for mineralization. We therefore also examined preservability of the much smaller embryos of a planktotrophic sea urchin from another family, Lytechinus pictus. L. pictus eggs are only 100 μm in diameter and have a more inflated and apparently more fragile fertilization envelope than H. erythrogramma. L. pictus cleavage-stage embryos exhibited identical responses to our test conditions as the much larger H. erythrogramma embryos. Dead two- and four-cell L. pictus embryos placed in reducing conditions retained their fertilization envelopes and blastomere morphology for up to 4 weeks (Fig. 3A), whereas killed embryos returned to normal seawater underwent autolysis within a few hours postdeath (Fig. 3B). These data and taphonomy of later-stage embryos described below show that size is not a key factor in preservation.
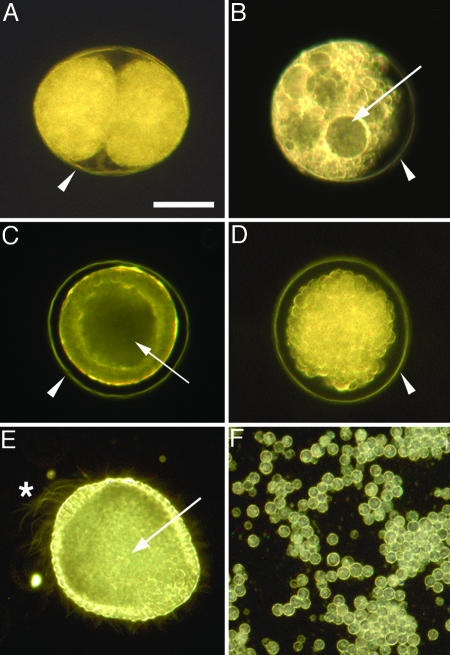
Fig. 3.
Unhatched embryos of the indirect developing sea urchin L. pictus are preserved under reducing conditions but not after hatching. Where present, the fertilization envelope is indicated by an arrowhead (A–D). Embryos in A, B, D, and F were killed by 12 min in seawater containing 1% ammonium, then treated as described. (A) Embryo killed at two-cell stage, then placed in seawater containing 100 mM β-ME, photographed after 26 days. Blastomere arrangement and structure are well preserved. (B) Embryo killed at two-cell stage, then placed in normal seawater, photographed after 5 h. The fertilization envelope is intact, but the embryo has swollen to fill the space within the fertilization envelope and is undergoing autolysis; cell structure has already disappeared (compare Fig. 2D). (C) Live unhatched blastula (17 h postfertilization) focused to show columnar cell shape of the blastula wall and the internal hollow blastocoel (arrow). (D) Unhatched blastula killed at the same stage as C, then placed in seawater containing 100 mM β-ME, photographed after 1 day. Embryo is intact within the fertilization envelope, but the cells have rounded up. Focusing through the embryo revealed the blastocoel has collapsed. (E) Live hatched blastula (24 h postfertilization) focused at the internal hollow blastocoel (arrow). The embryo was slightly flattened under a coverslip to immobilize it; the beating cilia are visible, including the large apical tuft cilia at the animal pole (asterisk). Differentiated epithelial cell shape at the animal and vegetal poles can be seen, and some internalized micromeres near the vegetal end of the blastocoel can be seen. (F) Cells remaining from a culture of hatched blastulae killed at the same stage as E, then placed in seawater containing 100 mM β-ME, photographed after 5 h. Cells rounded up and disaggregated. (Scale bar: 50 μm.)
Role of the Fertilization Envelope.
An unexpected factor in potential preservation of embryos is the crucial role of the fertilization envelope. Under reducing conditions both large lipid-rich embryos of a direct developer and small protein yolk-based embryos of an indirect developer could be preserved for prolonged times when the fertilization envelopes were intact. To further assess the role of the fertilization envelope we tested preservation of blastula-stage embryos from both small- and large-egged species before and after hatching. Examining embryos after release from the fertilization envelope mediated by the normal hatching process circumvented artefacts because of weakening or distortion of embryos that we have sometimes observed during mechanical or chemical removal of the fertilization envelope at earlier stages.
If killed prehatching blastulae were returned to normal seawater, the individual cells underwent autolysis, followed by degeneration of the fertilization envelopes and bacterial decay. Under reducing conditions, the preservation potential of killed prehatching blastulae was similar to cleavage-stage embryos, but retention of normal morphology was not as good as in cleavage stages. Under reducing conditions, the normal columnar shape of cells of unhatched L. pictus blastulae was lost; cells rounded up, cell–cell adhesion was diminished, and the blastocoels collapsed, producing a stereoblastula-like morphology (compare Fig. 3 C and D). Interpretation of developmental morphology in potential fossils at this stage would thus be misleading. The clusters of blastomeres in these preserved L. pictus blastulae resemble some of the rarer Doushantuo fossil embryo-like forms with hundreds of cells and poorer preservation than the more common cleavage-stage forms (1, 30). Unhatched H. erythrogramma blastulae behaved similarly. It thus appears that cell–cell adhesion is not sufficient in later-stage embryos to maintain faithful morphological architecture under reducing conditions. Unhatched L. pictus blastulae exhibited similar preservational potential to cleavage stages, remaining well preserved in reducing conditions for at least 3 weeks. Unhatched H. erythrogramma blastulae showed slightly less preservational potential, caused in part by the accumulation of large lipid droplets. Some experimental samples began to degrade after 1 week.
Reducing conditions did not support preservation of any posthatching stages lacking a fertilization envelope for any of the three sea urchin species we examined (Figs. 3 E and F and 4). The morphology of newly hatched L. pictus blastulae is very similar to unhatched blastulae (Fig. 3E), and so is the behavior of the cells when placed in reducing conditions after killing the embryos. In seawater containing β-ME, cells from hatched blastulae quickly rounded up and lost the tight cell–cell adhesion of the living embryo. In the absence of the constraining fertilization envelope, the embryos rapidly fell apart (Fig. 3F). Killed hatched H. erythrogramma blastulae behaved similarly under reducing conditions. Individual cells might be preserved for extended periods in reducing conditions, but it would be difficult indeed to identify their fossilized remains.
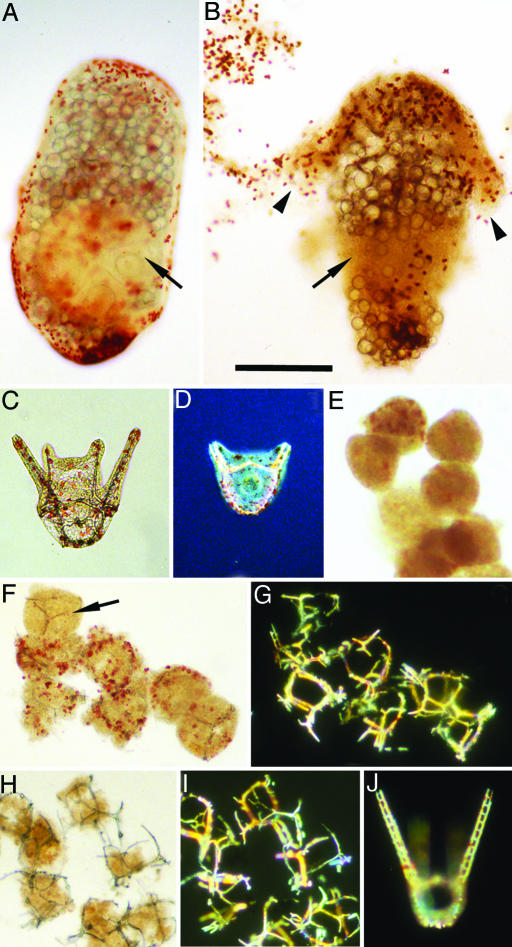
Fig. 4.
Reducing conditions do not preserve Heliocidaris larvae. (A and B) H. erythrogramma. (A) Normal 2-day larva focused to show the internal pentameral adult rudiment (arrow); large lipid droplets that serve as “yolk” are also visible (19). (Metamorphosis would occur at day 4.) (B) Larva from a culture killed at 2 days by treatment with 1% ammonium for 20 min, then placed in seawater containing 100 mM β-ME, photographed after 2 days. Larvae become fragile, lose structural integrity, and disintegrate. In the specimen shown, adult rudiment structure is lost (arrow) and pigmented epithelium (arrowheads) is detaching. (C–J) H. tuberculata. (C) Normal 48-h pluteus; skeletal rods in arms and body are visible within the transparent pigmented epithelium. (D) Normal 36-h pluteus, starting stage for experiments in E–J. Photographed with Nomarski optics to display internal skeletal structure; hindgut is visible in body of larva. (E–I) Embryos from the same culture as D, killed by treatment with 1% ammonium for 20 min, then placed in seawater containing 100 mM (E) or 10 mM β-ME (F and G) or returned to normal seawater (H and I), photographed after 1 day. (E) In strong reducing conditions, structural integrity and morphology are rapidly lost; skeletal elements rapidly disappear. (In sample shown, no skeletal elements were detected under polarizing optics.) (F) In less stringent reducing conditions, structural integrity and pigmentation disappear more slowly. Pluteus morphology and skeletal organization are lost, but skeletal elements (arrow) are stable. (G) Same field as F under polarizing optics to display skeletal elements. (H) In normal seawater, cytoplasm is subject to both internal and external decay processes, including predation by protists, and rapidly disappears. Skeletal organization is lost, but skeletal elements are preserved for prolonged periods. (I) Same field as H under polarizing optics. (J) Normal 3-day pluteus from untreated controls under polarizing optics. (Scale bar: 200 μm.).
Although they possess differentiated cell layers and well integrated internal tissue architecture, H. erythrogramma larvae also lost morphological integrity within a day or less under reducing conditions (Fig. 4 A and B). Likewise pluteus larvae from the indirect-developing, planktotrophic sister species, Heliocidaris tuberculata (Fig. 4 C–J), were not preserved in seawater containing β-ME. Moreover, calcitic skeletal elements rapidly dissolved in the highly reducing conditions that would potentially support phosphatization processes of fossilization of soft tissues (Fig. 4E). Skeletal elements were stable in less severe reducing conditions or in normal seawater (Fig. 4 F–I), environments incompatible with preservation of cell structure.
Discussion
Our results show that several factors may affect fossilization of embryos (Table 1). Both the correct chemical environment and the presence of a fertilization envelope are key. Even when contained within a fertilization envelope, soft-bodied embryos and larvae in normal seawater are subject to internal autolytic decay processes. Without a fertilization envelope, they are subject to both autolysis and external action by bacterial decay and action of protists. In reducing conditions compatible with mineralization, autolysis is blocked and extended preservation is possible, but only if the embryo is enclosed within the fertilization envelope. The fertilization envelope may facilitate establishment of a geochemical microenvironment that inhibits decay and allows authigenic mineralization.
Table 1.
Consequences of death and postdeath conditions for soft-bodied embryos
| Condition | Outcome |
|---|---|
| Mode of death | |
| Fast | Normal cell cleavage pattern retained |
| Slow | Abnormal cell cleavage pattern |
| Fertilization envelope present | |
| Normal seawater | Rapid autolysis |
| Reducing conditions | Extended preservation |
| Cleavage stages | Cell arrangement retained |
| Prehatching blastula | Cell arrangement lost |
| Fertilization envelope absent | |
| Normal seawater | Rapid decay |
| Reducing conditions | Rapid loss of morphology; individual cells preserved |
These data suggest strong taphonomic biases in distribution of fossil embryos. First, fossils of cleavage-stage embryos in fertilization envelopes should reflect a broad phylogenetic spectrum. Throughout much of marine animal diversity, early developmental stages are surrounded by envelopes that harden at fertilization and surround the embryos until hatching, including embryos of many species within cnidarians, annelids, phoronids, bryozoans, nematodes, arthropods, hemichordates, echinoderms, ascidians, and vertebrates (31–34). This wide distribution among living marine clades suggests that fertilization envelopes were likely also widely distributed in Late Precambrian and Cambrian marine embryos. The presence of large numbers of fossilized embryos in early cleavage stages in the Doushantuo fauna (1, 6, 11) fits with the feasibility of preservation suggested by our data.
Second, hatched embryos and soft-bodied larvae are unlikely to be preserved even under reducing conditions. In light of these results, claims of fossilized larvae among the Doushantuo fauna (2, 3), already the subject of critical analysis (5, 14, 35), appear even more unlikely. In addition, the nonequivalence of preservational potential for different developmental stages means that there would likely be a gap in the fossil record for ontogeny of many species. Our data coupled with previous taphonomic studies (15–18) suggest we should expect to find fossils of early stages and later cuticularized developmental stages but not hatched blastulae or primary larvae. This preservation bias may produce an artifact of interpretation of fossil faunas in which common cleavage-stage embryo fossils might not relate to similar-sized fossils of preadult stages.
Our data provide clues for interpreting the morphology of fossil embryos. Rapid death maintains blastomere numbers and shape, whereas slow death produces aberrant embryos. Thus, although concern has been expressed over the patterns of cleavage exhibited by Doushantuo embryos (36, 37), the equal size of the blastomeres within these embryos suggests rapid death and a faithful representation of the living embryo. The embryos from the Lower Cambrian of Shaanxi (6, 11, 38), exhibiting unequal blastomere size, are more difficult to interpret. The irregular pattern could be explained by slow death. However, some cleavage-stage embryos, such as those of spiralians, have different-sized blastomeres; also, in stages where hundreds of cells may be present, some embryos have cells of unequal size that are less evenly spaced. Nonetheless, a general feature of early cleavage embryos is that cells have a regular and coherent arrangement, unlike the irregular cell patterns such as in Fig. 1 J and K. Moreover, embryos that have undergone abnormal fertilization, as in polyspermy (Fig. 1L), exhibit abnormal cleavages unrelated to the events that killed or preserved them.
We found that many modes of rapid death do not disrupt embryos. However, hypotonic seawater, a plausible cause of death in situ, resulted in changes to blastomere volume and shape (Fig. 1G). Doushantuo cleavage embryos show a range of morphologies in which the blastomeres are more or less tightly adpressed to one another (1) or to the fertilization envelope (39). Another possible artifact might arise from our observation that even under reducing conditions fidelity of normal architecture was not fully maintained in blastula stages. Such differences in morphology in fossil forms might represent different species or even a change in cleavage pattern during development of a single species, but also might be artefacts of taphonomic variables. Attempts to discriminate developmental series of different species in fossil deposits (11) have not considered such artefacts.
In some cases the fossils may provide clues to nonideal prefossilization conditions. For example, Xiao and Knoll (25, 30) inferred organic degradation in some of the Doushanto embryos, the appearance of which is similar to some of our experimental embryos under nonpreserving conditions (Fig. 3 B and D). Some of the poorly preserved fossils interpreted as hydrozoan gastrulae (2) also resemble these degraded embryos.
Understanding potential taphonomic biases in the preservation of Precambrian and Cambrian fossil embryos provides a window on the metazoan radiation and the origins of larvae (40). Hypotheses of developmental evolution among metazoan phyla formulated on the basis of a fossil record devoid of primary larvae (12, 35) may be spurious. Rather, the evolution of life history and developmental modes in early metazoans may have to be inferred indirectly from fossil embryos.
We observed that decay and preservation potential were similar in both the large lipid-rich direct developing H. erythrogramma embryos and small yolk-rich embryos from two species of planktotrophic indirect developers, indicating that size and cytoplasmic composition may have little influence on taphonomy of sea urchin embryos and larvae. The Doushantuo fauna reflects a developmental-stage bias consistent with taphonomic observations, but the fauna reported to date contains only larger embryos. This finding may result from a preponderance of large-egged direct developers in the fauna, a biostratinomic artifact, or an artifact of sampling microfossils of smaller sizes.
The distribution of fossil embryo sizes is significant because in modern marine fauna, large egg size (>300 μm) and lecithitrophy, and small egg size and planktotrophy, are strongly linked with direct and indirect development, respectively. We show that the primary larvae of indirect developers are unlikely to be preserved. Reinterpretation of the embryo fossil record in this light suggests that the absence of primary larvae is probably a taphonomic artifact and, as such, it may never prove possible to directly test hypotheses on the life-history strategy adopted by early metazoans. However, fossil embryos in the small size range can be used as a marker for the presence of feeding larvae. A shift associated with the Cambrian radiation from predominantly direct development to mainly planktonic feeding larvae should leave a signal of a change in size distribution in fossilized cleavage-stage embryos. Based on extant species, a size shift from 300 μm or more diameters to a smaller diameter range from 60 to 200 μm would be predicted.
There are convincing arguments that feeding larvae were present in several taxa by the early Ordovician (41, 42); thus comparisons of embryo fossils from the late Precambrian through the early Ordovician (13, 42) would allow this question to be answered. If small-sized embryos typical of planktotrophic indirect-developers are present in late Cambrian to early Ordovician sediments but absent in late Precambrian to early Cambrian deposits, this pattern would be consistent with a later appearance in evolution. In contrast, if small-sized embryos cannot be found in deposits from time periods when feeding larvae are known to exist, then their absence from other deposits is likely to be a taphonomic bias and would not be informative. Thus even though we cannot expect to find larvae themselves in the fossil record, the robust predictions supported by size classes of modern embryos will allow interpretation of life history and developmental mode of fossil fauna, once it is known if the size range of fossil embryo faunas is real or if microfossils representing small-sized embryos are present but heretofore unsampled.
Experimental Procedures
Embryo Culture and Taphonomy Conditions.
H. erythrogramma and H. tuberculata were collected near Sydney, Australia. L. pictus was obtained from California (Marinus, Long Beach, CA). Heliocidaris embryos were cultured at 21–24°C, and L. pictus were cultured at 14–19°C. Embryos or larvae were killed rapidly or over time periods up to a few hours under conditions that were toxic (0.1% or 1% ammonium in seawater, added as ammonium chloride), partially anaerobic (seawater in which N2 was bubbled for several hours before embryos were suspended in it), or hypotonic (seawater diluted to 50% with deionized water). To examine preservation, killed embryos were placed in normal seawater under aerobic conditions or in seawater containing 100 mM β-ME to mimic the reducing conditions that permit mineralization in nature (15–18, 23, 24).
Microscopy.
Light microscopy was carried out on Zeiss axioplan compound microscopes or a Wild (Heerbrugg, Switzerland) stereo microscope, using bright-field, dark-field, or polarizing optics. Living swimming embryos or larvae were immobilized under cover slips or by addition of a small amount of 2% paraformaldehyde to the seawater. Images were taken on 35-mm slide film or captured digitally with a SPOT RT Slider digital camera and spot software (Fryer, Chicago), and processed in photoshop (Adobe Systems, San Jose, CA) on a Macintosh computer. Samples for scanning electron microscopy were fixed in 2.5% glutaraldehyde in seawater, dehydrated through an ethanol series, dried by the critical point method with amyl acetate as the intermediate fluid, and sputter-coated with a gold–palladium alloy.
Acknowledgments
We thank our colleagues at the University of Sydney and the Sydney Aquarium for their help and support in experiments carried out in Sydney; Dennis Peters, Department of Chemistry, Indiana University, for his help in understanding the redox properties of H2S and thiols; and two anonymous reviewers for insightful comments. This work was supported in part by grants from the National Science Foundation (to R.A.R.) and the Natural Environment Research Council (to P.C.J.D.).
Abbreviation
- β-ME
β-mercaptoethanol
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Xiao S., Zhang Y., Knoll A. H. Nature. 1998;391:553–558. [Google Scholar]
- 2.Chen J., Oliveri P., Li C.-W., Zhou G.-Q., Gao F., Hagadorn J. W., Peterson K. J., Davidson E. H. Proc. Natl. Acad. Sci. USA. 2000;97:4457–4462. doi: 10.1073/pnas.97.9.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J., Oliveri P., Gao F., Dornbos S. Q., Li C. W., Bottjer D. J., Davidson E. H. Dev. Biol. 2002;248:182–196. doi: 10.1006/dbio.2002.0714. [DOI] [PubMed] [Google Scholar]
- 4.Condon D., Zhu M., Bowring S. A., Wang W., Yang A., Jin Y. Science. 2005;308:95–98. doi: 10.1126/science.1107765. [DOI] [PubMed] [Google Scholar]
- 5.Xiao S., Yuan X., Knoll A. H. Proc. Natl. Acad. Sci. USA. 2000;97:13684–13689. doi: 10.1073/pnas.250491697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bengtson S., Yue Z. Science. 1997;277:1645–1648. [Google Scholar]
- 7.Zhang X. G., Pratt B. R. Science. 1994;266:637–639. doi: 10.1126/science.266.5185.637. [DOI] [PubMed] [Google Scholar]
- 8.Peterson K. J., Butterfield N. J. Proc. Natl. Acad. Sci. USA. 2005;102:9547–9552. doi: 10.1073/pnas.0503660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong X., Donoghue P. C. J., Cunningham J. A., Liu J., Cheng H. Evol. Dev. 2005;7:468–482. doi: 10.1111/j.1525-142X.2005.05050.x. [DOI] [PubMed] [Google Scholar]
- 10.Dong X., Donoghue P. C. J., Cheng H., Liu J.-B. Nature. 2004;427:237–240. doi: 10.1038/nature02215. [DOI] [PubMed] [Google Scholar]
- 11.Steiner M., Zhu M., Li G., Qian Y., Erdtmann B.-D. Geology. 2004;32:833–836. [Google Scholar]
- 12.Conway Morris S. BioEssays. 1998;20:676–682. doi: 10.1002/(SICI)1521-1878(199808)20:8<676::AID-BIES11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 13.Donoghue P. C. J., Kouchinsky A., Waloszek D., Bengtson S., Dong X.-P., Val’kov A. K., Cunningham J. A., Repetski J. E. Evol. Dev. 2006;8:232–238. doi: 10.1111/j.1525-142X.2006.00093.x. [DOI] [PubMed] [Google Scholar]
- 14.Bengtson S. In: The New Panorama of Animal Evolution. Legakis A., Sfenthourakis S., Polymeni R., Thessalou-Legaki M., editors. Moscow: Pensoft; 2003. pp. 289–300. [Google Scholar]
- 15.Sagemann J., Bale S. J., Briggs D. E. G., Parkes R. J. Geochim. Cosmochim. Acta. 1999;63:1083–1095. [Google Scholar]
- 16.Briggs D. E. G., Kear A. J. Science. 1993;259:1439–1442. doi: 10.1126/science.259.5100.1439. [DOI] [PubMed] [Google Scholar]
- 17.Briggs D. E. G., Kear A. J. Palaios. 1994;9:431–456. [Google Scholar]
- 18.Martin D., Briggs D. E. G., Parkes R. J. Geology. 2003;31:39–42. [Google Scholar]
- 19.Williams D. H. C., Anderson D. T. Aust. J. Zool. 1975;23:371–403. [Google Scholar]
- 20.Villinski J. T., Villinski J. C., Byrne M., Raff R. A. Evolution. 2002;56:1764–1775. doi: 10.1111/j.0014-3820.2002.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 21.Wessel G. M., Conner S., Laidlaw M., Harrison J., LaFleur G. J. Biol. Reprod. 2000;63:1706–1712. doi: 10.1095/biolreprod63.6.1706. [DOI] [PubMed] [Google Scholar]
- 22.Kudo S. Zygote. 2000;8:257–265. doi: 10.1017/s0967199400001052. [DOI] [PubMed] [Google Scholar]
- 23.Sahling H., Rickert D., Lee R. W., Linke P., Suess E. Mar. Ecol. Progr. Ser. 2002;231:121–138. [Google Scholar]
- 24.Kelley D. S., Baross J. A., Delaney J. R. Annu. Rev. Earth Planet. Sci. 2002;30:385–491. [Google Scholar]
- 25.Xiao S. H., Knoll A. H. Lethaia. 1999;32:219–240. doi: 10.1111/j.1502-3931.1999.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 26.Latimer W. M. The Oxidation States of the Elements and Their Potentials in Aqueous Solutions. Englewood Cliffs, NJ: Prentice–Hall; 1952. [Google Scholar]
- 27.Alberty R. A. Arch. Biochem. Biophys. 1998;358:25–39. doi: 10.1006/abbi.1998.0831. [DOI] [PubMed] [Google Scholar]
- 28.Alberty R. A. Biophys. Chem. 2004;111:115–122. doi: 10.1016/j.bpc.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Tewari Y. B., Goldberg R. N. J. Chem. Thermodyn. 2003;35:1361–1381. [Google Scholar]
- 30.Xiao S. H., Knoll A. H. J. Paleontol. 2000;74:767–788. [Google Scholar]
- 31.Kume M., Dan K. Invertebrate Zoology. New York: Garland; 1998. [Google Scholar]
- 32.Dong C. H., Yang S. T., Yang Z. A., Zhang L., Gui J. F. Dev. Biol. 2004;265:341–354. doi: 10.1016/j.ydbio.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 33.Nakajima Y., Humphreys T., Kaneko H., Tagawa K. Zool. Sci. 2004;21:69–78. doi: 10.2108/0289-0003(2004)21[69:DANOOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 34.Urata M., Yamaguchi M. Zool. Sci. 2004;21:533–540. doi: 10.2108/zsj.21.533. [DOI] [PubMed] [Google Scholar]
- 35.Conway Morris S. In: Gastrulation: From Cells to Embryo. Stern C., editor. Plainview, NY: Cold Spring Harbor Lab. Press; 2004. pp. 703–711. [Google Scholar]
- 36.Conway Morris S. Curr. Biol. 1997;7:R71–R74. doi: 10.1016/s0960-9822(06)00039-x. [DOI] [PubMed] [Google Scholar]
- 37.Valentine J. W. On the Origin of Phyla. Chicago: Univ. of Chicago Press; 2004. [Google Scholar]
- 38.Yue Z., Bengtson S. Lethaia. 1999;32:181–195. [Google Scholar]
- 39.Yin C., Bengtson S., Zhao Y. Acta Palaeontol. Polonica. 2004;49:1–12. [Google Scholar]
- 40.Sly B. J., Snoke M. S., Raff R. A. Int. J. Dev. Biol. 2003;47:623–632. [PubMed] [Google Scholar]
- 41.Signor P. W., Vermeij G. J. Paleobiology. 1994;20:297–319. [Google Scholar]
- 42.Peterson K. J. Geology. 2005;32:929–932. [Google Scholar]