Abstract
Environmental and genetic aberrations lead to neural tube closure defects (NTDs) in 1 in every 1000 births1. Mouse and frog models for these birth defects have suggested that Van Gogh-like 2 (Vangl2, also known as Strabismus) and other components of planar cell polarity (PCP) signalling control neurulation by promoting the convergence of neural progenitors to the midline2-8. Here we report a novel role for PCP signalling during neurulation in zebrafish. We demonstrate that non-canonical Wnt/PCP signalling polarizes neural progenitors along the anterior-posterior axis. This polarity is transiently lost during cell division in the neural keel but is re-established as daughter cells reintegrate into the neuroepithelium. Loss of zebrafish Vangl2 (in trilobite mutants) abolishes the polarization of neural keel cells, disrupts re-intercalation of daughter cells into the neuroepithelium, and results in ectopic neural progenitor accumulations and NTDs. Remarkably, blocking cell division leads to rescue of trilobite neural tube morphogenesis despite persistent defects in convergence and extension. These results reveal a role for PCP signalling in coupling cell division and morphogenesis at neurulation and suggest a novel mechanism underlying NTDs.
During zebrafish neurulation, the neural plate folds toward the midline. This results in the apposition of apical surfaces from opposite sides of the neural plate and the formation of the neural keel (Supplementary Fig.1). As cells divide, one daughter cell remains in the ipsilateral side of the neural keel whereas the other daughter cell intercalates across the midline and integrates into the contralateral neuroepithelial layer9-11. To explore the molecular basis of neural progenitor cell morphogenesis, we used a candidate gene approach and asked if the PCP signalling component Vangl2 might be involved12, 13. We eliminated all Vangl2 activity by generating maternal-zygotic trilobite (MZtri) mutants using a germ line-replacement strategy14. MZtri embryos proved more severely affected than zygotic mutants (Supplementary Fig.2). Comparison of wild-type (WT) and mutant embryos at the 20-somite stage revealed that MZtri embryos do not generate a normal neural tube (Fig. 1g,h). The MZtri neural anlage develops as an outer pseudo-stratified neuroepithelial layer surrounding an ectopic mass of disorganized cells (Fig. 1h). As early as the neural keel stage, the MZtri neural primordium appears broader and thicker than in WT (Fig. 1c,d). This trend continues through neural rod stages when cells appear to accumulate in the centre of the wide MZtri neural anlage (Fig. 1e,f). The floorplate of MZtri mutant embryos also appears broader than in WT (Fig.1e-h), as is evident in sections through sonic hedgehog stained WT and MZtri embryos (Supplementary Fig.8e,g). Expanded neural midline structures are also characteristic of frog and mouse PCP signalling mutants2, 8.
Figure 1.

PCP signalling is required for zebrafish neural tube formation. (a-h) Confocal micrographs of transverse sections through rhodamine-phalloidin stained embryos, comparing WT and MZtri neural tube morphogenesis at 5-somite/neural plate (a,b), 10-somite/neural keel (c,d), 15-somite/neural rod (e,f), and 20-somite/neural tube (g,h) stages. The neural anlage has been outlined in all panels. (i-j) Sections through mGFP-injected 20-somite staged embryos showing ectopic cell accumulations within the developing neural tube of MZslb;MZppt embryos (i), and severe disorganization of the neural anlage in MZslb;MZppt embryos that were injected with 6ng of Wnt4 MO (j). (k-m) Rhodamine-phalloidin stained sections through 20-somite staged MZtri embryos cultured overnight in either 4% DMSO (k), or in the presence cell division inhibitors25 (l-m). Note rescue of the expanded floorplate (arrows in k-m) and neural tube morphogenesis defects upon blocking cell division. The extent of ectopic cellular accumulations in PCP signalling mutants has been highlighted (h-l). Scale bars, 50 μm.
Because Vangl2 has been shown to modulate the non-canonical Wnt signalling pathway, we asked whether Wnt signals also regulate neural tube morphogenesis. Using a modified germ line-replacement protocol (Supplementary Fig.3), we generated wnt11/silberblick(slb)15 and wnt5/pipetail(ppt)16 MZ compound mutants (Supplementary Fig.4). MZslb;MZppt embryos demonstrated a similar, yet less severe neurulation phenotype as MZtri mutants (Fig. 1i). Reduction of Wnt4 activity17 in an MZslb;MZppt background enhanced the mutant phenotype, and at 20 somite-stages, MZslb;MZppt;wnt4-morphant embryos displayed a neurulation phenotype very similar to MZtri mutants (Fig. 1j, Supplementary Fig.4). These results indicate that non-canonical Wnt signalling is required for normal zebrafish neurulation.
In the frog, neural tube closure requires PCP signalling within the neural plate18. To determine whether MZtri neurulation defects are autonomous to the neuroectoderm or secondary to mesoderm or endoderm CE defects, we examined neurulation in embryos that lack endoderm and trunk and head mesoderm. Such embryos were generated by misexpression of Lefty, an inhibitor of Nodal signalling19, 20. MZtri+lefty embryos were considerably shorter than WT+lefty controls (compare Fig.2a and b), and displayed neurulation defects similar to MZtri mutants (Fig.2b'). In a complementary assay, we asked if mutant mesendoderm can induce the MZtri neurulation phenotype. We generated chimeric embryos in which only the endoderm and trunk mesoderm lineages were derived from MZtri mutant cells (Fig.2c). In these embryos, the neural tube developed with normal neuroepithelial morphology, a well-formed neurocoel, and no evidence of ectopic cell accumulations (Fig.2c'). These results indicate that MZtri neurulation defects are due to the lack of Vangl2 function in ectodermal tissues.
Figure 2.

Cell autonomy of PCP signalling within the neural keel. (a-b) Whole-mounts and transverse sections through the trunk of 24 hour post-fertilization WT (a) and MZtri (b) embryos injected with 100 pg of lefty mRNA. Convergence of the neural plate into a neural rod occurs normally in WT+lefty embryos, despite the absence of underlying mesendoderm (a'). This convergence is disrupted in MZtri+lefty mutants, which show neurulation defects in the absence of trunk mesoderm (b'). Brackets in a and b indicate the extent of trunk axial extension. Identical results were obtained using a different genetic combination: maternal-zygotic one-eyed-pinhead (MZoep) mutants were used to eliminate Nodal signalling, and PCP signalling was perturbed through diego mRNA injection (not shown). (c) mGFP-labelled MZtri cells were transplanted into MZoep host embryos at mid-blastula stages to generate MZtri->MZoep chimeric embryos. MZoep mutants lack Nodal signalling and do not form endoderm or trunk mesoderm lineages29, therefore endoderm and trunk somites in chimeric embryos develop entirely from GFP-positive MZtri donor cells. (c') Transverse sections of 24 hpf MZtri->MZoep chimeras were counter-stained with rhodamine-phalloidin to visualize neural tube formation (outlined in c'). The absence of MZtri neurulation defects indicates a requirement for PCP signalling autonomous to the neuroectoderm. (d-g) Transverse sections through the trunk of WT and MZtri chimeric embryos at 24 hpf. (d) Bilateral distribution of mRFP-labelled WT cells (arrowheads) within the neural tube of an mGFP-labelled WT host. (e) Unilateral accumulation of mRFP-labelled MZtri cells within the neural tube of mGFP-labelled WT host embryos. (f-g) mRFP-labelled MZtri (f) or WT (g) cells transplanted into mGFP-labelled MZtri host embryos accumulate unilaterally within the MZtri neural anlage. Scale bars, 50 μm.
Several potential mechanisms might underlie the ectopic accumulation of cells seen in MZtri mutants, including abnormal delamination of neuroepithelial cells or failed re-integration of cells into the neuroepithelium following cell division. As a first test to distinguish between these possibilities, we used the photo-convertible Kaede fluorophore21 to label half of the neuroepithelium at neural plate/early neural keel stages and then analyzed the location of the labelled cells and their descendants in the neural tube (Fig.3a-d). Consistent with previous studies of zebrafish neurulation9-11, we found that cell division in the neural keel results in the bilateral distribution of daughter cells across apposing neuroepithelial layers of the WT neural tube (n=10; Fig.3a,b). In dramatic contrast, labelled cells were not found in the contralateral neuroepithelium of MZtri embryos (n=29), and a sharp midline boundary was maintained even among cells accumulating ectopically in the neural anlage (Fig.3c,d). These results are consistent with a defect in the integration of MZtri neural progenitors into the contralateral neuroepithelium.
Figure 3.
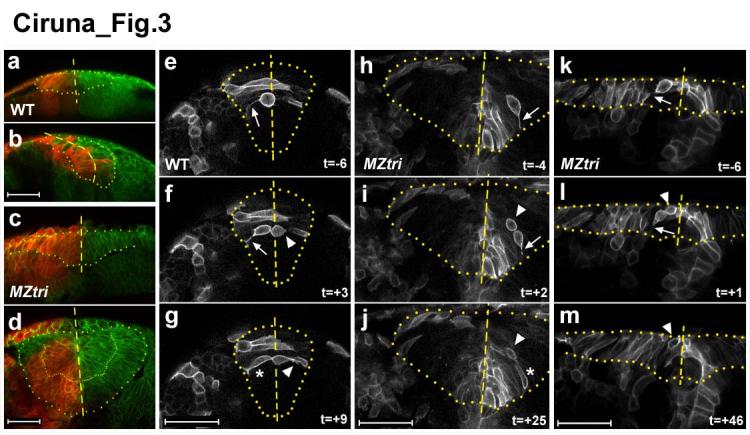
The cellular basis of MZtri neurulation defects. (a-d) Lineage tracing of WT (a,b) and MZtri (c,d) neural plate cells after red photo-conversion of the Kaede fluorophore. WT neural progenitors routinely crossed the midline into the contralateral side of the neural tube (b). MZtri neural progenitor cells never integrated into the contralateral neuroepithelium (d). Cells accumulating ectopically in the centre of the MZtri neural anlage also respected the midline (dashed lines). (e-m) Confocal micrographs from time series depicting cell division during WT (e-g) and MZtri (h-m) neurulation. Time is indicated as minutes preceding or following the completion of cytokinesis. The boundary of the neural keel has been highlighted, and the midline indicated. Cells in the WT neural keel round up their cell bodies and divide apically (e). Basal daughter cells remain connected to the basement membrane via a cellular process (arrow in e,f) and re-insert into the neuroepithelium (asterisk in g). Apical daughter cells (arrow head in f,g) lose contact within the basal membrane, adopt medial-lateral polarity, and intercalate across the midline of the neural keel. MZtri cells divide apically, and basal daughter cells behave as in WT (arrow in h,i,k,l, asterisk in j). MZtri apical daughter cells (arrowhead in i,j,l,m) do not intercalate into the contralateral neuroepithelium and remain in the place they were born. Scale bars, 50 μm.
To more directly follow the behaviour of neural progenitors during and after mitosis, we imaged cell behaviour in the neural keel of WT (n=14) and MZtri mutant (n=11) embryos (Fig.3e-m). As observed previously, we found that WT cells rounded up and divided apically, i.e. along the medial-lateral axis of the neural keel, and that daughter cells became incorporated into opposite sides of the neural tube (Fig. 3e-g, Supplementary video 1)10, 11. In addition, we found that the more basal daughter cell maintained contact with the basement membrane via a thin cellular process (arrow, Fig.3e.f) and returned to its original position within the neuroepithelium. In contrast, the more apical daughter cell lost contact with the basement membrane, became polarized across the medial-lateral axis and intercalated across the midline into the contralateral side of the neural keel (arrowhead, Fig.3f,g). Intercalation of apical daughter cells across the midline averaged 8 minutes following completion of cytokinesis (data not shown).
In MZtri embryos, cell division appeared normal. Cells divided apically, and basal daughter cells maintained their ipsilateral position within the neuroepithelium (arrow, Fig.3h,i). In notable contrast to wild type, however, apical MZtri daughter cells failed to re-integrate into the neuroepithelium following mitosis (arrowhead, Fig.3i,j). These daughter cells accumulated in the middle of the MZtri neural anlage and remained in the place where they were born over the duration of the image series (Fig.3j, Supplementary Figure 5). To determine whether MZtri intercalation defects were secondary to abnormal neural tube morphogenesis, we examined cell division at the onset of neural keel formation, prior to an obvious MZtri neurulation phenotype. We observed that following mitosis, apical MZtri daughter cells failed to intercalate across the midline (Fig.3k-m, Supplementary Video 2). These results indicate that the PCP pathway is required for the intercalation of neural progenitor cells into the contralateral neuroepithelial layer following cell division in the neural keel.
PCP signalling functions to polarize cells, but molecular evidence for such a role during neurulation has been elusive. We therefore analyzed the subcellular localization of PCP signalling components22, 23 and asked if the failure of MZtri cell re-intercalation is due to polarity defects. We found that EGFP-tagged Prickle24 (Gfp-Pk), a PCP effector molecule, showed striking asymmetric localization in wild-type cells of the notochord and neural keel (Fig.4a,d). This fusion protein was functional and rescued zebrafish Pk1-morphants (Supplementary Fig.6). Gfp-Pk was present predominantly in the cytoplasm but became asymmetrically localized to distinct, dynamic puncta along the anterior membrane of neural keel cells (Supplementary Video 3; arrows, Fig.4a). Interestingly, membrane localization of Gfp-Pk was lost during cell division in the neural keel (Fig.4e), but was subsequently re-established in both daughter cells (Fig.4f). To determine if this localization is dependent on PCP signalling, we analyzed MZtri and MZslb;MZppt;wnt4-morphant embryos (Fig.4b,c). In both contexts the asymmetric localization of Gfp-Pk was severely reduced or absent. These results establish membrane localization of Gfp-Pk as the first molecular marker for the planar polarity in neural progenitors and reveal that PCP signalling polarizes cells across the antero-posterior (AP) axis.
Figure 4.
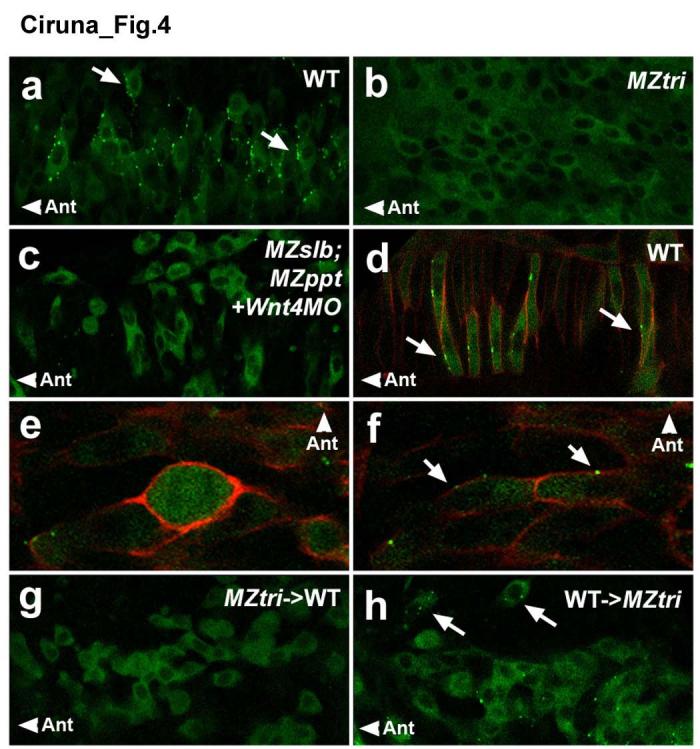
Anterior membrane localization of Gfp-Pk as a marker of planar polarity. Confocal images taken at the level of the anterior spinal cord, through the dorsal-ventral plane of the neural keel (a-c, e-h) or notochord (d) of 8- to 10-somite staged embryos. (a-c) Scatter labelling of Gfp-Pk in the neural keel of (a) a WT embryo, (b) an MZtri mutant, or (c) an MZslb;MZppt mutant injected with 6 ng of Wnt4 MO. (d) Scatter labelling of Gfp-Pk plus mRFP in WT notochord, demonstrating anterior membrane translocation of Gfp-Pk (arrows) in cells that undergo well-characterized CE movements in response to PCP signalling. (e-f) A Gfp-Pk plus mRFP labelled WT neural keel cell demonstrating transient loss of polarity markers during mitosis (e), but re-establishment of membrane-localized Gfp-Pk in both daughter cells (arrows in f). (g-h) Chimeric analysis of the autonomy of PCP signalling, using Gfp-Pk as a marker of planar polarity. (g) MZtri cells transplanted into WT host embryos do not localize Gfp-Pk to the membrane, as Vangl2 is required for Pk translocation. (h) WT cells transplanted into MZtri hosts show reduced membrane Gfp-Pk localization and abnormal polarity (arrows). “Ant” marks the anterior direction.
To determine the cell autonomy of Vangl2 function and Gfp-Pk localization during neurulation, we generated chimeras of WT and MZtri cells (Supplementary Table 1). When WT donor cells were transplanted into WT hosts, the majority of donor clones distributed daughter cells into the contralateral side of the neural tube (88% of clones, n=16; Fig.2d). In contrast, only a minority of MZtri donor clones in the neural tube of wild-type hosts (38%, n=37) showed contralateral cell intercalation, despite the normal morphogenesis of the surrounding WT neuroepithelial tissue (Fig.2e). Time-lapse analysis of MZtri cell behaviour in WT neural keels showed that most MZtri daughter cells (n=4/6) failed to intercalate across the midline following mitosis, indicating a cell autonomous role for Vangl2 (Supplementary Fig.7a-c). In cases where MZtri cells showed some evidence of medial-lateral intercalation, the rate of cell movement was severely delayed (n=2/6; Supplementary Figure 7d-f).
The majority of MZtri donor clones in MZtri hosts showed no evidence of cell intercalation across the midline (95%, n=41; Fig.2f). Similarly, in transplantations of WT cells into MZtri hosts, 93% of donor clones did not intercalate into the contralateral neuroepithelium (n=61; Fig.2g). Time-lapse analysis of WT cell behaviour in a MZtri neural keel confirmed that WT neural progenitors fail to polarize and re-intercalate following mitosis (n=7; Supplementary Fig.7g-i). Strikingly, WT cells transplanted into MZtri hosts also showed a reduction in membrane-localized Gfp-PK and a loss in AP polarity (Fig.4h), indicating that non-autonomous effects on neurulation are not simply the result of a passive obstruction to cell intercalation. These results suggest that Vangl2 is required both autonomously in cells that must re-integrate into the neuroepithelium and non-autonomously in neighbouring cells in order to establish or maintain planar polarity in neural progenitors.
Our results show that PCP signalling is required for the re-polarization and re-integration of neural progenitors following cell division in the neural keel. Indeed, the predominant role of PCP signalling might be to counteract the morphogenetic consequences of mitosis, which results in loss of polarity and the exclusion of apical daughter cells from the neuroepithelium. The strictest inference of this model suggests that MZtri neurulation defects can be suppressed by blocking cell division, thus precluding the need for PCP signalling. We therefore attempted to block cell division in WT and MZtri embryos through application of the DNA synthesis inhibitors aphidicolin and hydroxyurea25. Mitotic inhibitors were applied at late gastrulation stages in order to target maximal effects on neurulation. Inhibitor treatment significantly reduced the incidence of contralateral cell intercalation of wild-type neural progenitors (Supplementary Table 1), but neurulation proceeded normally in treated WT embryos (Supplementary Fig.8). Strikingly, blocking cell division suppressed MZtri neurulation defects in 90% of embryos (n=41; Fig.1k-m; Supplementary Fig. 8), and rescued neural tube morphogenesis, aberrant floorplate expansion and ectopic neural progenitor accumulations in 62% of these cases (Fig.1m). This result indicates that PCP signalling is no longer required for neural tube formation if cell division is blocked.
Taken together, our studies suggest that through re-establishing cell polarity and directing intercalative behaviour, PCP signalling functions to correct for the morphogenetic consequences of mitoses on neural tube morphogenesis. First, in the neural plate, midline progenitors distribute daughter cells along the medial-lateral axis, thus broadening the neural midline and future floorplate11. PCP-mediated convergence of midline cells counters this broadening2-8. Second, neural keel cells divide apically and position daughter cells outside of the neuroepithelium. PCP-mediated re-integration of neural progenitors assures that these cells do not accumulate in the midline. Although our results do not exclude additional roles for PCP signalling during neural development, blocking cell division abrogates the need for PCP signalling during neurulation and suppresses the floorplate and neural tube phenotypes associated with MZtri mutants.
Our results contrast with a recent study that analyzed the role of PCP signalling during earlier stages of zebrafish development. Gong et al. demonstrated that PCP signalling functions upstream of mitosis to orient the plane of cell division at gastrulation26. At this stage, PCP signals instruct neural precursors to divide along the animal-vegetal axis, thus driving extension of the zebrafish axis. Conversely, our study indicates that PCP signalling functions after mitosis to counteract the morphogenetic consequences of cell divisions during neurulation. Although the molecular basis of this process is unknown, an intriguing possibility is that PCP signalling regulates molecules involved in the establishment of apical-basal polarity or cell adhesion, two processes that might be disrupted by mitosis27. It also remains to be determined whether components of the mitotic apparatus directly regulate the re-establishment of planar polarity. No matter what the precise molecular underpinnings might be, our study shows that PCP signalling is required to compensate for the morphogenetic consequences of cell division on neural tube morphogenesis by promoting the polarity and intercalation of neural progenitors. Intriguingly, neural tube closure defects in curly tail (ct) mice, a genetic model for human NTDs, can also be modulated with agents that slow the rate of embryonic cell division28. Pharmacological inhibition of cell division at gastrula stages exacerbates the Ct NTD phenotype, whereas treatment during neurulation rescues neural tube closure defects28. Although the nature of the Ct mutation and the mechanism of pharmacological rescue remain unclear, our results raise the possibility that the uncoupling of PCP signalling and cell division during neurulation represent a common origin of neural tube closure defects.
Methods
Strains. The following mutants were used: ppt(sk13) (Supplementary Fig. 4), slb(tx226)15, tri(tk50f)13, and MZoep(tz57)29. Germ line-replacement chimeras for tri were generated as previously described14. The generation of germ line-replacement chimeras for slb;ppt compound mutants is described in Supplementary Figure 3.
Embryo microinjection and transplantation. Plasmids containing membrane-localized GFP (mGFP), membrane localized RFP (mRFP), EGFP tagged Prickle (Gfp-Pk)24, Kaede21, or lefty19, 20 were linearized and sense strand-capped mRNA was synthesized using the mMESSAGE mMACHINE system (Ambion). Morpholino antisense oligonucleotides (Gene Tools) were designed for zebrafish Pk130 and Wnt417 as previously described. Zebrafish embryos were dechorionated by pronase treatment and injected at the one-cell stage. Scatter labelling was obtained by injecting a subset of blastomeres at the 16- to 32-cell stage. Cell transplantations were performed at mid-blastula stages, as previously described14. For chimeric analyses of Vangl2 function, one cell-staged WT and MZtri embryos were injected with 100 pg of either mRFP or mGFP mRNA. At mid-blastula stages, ∼30-50 donor cells were transplanted into a single location above the margin of host embryos, to ensure uni-lateral distribution of donor cell clones within the anterior spinal cord of host embryos.
Cell division inhibitors. To block cell division at neurulation, embryos were cultured in a solution of 150 uM aphidicolin (Sigma) and 20 mM hydroxyurea (Sigma) in 4% DMSO25 beginning at 80% epiboly-stages.
Sectioning and microscopy. For transverse sections, embryos were fixed overnight in 4% paraformaldehyde, embedded in 2% agarose, and sectioned on a vibratome into 200 μm slices. Live embryos were mounted in 0.8% agarose prior to imaging. Fluorescent-images of mGFP-, mRFP-, and Gfp-Pk-injected embryos, or rhodamine-phalloidin (Molecular Probes) stained samples were obtained using a Zeiss LSM510 confocal microscope. For live imaging of cell divisions within the neural keel, embryos were scatter-labelled with mGFP and imaging was performed in a transverse plane through the trunk of 6-12 somite staged embryos at the 1st to 6th somite level.
Lineage tracing. Embryos were injected with 100 pg of Kaede mRNA and 100 pg of mGFP mRNA (for contrast) at the 1-cell stage, developed in the dark until 5-6 somite stages, and live-mounted in 0.8% agarose. The Kaede fluorophore was converted from green to red by focussing a 60 second pulse of UV light specifically on one lateral half of the neural plate, using the pinhole of a Zeiss LSM510 confocal microscope. Embryos were imaged both immediately and 10 hours after UV treatment.
Supplementary Material
Acknowledgements
We thank W. Talbot and D. Lyons for sharing their protocol for pharmacological inhibition of cell division, A. Chitnis for useful discussion, and L. Solnica-Krezel, W. Talbot, J. Wallingford, A. Giraldez, D. Prober and J. Rihel for helpful comments on the manuscript. This work was supported by grants from the NIH to AFS and MM. AFS was a Scholar of the McKnight Foundation for Neuroscience and is an Irma T. Hirschl Trust Career Scientist and an Established Investigator of the American Heart Association. BC was supported by a long-term fellowship from the Human Frontier Science Program.
Footnotes
Supplementary Information accompanies the paper on www.nature.com/nature.
Competing interests statement The authors declare that they have no competing financial interests.
References
- 1.Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–93. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- 2.Greene ND, Gerrelli D, Van Straaten HW, Copp AJ. Abnormalities of floor plate, notochord and somite differentiation in the loop-tail (Lp) mouse: a model of severe neural tube defects. Mech Dev. 1998;73:59–72. doi: 10.1016/s0925-4773(98)00029-x. [DOI] [PubMed] [Google Scholar]
- 3.Kibar Z, et al. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–5. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- 4.Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet. 2001;10:2593–601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- 5.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–4. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- 6.Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- 7.Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev Biol. 2002;247:165–81. doi: 10.1006/dbio.2002.0673. [DOI] [PubMed] [Google Scholar]
- 8.Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–25. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- 9.Kimmel CB, Warga RM, Kane DA. Cell cycles and clonal strings during formation of the zebrafish central nervous system. Development. 1994;120:265–76. doi: 10.1242/dev.120.2.265. [DOI] [PubMed] [Google Scholar]
- 10.Concha ML, Adams RJ. Oriented cell divisions and cellular morphogenesis in the zebrafish gastrula and neurula: a time-lapse analysis. Development. 1998;125:983–94. doi: 10.1242/dev.125.6.983. [DOI] [PubMed] [Google Scholar]
- 11.Geldmacher-Voss B, Reugels AM, Pauls S, Campos-Ortega JA. A 90-degree rotation of the mitotic spindle changes the orientation of mitoses of zebrafish neuroepithelial cells. Development. 2003;130:3767–80. doi: 10.1242/dev.00603. [DOI] [PubMed] [Google Scholar]
- 12.Park M, Moon RT. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat Cell Biol. 2002;4:20–5. doi: 10.1038/ncb716. [DOI] [PubMed] [Google Scholar]
- 13.Jessen JR, et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–5. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciruna B, et al. Production of maternal-zygotic mutant zebrafish by germ-line replacement. Proc Natl Acad Sci U S A. 2002;99:14919–24. doi: 10.1073/pnas.222459999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heisenberg CP, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 16.Rauch GJ, et al. Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harb Symp Quant Biol. 1997;62:227–34. [PubMed] [Google Scholar]
- 17.Matsui T, et al. Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development. Genes Dev. 2005;19:164–75. doi: 10.1101/gad.1253605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallingford JB, Harland RM. Xenopus Dishevelled signaling regulates both neural and mesodermal convergent extension: parallel forces elongating the body axis. Development. 2001;128:2581–92. doi: 10.1242/dev.128.13.2581. [DOI] [PubMed] [Google Scholar]
- 19.Thisse C, Thisse B. Antivin, a novel and divergent member of the TGFbeta superfamily, negatively regulates mesoderm induction. Development. 1999;126:229–40. doi: 10.1242/dev.126.2.229. [DOI] [PubMed] [Google Scholar]
- 20.Meno C, et al. Mouse Lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol Cell. 1999;4:287–98. doi: 10.1016/s1097-2765(00)80331-7. [DOI] [PubMed] [Google Scholar]
- 21.Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc Natl Acad Sci U S A. 2002;99:12651–6. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strutt DI. The asymmetric subcellular localisation of components of the planar polarity pathway. Semin Cell Dev Biol. 2002;13:225–31. doi: 10.1016/s1084-9521(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 23.Jiang D, Munro EM, Smith W. C. Ascidian prickle regulates both mediolateral and anterior-posterior cell polarity of notochord cells. Curr Biol. 2005;15:79–85. doi: 10.1016/j.cub.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 24.Jenny A, Darken RS, Wilson PA, Mlodzik M. Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. Embo J. 2003;22:4409–20. doi: 10.1093/emboj/cdg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons DA, et al. erbb3 and erbb2 are essential for schwann cell migration and myelination in zebrafish. Curr Biol. 2005;15:513–24. doi: 10.1016/j.cub.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 26.Gong Y, Mo C, Fraser SE. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 2004;430:689–93. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- 27.Djiane A, Yogev S, Mlodzik M. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell. 2005;121:621–31. doi: 10.1016/j.cell.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 28.van Straaten HW, Copp AJ. Curly tail: a 50-year history of the mouse spina bifida model. Anat Embryol (Berl) 2001;203:225–37. doi: 10.1007/s004290100169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gritsman K, et al. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell. 1999;97:121–32. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- 30.Carreira-Barbosa F, et al. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–46. doi: 10.1242/dev.00567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


