Abstract
The African American Study of Kidney Disease and Hypertension (AASK) is a multicenter randomized clinical trial designed to test the effectiveness of three anti-hypertensive drug regimens and two levels of BP control on the progression of hypertensive kidney disease. Participants include African-American men and women aged 18 to 70 yr who have hypertensive kidney disease and GFR between 20 and 65 ml/min per 1.73 m2. The three anti-hypertensive drug regimens include an angiotensin converting enzyme inhibitor (ramipril), a dihydropyridine calcium channel blocker (amlodipine) or a beta-blocker (metoprolol) as initial therapy. The BP control levels are a lower goal (mean arterial pressure, ≤92 mmHg) and a usual goal (mean arterial pressure, 102 to 107 mmHg inclusive). The primary outcome is rate of change in renal function as measured by GFR, assessed by 125 I-iothalamate clearance. The main secondary patient outcome is a composite including the following events: (1) reduction in GFR by 50%, (2) end-stage renal disease, or (3) death.
The African American Study of Kidney Disease and Hypertension (AASK) is a full-scale randomized clinical trial sponsored by the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH). The AASK is investigating whether any of three BP medication regimens or either of two levels of BP control can slow the progression of kidney disease in patients with hypertensive nephrosclerosis. AASK has randomized 1094 African-Americans (AA) with hypertension and reduced renal function (125I-iothalamate GFR 20 to 65 ml/min per 1.73 m2) at 21 clinical centers in 13 states across the United States.
Hypertensive nephrosclerosis has been reported to be the second leading cause of renal failure in the United States and, until recently, the leading cause of renal failure, or ESRD, in AA (1,2). Over the last 20 yr, new anti-hypertensive medications have contributed to lower rates of mortality and morbidity due to stroke and heart disease. However, the rate of ESRD due to hypertension has continued to increase and remains higher in AA than other subgroups in the United States (1,3). Although AA make up only 12% of the US population, 28% of the patients on hemodialysis are AA, and AA develop ESRD (defined by the need for dialysis or transplantation) at a rate four times greater than that for Caucasians (4). It is not clear what accounts for increased susceptibility of AA to ESRD (5-11). It is not entirely explained by the higher prevalence of hypertension (12,13) or socioeconomic factors in the AA population (14).
It has been demonstrated that long-term BP control to recommended standards for controlling cardiovascular disease with conventional anti-hypertensive medications (including ganglionic blocking agents, reserpine, diuretics, vasodilators, and beta-blockers) can help preserve renal function (15-21). However, large-scale trials in patients with hypertensive renal disease have generally not been designed to assess change in renal function in relation to BP control, and have relied on indirect estimates of GFR by serum creatinine concentration.
Two previous randomized trials (22,23) have examined the effects of low BP goals similar to the lower goal of the AASK on renal disease progression. The rationale for the lower BP goal in the AASK was provided in part by a subgroup analysis of 53 AA in one of these trials, the Modification of Diet in Renal Disease (MDRD) Study, which suggested a benefit of the lower BP goal in AA (22,24). However, the question of whether controlling BP to levels below current standards further slows the progression of renal disease in patients with hypertension and renal insufficiency (25) has not been resolved. It is also unknown whether specific classes of anti-hypertensive medications are more effective in slowing renal disease progression in this population independent of their effects on BP.
The AASK Pilot was conducted with 94 patients at 11 clinical centers in 1992 to 1993 (26). The full-scale AASK is being carried out in 21 clinical centers (see Appendix 1). The clinical centers include all four of the historically AA medical schools: Howard University, Martin Luther King-Drew Medical College, Meharry Medical College, and the Morehouse School of Medicine.
This report presents the design and statistical analysis plan for the full-scale phase of the AASK. Special attention is given to challenges for the analysis plan associated with the expectation of differences between short- and long-term effects of the interventions.
Materials and Methods
The AASK pilot study was conducted to document the feasibility of meeting the study objectives and to evaluate study procedures (26). Enrollment for the full-scale study began in March 1995, and 1094 patients were randomized between June 1995 and September 1998 (27). Patients will continue to be followed through September 2001. In this 3 × 2 factorial trial, the three anti-hypertensive medication regimens began with an angiotensin converting enzyme inhibitor (ACEI: ramipril), a dihydropyridine calcium channel blocker (DHPCCB: amlodipine) or a beta-blocker (BB: metoprolol). The two levels of BP control are a usual goal (mean arterial pressure [MAP] 102 to 107 mmHg), which corresponds to a BP of approximately 135/85 to 140/90 mmHg, or lower goal (MAP ≤92 mmHg), which corresponds to a BP of approximately 115/80. Enrollment by randomized group is shown in Table 1. Fewer patients were enrolled into the DHPCCB group because, as described later, the anticipated hemodynamic effect of these agents on GFR increases the statistical power of comparisons of the DHPCCB group compared with the other treatment groups.
Table 1.
Enrollment by randomized group
| Initial (Blinded) Therapy in Drug Regimen |
|||||
|---|---|---|---|---|---|
| Angiotensin converting enzyme inhibitor (ACEI) | Beta-blocker (BB) | Calcium channel blocker (DHPCCB) | Total | ||
| MAP goal | MAP ≤ 92 | 215 | 215 | 110 | 540 |
| MAP 102 to 107 | 221 | 226 | 107 | 554 | |
| Total | 436 | 441 | 227 | 1094 | |
The usual goal reflects good BP control in an otherwise normal hypertensive population. The low goal of MAP ≤92 mmHg is a lower goal of unproven benefit. The lower limit of 102 mmHg in the usual goal provides a minimum targeted separation between the two MAP groups of 10 mmHg, and is intended to facilitate separation in achieved BP between the lower and usual BP groups.
Investigators and participants are unblinded to the BP group assignment. Patients are seen at least every other month, and BP are measured at each visit using random zero sphygmomanometers. If a patient's MAP is more than 5 mmHg over goal on two consecutive visits, an extra visit is held.
Anti-Hypertensive Medications
Animal data suggest that both ACEI and CCB may provide renoprotection independent of their effect on BP (28,29), and ACEI have been shown to be renoprotective for patients with diabetic renal disease (30,31). BB were selected as the reference to determine if ACEI or DHPCCB are renoprotective in AA with hypertensive nephrosclerosis, as there is less evidence for renoprotective effects of BB than the other two agents. BB also inhibit renin release and thereby lower intrarenal angiotensin II levels, but to a lesser extent than ACEI.
The AASK intent is to test whether the randomized drug regimens containing ACEI or DHPCCB better preserve renal function in AA with renal insufficiency attributed to hypertensive renal disease, independent of these drugs' effects on BP. Patients are randomized to one of the three blinded anti-hypertensive agents as a first step in a stepped-care regimen of hypertensive medication. At each visit, patients are prescribed either the low, medium, or high dose of their blinded anti-hypertensive medication, and staff members work to keep each patient's BP within its assigned range by increasing the dose of the blinded medication to the highest level that does not put the patient below goal, by adding or changing doses of the stepped-care regimens, or by using nonpharmacologic therapy. AASK medication masking is accomplished through a double-dummy system in which each patient takes one tablet (either BB or placebo) and one capsule (either ACEI, DHPCCB, or placebo) each day.
The ACEI ramipril doses are 2.5 mg, 5 mg, or 10 mg and the BB metoprolol doses are 50 mg, 100 mg, or 200 mg. For the DHPCCB amlodipine, only two doses are used, but the blinding requires that doses of 5 mg, 5 mg, and 10 mg be considered low, medium, and high, respectively.
It was anticipated that additional anti-hypertensive medications would be required to achieve the BP goals, especially the lower goal. The AASK stepped-care system includes the following anti-hypertensive medication steps: (1) diuretics (preferably furosemide); (2) alpha-adrenoreceptor antagonists (preferably doxazosin); (3) centrally acting alpha II agonist (preferably clonidine); and (4) vasodilator (preferably minoxidil or hydralazine). The use of additional stepped-care anti-hypertensive medications in the three anti-hypertensive treatment arms was expected to be similar, although anti-hypertensive requirements were expected to be greater in the lower than the usual BP group. When clinically possible, the drugs are added step by step, with each step maximized before adding the next step. Study coordinators and a study-wide Adherence Committee work to promote adherence by counseling patients and providing feedback on BP level attained and results of pill counting. Pill counts are done at each protocol visit.
Eligibility and the Patient Timeline
AA between 18 and 70 yr with presumed hypertensive chronic renal disease and 125I-iothalamate GFR between 20 and 65 ml/min per 1.73 m2 were eligible to enroll in the AASK. This represents between a 33% to 80% decline from normal renal function. From the AASK pilot study, which included renal biopsies (26), it was determined that hypertensive nephrosclerosis could be confirmed in this population based on clinical grounds. Thus, biopsy evidence of hypertensive nephrosclerosis was not sought for the full-scale study. Other eligibility and exclusion criteria are shown in Table 2.
Table 2.
Inclusion and exclusion criteria
| Inclusion Criteria: |
| 1. African-American men and women (including black individuals born in the Caribbean, Africa, Canada, etc.) age 18 to 70 years. Each center will attempt to include equal numbers of men and women, at least 1:3 of each. |
| 2. Hypertension is defined as a sitting diastolic BP of 95 mmHg or more. The average of the last two of three consecutive readings on a random zero sphygmomanometer machine at any visit is the level used. Hypertensive participants on anti-hypertensive therapy at Baseline need only one qualifying clinic visit. Those not currently on medications at Baseline must qualify on each of two consecutive clinic visits. |
| 3. Reduced renal function, defined as a prerandomization (G1 visit) 125I-iothalamate GFR between 20 to 65 ml/min 1.73 per m2. |
| 4. Willingness and ability to cooperate with the protocol. |
| Exclusion Criteria: |
| 1. History of malignant or accelerated hypertension within 6 mo prior to study entry; previous chronic peritoneal or hemodialysis or renal transplantation. |
| 2. Known secondary causes of hypertension. |
| 3. Any known history of diabetes mellitus type I and II, or fasting (8–12 h) glucose >140 mg/dl on two occassions, or glucose >200 mg/dl on one occasion prior to randomization. |
| 4. A ratio of urinary protein (mg/dl) to creatinine (mg/dl) exceeding 2.5 in a 24-h urine sample collected shortly before the initial GFR visit. (This ratio is used as an estimate of > 2.5 g/d proteinuria without needing to factor for validity of the collection.) |
| 5. Clinical or renal biopsy evidence of any renal disease other than hypertensive nephrosclerosis. Persons with arteriographically documented renal arterial atherosclerotic disease less than 50% stenosis of the renal artery should be considered eligible for study participation if the principal investigator at the center feels the disease is not clinically significant. |
| 6. History of drug abuse in the past 2 yr, including narcotics, cocaine, or alcohol (>21 drinks/wk). |
| 7. Serious systemic disease that might influence survival or the course of renal disease. (Chronic oral steroid therapy is an exclusion, but steroid-containing nasal sprays are not. In active sarcoidosis is not an exclusion.) |
| 8. Clinical evidence of lead intoxication. |
| 9. Arm circumference >52 cm, which precludes measuring blood pressure with the “thigh” blood pressure cuff. Arm length such that if the cuff that is appropriate for the arm circumference extends into the antecubital space so that the cuff would interfere with placement of the stethoscope over the brachial artery for blood pressure measurement. |
| 10. Clinical evidence of congestive heart failure, current or within the preceding 6 mn. Ejection fraction below 35% measured by any method. Heart block greater than first degree or any other arrhythmia that would contraindicate the use of any of the randomized drugs. |
| 11. Reactive airway disease, current or in the preceding 6 mo requiring prescribed treatment for more than 2 wk. |
| 12. Impairment or difficulty in voiding, precluding adequate urine collections. |
| 13. Intake of nonsteroidal anti-inflammatory agents (NSAIDs) more than 15 d/mo, excluding aspirin. Inability to discontinue NSAIDs or aspirin for 5 d prior to GFR measurement. |
| 14. History of severe adverse reaction to any of the randomized drugs required for use in the protocol or contraindication of their use. |
| 15. Pregnancy or likelihood of becoming pregnant during the study period; lactation. |
| 16. Serum potassium level >5.5 mEq/L at the SV2 and confirmed at G1 for those not on ACE inhibitors during baseline, or serum potassium level >5.9 mEq/L at the SV2 and confirmed at G1 for those on ACE inhibitors during baseline. |
| 17. Leukopenia <2,500/mm3 at SV2 and confirmed at the end of baseline. |
| 18. Medically indicated need for any of the randomized drugs for any other reason (including angina pectoris, migraine, arrhythmia). |
| 19. Allergy to iodine. |
| 20. Suspicion that the participant will not be able to adhere to medications or comply with the protocol visit schedule. |
| 21. Participation in another intervention study. |
The majority of AASK participants were not private patients of AASK investigators, but were identified through chart reviews, lab data reviews, or referrals from outside physicians. Participants were located through a variety of methods, including public appeals through the media, and mass mailing of brochures. Recruitment techniques have been described (27). The study protocol and consent were approved by each clinical center's Investigational Review Board, and each patient signed an informed consent to enter the study.
Patients identified were first screened informally during conversations with AASK staff and then attended a formal screening, known as the SV-2 visit at which eligibility criteria were systematically checked. After this, a baseline period was held to confirm eligibility. The baseline period included (1) BP medication back-titration visits which continued until a patient's diastolic BP was greater than 95 mmHg; (2) baseline laboratory and quality of life measures; and (3) two GFR visits. The first GFR determined study eligibility.
Randomization
Data entry at each clinical center was accomplished by remote data entry (over the Internet) into the AASK database, an Oracle database, at the Data Coordinating Center (DCC). When all baseline data were entered, the clinical center staff members could access the DCC database to request a randomization assignment. The DCC programs checked that a patient's baseline data were complete, that eligibility requirements had been met, and that a copy of the signature sheet of the patient's consent form had been received. The computer screen then displayed the BP group to which the patient had been randomized (usual or low) and the location of a pair of centrally supplied blinded pill bottles (one with tablets and one with capsules) containing the patient's blinded medication and placebo. Locations for low, medium, and high doses of medication were provided so that any dosage could be selected and dosage could be switched at any time. The time from SV2 to randomization ranged from 2 wk to 6 mo. Randomization schedules were stratified by clinical center. Random permuted blocks with randomly varying block sizes were utilized.
Follow-Up Visit Schedule
Soon after randomization, each participant received randomized blinded drugs (visit FV-0). Participants were then seen at a minimum of once a month for the first 6 mo and then at a minimum of once every 2 mo thereafter. Additional interim visits are held as necessary for BP control. At each visit, AASK-certified personnel measured seated BP three times and standing BP once with a random zero (RZ) sphygmomanometer. Pill counts of all anti-hypertensive medications are done at every protocol visit. GFR is measured at 3, 6, and 12 mo, and every 6 mo thereafter. Central serum measurements of sodium, potassium, chloride, bicarbonate, urea nitrogen, glucose, creatinine, total protein, albumin, aspartate transaminase, lactate dehydrogenase, alkaline phosphates, total bilirubin, calcium, phosphorus, uric acid, magnesium, gamma glutamyltransferase, total cholesterol, HDL cholesterol, triglycerides, and LDL cholesterol were done every 6 mo. Central 24-h urine measurements of urine protein, sodium, potassium, creatinine, and urea nitrogen were also done every 6 mo. Fasting lipid profiles and quality of life measurements (SF-36) were done annually. Throughout follow-up, all medications each patient is taking were logged into the database.
Clinical Centers and Central Facilities
Each clinical center is staffed with a physician PI, one or more physician coinvestigators, a study coordinator, a data entry person, a recruitment/adherence coordinator, a BP interventionist, and a GFR technician. The amount of staffing varied and depended on recruitment goals. One staff member often filled several roles. Each center had a customized recruitment goal, which depended primarily on the population of AA from which it could recruit.
Central facilities include the Data Coordinating Center (DCC) at the Department of Biostatistics and Epidemiology at the Cleveland Clinic Foundation, the Drug Distribution Center, the Central Biochemistry Laboratory, and the Central GFR Laboratory. The DCC coordinated training, documentation, database management, and development of statistical plans, statistical analyses, quality control, reports, and publications. The Drug Distribution Center from the Department of Hospital Pharmacy at the Cleveland Clinic supervises a drug encapsulator (Clinical Encapsulating Services) and a drug packager (McKesson Bioservices). The Drug Distribution Center also coordinated after-hours emergency unblinding through the Cleveland Clinic's Hospital Pharmacy.
Twenty-four-hour urine aliquots and serum samples are sent to the AASK Central Biochemistry Laboratory in the Department of Laboratory Medicine at the Cleveland Clinic for analysis and for storage of afterthought specimens (annual urinalysis and complete blood count (CBC) whole blood measures are done at each center's local laboratory). AASK serum and urine specimens for measurement of renal function are shipped to an AASK Central GFR Lab in the Department of Hypertension and Nephrology at the Cleveland Clinic.
Quality Control
Quality control requirements for the AASK are specified in the study protocol and carried out under the leadership of the AASK Quality Control Committee and the DCC. All staff members were trained and certified at the start of the study. Special Quality Control systems were established for monitoring BP measurement, drug distribution, biochemistry, GFR and data quality control, and site visits were performed.
BP Quality Control. AASK seated BP measurements are taken after measurement for proper cuff size. A patient rests quietly for 5 min, pulse is taken, and steps for determining the random zero sphygmomanometer's peak inflation level and zero value are followed. Protocol procedures are followed as seated BP is measured three times. The average of the last two measures is considered the AASK BP level.
Each of the 21 AASK Clinical Centers has a centrally trained BP trainer, who is responsible for the overall BP measurement quality at that center. The 21 trainers are recertified annually by a BP consultant. The trainers are responsible for training and supervising anyone who will measure BP at their centers. Quarterly quality control readings are done with each BP measurer working side-by-side with the BP trainer. The DCC provides the Quality Control Committee with various measures of BP quality, including intratest coefficients of variation and digit preference in the BP measurements and in the sphygmomanometer's zero values.
Drug Distribution Quality Control. At every visit at which blinded drugs are dispensed, clinical centers enter into the database random drug code numbers assigned by the DCC that are on the bottles given to the patient. Database programs determine whether the correct bottles were dispensed.
Biochemistry Quality Control. For quality control of the Central Biochemistry Lab's serum and urine measurements, the clinical centers send split samples to the central lab twice annually. The lab measures the second sample blinded, and the DCC compares the two measurements for the Quality Control Committee.
GFR Quality Control. Central training and certification are required for each person who will be measuring GFR on AASK patients. A GFR consists of four “periods” of time, each of which begins with a serum collection and ends with a urine collection. An estimated GFR is calculated for each of the four periods; the AASK GFR is a weighted average of the GFR of the individual periods. The DCC monitors various aspects of GFR quality, including the coefficient of variation of the four estimated GFR. Each clinical center's rate of missed GFR measurements is monitored by the Quality Control and Adherence Committees.
For quality control of the Central GFR Lab, the clinical centers send split samples to the central GFR lab twice annually. The GFR lab measures the second sample blinded to whose specimen it is, and the DCC compares the results of the two GFR.
Data Quality Control. Data are entered at the clinical centers and are double-entered, or rekey verified, at the clinical centers. Edit checks are applied at the time of data entry and during data analysis. The DCC and Quality Control Committee monitor time to data entry, time to response to data discrepancy inquiries, and the time it takes the DCC to correct data.
Site Visits. Each clinical center had at least two site visits: One in the first 2 yr of the study focusing on recruitment issues and a second visit, later in the study focusing on quality control. The two-day quality control site visits consist of a brief data audit and a detailed discussion of patient adherence, patient retention, protocol adherence as to choice of anti-hypertensive agent, the clinical center's protocol adherence, achieved separation between BP groups, and quality control.
Objectives of the AASK
Primary Outcome and Objectives
The primary objective of the AASK is to determine if the low BP goal (compared with the usual BP goal) or the use of a specific anti-hypertensive regimen reduces the mean rate of change in GFR during follow-up. The primary analysis will compare the rate of change in GFR between the following treatment groups: (1) low versus usual BP goals; (2) ACEI versus BB regimens; and (3) DHPCCP versus BB regimens.
Assuming comparable BP levels can be achieved within the anti-hypertensive agent arms, comparisons (2) and (3) will treat the BB arm as a control group to determine if the ACEI or DHPCCB regimens have renoprotective effects independent of BP level. The ACEI DHPCCB groups will also be directly compared in secondary analyses. However, as described below, this comparison is expected to be complicated by opposite hemodynamic effects projected from the ACEI and DHPCCB interventions. The study's primary renal analysis considers GFR slopes; the study's main patient-outcome analysis considers rates of renal events, which includes reduction in GFR by 50%, reaching ESRD, or death.
Assessment of Renal Function for GFR Slope
Assessment of the effects of the AASK interventions is complicated by the expectation that the interventions will have differing hemodynamic effects on GFR during the first 3 mo that the patient is on the intervention. These hemodynamic effects are distinct from each intervention's hypothesized long-term effects on the progression of kidney disease. Past studies have suggested that, initially, ACEI may reduce GFR by 2 to 6% (32-34), DHPCCB may increase GFR by 2 to 4% (35,36), BB may reduce GFR by 1 to 2% (35,37), and the low BP goal may reduce GFR by 2% (38). Some research suggests that the initial hemodynamic effects persist as long as the patients remain on their respective interventions and that, at least for ACEI, the effect is reversible on termination of the therapy (39,40).
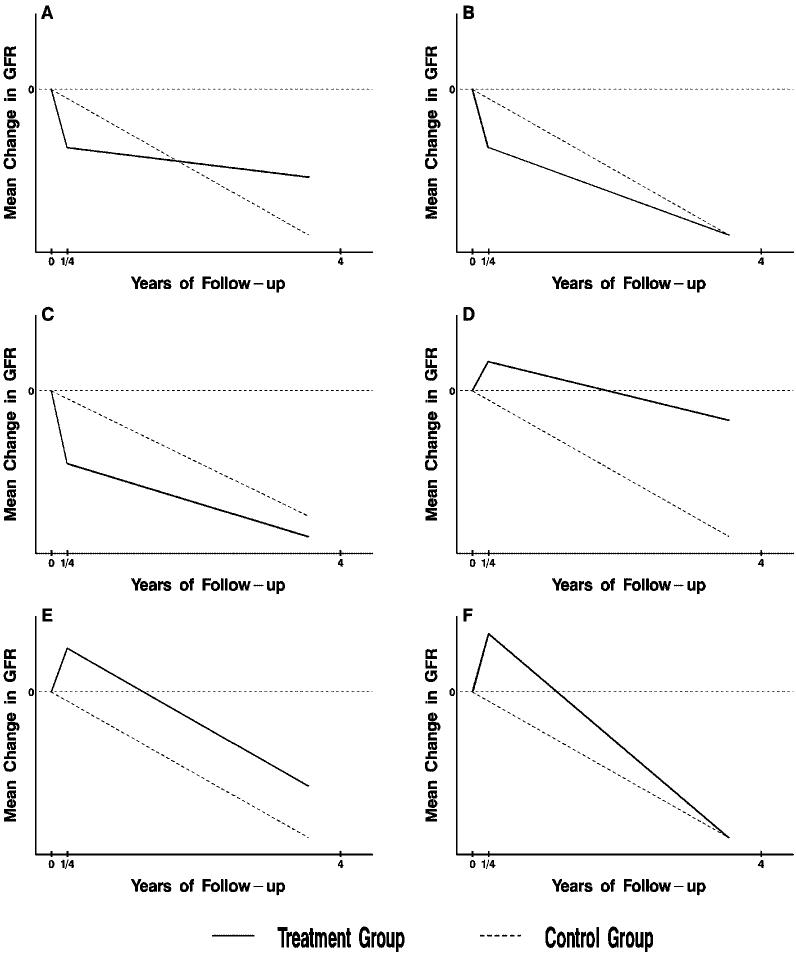
Several possible scenarios for the comparison of mean changes in GFR between treatment and reference groups are presented in Figure 1. Scenario A represents expected comparisons of the BP goals (or the ACEI versus BB regimens) under the research hypothesis of long-term beneficial effects of the lower goal (or the ACEI regimen), following the expected initial hemodynamic effects. Scenarios B and C are also consistent with the research hypothesis of beneficial effects of these interventions. In scenarios B and C, the planned AASK follow-up of 3 to 6 yr is not sufficient for the hypothesized long-term beneficial effects to overcome the regimens' initial hemodynamic effects. Scenarios D through F represent potential results for the DHPCCB versus BB comparison based on the expected initial increase in GFR in the DHPCCB group.
Figure 1.
Possible scenarios for the comparison of two treatment groups. Shown are six alternative scenarios for the effects of a treatment compared with a reference group on the mean change in GFR from baseline to 4 yr under the two-slope model. The chronic slope is depicted by the slope from 3 mo (1/4 year) to 4 yr, and the total mean slope as the average rate of change from baseline to 4 yr. Panels A and D represent definitive scenarios in which the comparisons between treatment groups of the mean chronic and total slopes are in agreement. The remaining panels depict inconclusive scenarios in which the comparisons of the chronic and total means slopes are not in agreement.
To clarify these scenarios, we divided the follow-up period into two phases. The acute phase consists of the first 3 mo after randomization, during which hemodynamic effects are expected to occur. The chronic phase consists of the remainder of follow-up after 3 mo. Two separate primary hypotheses are stipulated for each of the three primary treatment group comparisons. In H01, there will be no difference between treatment groups in the mean rate of decline in GFR during the chronic phase. In H02, there will be no difference between the treatment groups in the mean total rate of decline in GFR from baseline to the end of follow-up (taken to be 4 yr).
The hypothesis H01 may be criticized because it pertains to a change in the outcome variable starting 3 mo after randomization, when a patient's renal function has already been modified by the randomized treatment. However, if the hemodynamic effects persist while the patients remain on the respective treatments, the rate of change in GFR during the chronic phase should reflect the actual rate of disease progression independent of the hemodynamic effect.
By contrast, assessment of H02 is influenced by the initial hemodynamic effect in addition to the long-term effects during the chronic phase, and is thus dependent on the duration of the study; it may not accurately reflect the long-term course of the disease. Nonetheless, if the total mean rate of decline in GFR from baseline to the end of the study is not different between two treatment interventions, it is not clear that a difference in the rate of change of GFR during the chronic phase alone would provide convincing evidence that an intervention will continue to slow a patient's disease progression after the end of the study and ultimately delay the onset of ESRD.
Therefore, the AASK will be regarded as conclusively establishing a benefit of one intervention over another only if H01 and H02 are both rejected in the same direction. Thus, Figure 1, A and D represent unambiguous cases where the treatment would be declared beneficial; the remainder represent ambiguous scenarios where only one of H01 or H02 are false, or where both H01 and H02 are false, but in opposite directions.
Primary Analysis
The primary analysis of GFR will be carried out using a two-slope mixed-effects model (41,42) with different slopes in the acute and chronic phases. The fixed-effects component models the effects of the treatment groups on the mean GFR slopes during the two phases, whereas the random-effects component includes random intercepts, acute and chronic slopes for each patient, plus random deviations of the individual GFR measurements. Linear splines are used so that the regression lines in the acute and chronic phases join at 3 mo for both the fixed and random effects. Contrasts between mean slopes in the respective treatment groups will be used to test the effects of the treatments on the acute and chronic slopes, and on the total mean slope from baseline to 4 yr (48 mo). The total mean GFR slope will be estimated for each treatment group as the weighted average (3/48) βacute + (45/48) βchronic, where βacuteand βchronic denote the mean slopes per month during the acute and chronic phases, respectively.
The comparisons of the chronic slopes and the total mean GFR slope to 4 yr will be used to test the primary hypotheses H01 and H02. If the comparison of two treatment groups is significant in the same direction for both the chronic and total mean slopes, then we will conclude that the treatment group with the less steep slopes is likely to ultimately delay the onset of ESRD. To increase the precision of the estimated treatment effects, the following variables, which are expected to be associated with the GFR slopes, will be included as covariates: age, gender, history of cardiovascular disease, baseline MAP, baseline urine protein excretion, and clinical center. In accordance with the factorial design, both main effects and interactions between the BP level and anti-hypertensive agent factors will be tested. However, qualitative interactions between the treatment interventions are not expected, and it is recognized that the power to detect an interaction and for comparing individual cells should an interaction be detected will be limited. The primary analysis will be intent-to-treat, so that patients reaching stop points requiring modifications of their study treatments will continue to be followed and retained in their original randomized groups for analysis.
Multiple Hypothesis Tests and Interim Stopping
Six annual interim analyses are planned, including the final analysis. Lan-DeMets spending functions (43) will be used to maintain the total type I error rates at 5% separately for the comparisons of chronic and total mean slopes for each of the three primary treatment group comparisons. O'Brien-Fleming (44) stopping boundaries will be used, with two-sided tests for each comparison. The information fractions are obtained separately for the chronic and total mean GFR slopes by computing the ratio of the expected variance of the test statistic at the final analysis to the variance at the current interim analysis. The stopping rule stipulates that a treatment group comparison may be terminated if the stopping boundary is crossed in the same direction for both the chronic and total mean slopes. Secondary analyses will also be considered in deciding whether to terminate an intervention. If an intervention is terminated, we expect to reassign the patients on the discontinued intervention and to continue investigating the remaining interventions.
Conditional power will be evaluated at each interim analysis. Consideration will be given to terminating the study early if the conditional probability of obtaining a conclusive result (e.g., obtaining significance for the comparisons of both the chronic slopes and the total change in GFR) is found to be low for each of the three primary treatment group comparisons.
Hypothesis tests are carried out separately for H01 and H02 using comparisonwise two-sided significance levels of 5% for each of the three primary treatment group comparisons. We decided against using a multiple comparisons procedure for the ACEI versus BB and DHPCCB versus BB comparisons because the potential renoprotective effects of ACEI and DHPCCB have different biologic mechanisms. Thus, these comparisons evaluate distinct hypotheses. Our requirement that the comparisons of chronic and total mean slopes be separately tested at the 5% significance level is conservative, because the probability that the comparisons of chronic and total mean slopes would both reach significance at the 5% level under the joint null hypothesis is less than 0.05. We decided against relaxing the rejection criteria to obtain a joint significance level of 5% because it was felt that both the comparisons of chronic slopes and the total change in GFR must be significant at the 5% level to be convincing to the nephrology community.
Time-To-Event Analyses
The potential ambiguities of the analysis of GFR slopes illustrated in Figure 1 suggest that it might be advisable to consider alternative outcomes based on hard clinical endpoints. For studies of chronic renal disease, a logical choice is ESRD, which occurs when GFR declines to 7 to 10 ml/min per 1.73 m2, at which point a renal transplant or artificial dialysis is required to support life. However, the expected rate of ESRD in the AASK is too low for this outcome alone to provide sufficient power. As an alternative, a time-to-event analysis will be conducted based on the following composite: (1) Reduction of GFR by 50% or by 25 ml/min per 1.73 m2 from the mean of the two baseline GFR, confirmed by a repeat GFR; (2) ESRD; and (3) death.
Time-to-event analyses based on the time to a prespecified change in a marker of renal function have been used as the primary analysis in previous clinical trials (30,31). Events such as dialysis and death are considered to be “harder” endpoints than GFR slope and thus potentially more relevant to the patients involved. The time-to-event analysis will be carried out using Cox-regression (45) with age, gender, history of cardiovascular disease, baseline MAP, and baseline urine protein excretion included as covariates.
Assessments of Two-Slope Mixed Effects Model
The assumptions of the two-slope mixed effects model will be examined (42). Potential deviations include nonlinearity of the mean GFR decline during the chronic phase and misspecification of the random component of the model. The linearity of the decline in mean GFR will be assessed by fitting multislope spline models with changes in slope allowed at each protocol GFR measurement. Possible misspecifications in the random component will be assessed by comparing the variance components of the random effects between the treatment groups and other patient subgroups, evaluating alternative error structures for the random effects, and by comparing the model-based standard errors to robust sandwich-type standard errors. If major deviations from the two-slope model are detected, consideration will be given to generalizing the two-slope model to incorporate them.
Informative Censoring
Patients may become lost to further GFR follow-up in the AASK due to any of the following: (1) reaching ESRD, which precludes further GFR measurements, (2) death, and (3) otherwise dropping out and becoming lost to GFR follow-up. Informative censoring will occur if the dropout times are correlated with the GFR slopes conditionally on the treatment factors, baseline covariates, and observed GFR. If the distribution of dropout times differs between the randomized groups, informative censoring may lead to biased estimates of the treatment effects under the mixed effects model. The risk of informative censoring will be evaluated during the study by carrying out statistical simulations of the potential bias resulting from the observed distributions of drop-out times in the respective treatment groups. If the possibility of a substantial bias is detected, we will consider implementing a random-coefficient selection model (46) or a random-coefficient pattern mixture model (47) to adjust for the censoring process in the analysis.
Other Key Secondary Analyses
If the follow-up MAP level exhibits any difference between the anti-hypertensive agent arms, we will repeat the analyses comparing the anti-hypertensive agent groups after adding follow-up MAP as a covariate to assess the renal protective effects of the anti-hypertensive regimens independently of the level of BP. The change in proteinuria from baseline and the rate of cardiovascular hospitalization and death are secondary outcomes. All cardiovascular events including cardiovascular deaths and hospitalizations for myocardial infarctions, strokes, heart failure, revascularization procedures, and other hospitalized cardiovascular events are reviewed and classified by a blinded endpoints committee according to a prespecified protocol.
Statistical Power
Statistical power was evaluated by statistical simulation for a variety of scenarios based on published results on AA from the Hypertension Detection and Follow-up Program trial (15) and a study of AA with hypertensive nephrosclerosis completed shortly before the AASK trial (23), as well as patient data from the AASK Pilot (26) and the MDRD Study (22,24) which were available to the DCC. Table 3 presents the estimated power based on a representative set of assumptions, including: (1) A 3-yr uniform recruitment period with 3 yr of further follow-up, yielding a total sample size of 1094; (2) the initial decline in mean GFR from baseline to 3 mo is 2 ml/min per 1.73 m2 greater for the lower than the usual BP group, 2 ml/min per 1.73 m2 greater for the ACEI than the BB group, and 2 ml/min per 1.73 m2 less for the DHPCCB than the BB group; (3) the between-patient SD of GFR slopes is 3.8 ml/min per 1.73 m2 per yr, the within-patient variance of GFR is equal to 0.67 of the patients current GFR value; (4) the rate of loss to GFR follow-up is 4% per yr; (5) the rate of “cross-overs” between treatment arms is 4% per yr; (6) the mean chronic slope in the control groups for the respective comparisons (e.g., the BB group for the two anti-hypertensive agent comparisons, and the usual goal for the BP comparison) is between -2 and -4 ml/min per 1.73 m2 per yr; and (7) the death rate in the control groups is 10% per 5 yr. The table presents the power of the study to detect a 30% proportional reduction in GFR slope in the treatment group for each of the three main comparisons, with no effect assumed for patients with slopes greater than or equal to 0. For comparison, power is provided for acute effects of 0 in addition to the acute effects projected in assumption 2. A sided significance level of 5% is used for each analysis. For the time-to-event analysis, the mortality rate is also hypothesized to be 20% lower in the treatment than in the control groups.
Table 3.
Power of main AASK comparisonsa
| Analysis Method |
|||||
|---|---|---|---|---|---|
| Treatment Group Comparison | Assumed Acute Effect (ml/min per 1.73 m2 per 3 mo)b | Assumed Mean Slope in Reference Group (ml/min per 1.73 m2 per year) | Chronic Slope | Total Mean Slope from Baseline to 4 yr | Time-to-Event |
| –4 | 99 | 71 | 87 | ||
| –2 | –3 | 95 | 42 | 78 | |
| Low versus usual blood pressure goal | –2 | 78 | 14 | 65 | |
| –4 | 99 | 99 | 99 | ||
| 0 | –3 | 95 | 98 | 97 | |
| –2 | 78 | 86 | 91 | ||
| –4 | 97 | 62 | 79 | ||
| –2 | –3 | 90 | 35 | 68 | |
| –2 | 69 | 12 | 55 | ||
| ACEI vs. BB | |||||
| –4 | 97 | 99 | 98 | ||
| 0 | –3 | 90 | 95 | 93 | |
| –2 | 68 | 77 | 84 | ||
| –4 | 88 | 99 | 98 | ||
| +2 | –3 | 76 | 99 | 95 | |
| –2 | 51 | 95 | 84 | ||
| DHPCCB vs. BB | |||||
| –4 | 88 | 92 | 91 | ||
| 0 | –3 | 76 | 83 | 81 | |
| –2 | 51 | 60 | 68 | ||
See text for other assumptions of power analysis.
Assumed acute effects represent difference between projected mean change in GFR between baseline and 3 mo in the treatment group and the reference group for each comparison.
Assuming a mean GFR slope of -4 ml/min per 1.73 m2 per yr, the power for the primary treatment group comparisons ranges from 88% to 99% for the analysis of chronic slopes, and from 62% to 99% for the analysis of the total mean GFR slope. Due to the assumption that the size of the treatment effect will be proportional to the GFR slope in the control group, the power of both the analysis of chronic slopes and especially the total GFR slopes is reduced substantially if less steep mean slopes are assumed (48). The power for the secondary time-to-event analysis is also lower for the slower than for the faster assumed mean progression rates, but the dependence of the power of the time-to-event analysis on the mean slope is less than for the analysis of GFR slopes. The power of the analysis of total mean GFR slope is greater for the DHPCCB versus BB comparison than the ACEI versus BB or the lower versus usual BP goal comparisons due to the expectation of a positive initial hemodynamic effect of DHPCCB on GFR, but a negative initial hemodynamic effect for the other interventions. For this reason, a smaller sample size was used in the DHPCCB group than for the ACEI and BB groups.
Discussion
AA have a higher prevalence of hypertension than Caucasians, and if hypertension is present, are more likely to develop renal insufficiency (49). Once renal insufficiency is present, AA with hypertension have a more rapid rate of decline in renal function than Caucasians (15,50,51). Thus, identifying interventions that slow the decline in renal function in AA with hypertensive nephrosclerosis represents an important healthcare priority.
Although nonrandomized studies have suggested that lower BP preserves renal function in persons with hypertension (52), the absence of randomization to a specific BP goal makes the data difficult to interpret. The patients with lower or higher achieved BP may have other variables present that account for observed outcomes (e.g., milder hypertension or other determinants of renal disease progression). In the AASK, participants were randomly assigned to one of two BP goals, thus reducing confounding factors. The biopsies performed in the pilot trial verified that the study's entry criteria selected patients who actually had hypertensive nephrosclerosis (26), a feature not present in previous studies.
The MDRD Study reported a beneficial effect of random assignment to a low BP goal in patients with renal disease associated with proteinuria, although not in its intent-to-treat analysis including all randomized patients (22,53). The reported benefit of the low goal in MDRD patients with proteinuria provides a precedent for a benefit of a lower BP goal in a particular subpopulation with chronic renal disease. However, the MDRD had few AA, and hypertensive nephrosclerosis is usually not associated with a high degree of proteinuria. The randomized comparison of the BP goals in the AASK will test whether reducing BP to levels below those recommended for the general hypertensive population slows progression of renal disease in AA with hypertensive nephrosclerosis, and will also address the alternative hypothesis that there is a BP below which there will be a negative effect on renal outcomes (54).
Previous randomized trials have demonstrated that ACEI slow the progression of renal disease in patients with diabetic nephropathy and chronic renal insufficiency associated with proteinuria (30,31,55). DHPCCB are widely used anti-hypertensives in AA, and are also hypothesized to have renoprotective effects. The AASK will test whether ACEI and DHPCCB are renoprotective in AA with hypertensive nephrosclerosis in comparison with a reference group assigned to first-line therapy with a BB and the same target BP level. The use of a BB as the reference for testing the renoprotective effects of ACEI could be criticized because BB and ACEI share certain properties in that they both inhibit renin release, but the inhibition of the renin angiotensin system by BB is far less than ACEI.
Data from the AASK pilot study and other studies suggest that the interpretation of the analysis of GFR slope could be complicated by an acute (first 3 mo) increase in GFR in the DHPCCB group and acute declines in GFR in the ACEI group and in the low BP group. These acute modifications of GFR are generally thought to be hemodynamic effects without clinical significance, and for ACEI have been shown to be reversible after termination of therapy (38-40). For treatment group comparisons in which the early hemodynamic effect is in the opposite direction of the long-term effect on the decline in renal function, it is possible that effects of the treatment groups in the acute and chronic phases of the study could cancel, rendering the analysis of mean GFR slope inconclusive (see Figure 1).
In contrast to the analysis of GFR slope, which addresses the mean drug effect on renal function in all patients, including those with little or no GFR decline, the secondary outcome of time to a GFR event (halving of GFR), ESRD, or death is based on events of clear clinical effect, either large declines in renal function or death. Because the magnitude of the hypothesized acute effects on GFR were much smaller than the changes in GFR required to trigger a GFR event or ESRD, the composite outcome is expected to be less sensitive to the acute effects than the mean GFR slope (56). This point is illustrated by Table 3, which indicates that the power of the time-to-event analysis is greater than that of the total GFR slope when the acute effects are in the opposite direction of the hypothesized long-term effect, particularly when the magnitude of the mean slope in the reference group is small.
The AASK study is the first clinical trial to address a critical health care issue, the progressive loss of renal function in AA with hypertensive nephrosclerosis, and to demonstrate whether specific MAP goals or specific anti-hypertensive agents better preserve renal function.
Appendix 1. Sites, Co-Investigators, and Collaborators
Case Western Reserve University Principal Investigator: J. Wright, Study Coordinator: Y. Hall, Collaborators: R. Haynie, C. Mbanefo, M. Rahman, M. Smith, B. Crenshaw, R. Dancie, L. Jaen.
Emory University Principal Investigator: J. Lea, Study Coordinator: M. Douglas, Collaborators: A. Chapman, L. Dean, D. Hall, D. Watkins, B. Wilkening, L. Williams, C. Ross.
Harbor-UCLA Medical Center Principal Investigator: J. Kopple, Study Coordinator: L. Miladinovich, Collaborator: P. Oleskie.
Harlem Hospital Principal Investigator: V. Pogue, Study Coordinator: D. Dowie, Collaborators: H. Anderson, L. Herbert, R. Locko, H. Nurse, J. Cheng, G. Darkwa, V. Dowdy, B. Nicholas.
Howard University Principal Investigator: O. Randall, Study Coordinator: S. Xu, Collaborators: G. Ali, T. Retta, T. Alexander, M. Ketete, E. Mathew, D. Ordor, C. Tilghman.
Johns Hopkins University Principal Investigator: L. Appel, Study Coordinator: J. Charleston, Collaborators: C. Diggs, C. Harris, P. Miller, T. Shields, M. Sotomayer, P. Whelton.
Martin Luther King, Sr.-Charles R. Drew Medical Center Principal Investigator: K. Norris, Study Coordinator: M. Miller, Collaborators: H. Ward, D. Martins, H. Howell.
Medical University of South Carolina Principal Investigator: D. Cheek, Study Coordinator: D. Brooks, Collaborators: C. Gadegbeku, D. Ploth, N. Monestime, S. Murner, S. Thompson.
Meharry Medical College Principal Investigator: M. Faulkner, Study Coordinator: K. Phillips, G. Sanford, C. Weaver, Collaborator: O. Adeyele.
Morehouse School of Medicine Principal Investigator: W. Cleveland, Study Coordinator: W. Smith; Collaborators: A. Howard, K. Chapman, S. Plater.
Mount Sinai School of Medicine Principal Investigator: R. Phillips, Study Coordinator: A. Gabriel, M. Lipkowitz, A. Travis, J. Williams. Ohio State University
Principal Investigator: L. Hebert, Study Coordinator: L. Hiremath, Collaborators: M. Falkenhain, S. Ladson-Wofford, S. Nahman, K. Osei, A. Dodley, J. Parks, D. Veley.
Rush Presbyterian St. Luke's Medical Center Principal Investigator: G. Bakris, O. Adeyele, Study Coordinator: L. Fondren, L. Bagnuolo, J. Cohen, M. Powell, Collaborators - J. Lash, A. Smith, D. White, G. Henry, A. Johnson, T. Collins, S. Koshy, E. Afante.
University of Alabama, Birmingham Principal Investigator: S. Rostand, Study Coordinator: C. Johnson, B. Key, Collaborators: D. Thornley-Brown, R. Gay.
University of California, San Diego Principal Investigator: D. O'Connor, Study Coordinator: J. Mount. Collaborators: F. Gabbai, R. Parmer, F. Rao, J. Little, T. Makrogiannis,, A. Ogundipe, A. Stephenson.
University of Florida Principal Investigator: C. Tisher, Study Coordinator: L. Burgin, Collaborators: D. Allen, A. Diaz, C. Sarmiento.
University of Miami Principal Investigator: J. Bourgoignie, Study Coordinator: A. Doss, Collaborators: G. Contreras, D. Florence-Green, J. Junco, D. Merrill, J. Vassallo, A. de Velasco.
University of Michigan Principal Investigator: K. Jamerson, Study Coordinator: D. Cornish-Zirker, Collaborators: T. Graham, A. Johnson, F. Port, M. Keshishian, A. Ojo, S. Steigerwalt, S. Nesbitt, K. Manchester, W. Bloembergen.
University of Southern California Principal Investigator: S. Massry, Study Coordinator: A. Richardson; Collaborators: V. Campese, M. Smogorzewski.
University of Texas Southwestern Medical Center, Dallas Principal Investigator: J. Middleton, Study Coordinator: T. Lightfoot, Collaborators: E. Kuo, S. Leach, R. Toto, K. Jones, K. Hart, L. Littmon, B. McNeill, C. Ying.
Vanderbilt University Principal Investigator: J. Lewis,, Study Coordinator: N. Rogers, M. Sika, Collaborators: G. Schulman, S. McLeroy.
National Institute of Diabetes and Digestive and Kidney Diseases L. Y. Agodoa, J. P. Briggs, J. W. Kusek.
Steering Committee Chair J. Douglas
Data Coordinating Center (Cleveland Clinic Foundation) Principal Investigators J. Gassman, Study Coordinator: K. Brittain, S. Sherer, Collaborators: G. Beck, V. Dennis, T. Greene, M. Kutner, R. Stewart, L. Tuason, S-R. Wang, X. Wang, W. Zhang.
Central Biochemistry Laboratory F. Van Lente, J. Waletzky, C. O'Laughlin, C. Peck.Central GFR Laboratory
P. Hall, D. Pexa, H. Rolin; Blood Pressure Consultant: R. Byington; Psychological Consultant: P. Greene.
Data Safety and Monitoring Committee R. Luke, V. Chinchilli, C. Cook, B. Falkner, C. Ford, R. Glassock, T. Karrison, T. Kotchen, E. Saunders, M. Secundy, D. Wesson.
Acknowledgments
This study was funded under a cooperative agreement mechanism with NIDDK. We gratefully acknowledge support from the Office of Research in Minority Health and the donation of drug and some financial support to NIDDK by Pfizer Inc, Astra Zeneca Pharmaceuticals, and King Pharmaceuticals, Inc. The following NIH institutional grants also provided support: RR-00080, RR-00071, RR00032, RR11145, RR00827, RR00052, RR11104, and DK 2818.
References
- 1.US Renal Data System . USRDS 2001 Annual Data Report, Atlas of End Stage Renal Disesase in the United States. National Institutes of Health, National Institute of Diabetes, Digestive, and Kidney Diseases; Bethesda, MD: 2001. [Google Scholar]
- 2.Committee for the Study of the Medicare ESRD Program Division of Health Care Services . In: Kidney failure and the federal government. Rettig RA, Levinsky NG, editors. Vol. 91. Nat Acad Press; 1991. pp. 116–117. [PubMed] [Google Scholar]
- 3.Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure The sixth report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. Arch Intern Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 4.Blythe WB, Maddux FW. Hypertension as a causative diagnosis of patients entering end-stage renal disease programs in the United States from 1980 to 1986. Am J Kidney Diseases. 1991;18:33–37. doi: 10.1016/s0272-6386(12)80287-3. [DOI] [PubMed] [Google Scholar]
- 5.Dustan HP, Curtis JJ, Luke RG, Rostand SG. Systemic hypertension and the kidney in black patients. Am J Cardiol. 1987;60:731–771. doi: 10.1016/0002-9149(87)90464-4. [DOI] [PubMed] [Google Scholar]
- 6.Messerli FH, Garavaglia GE, Schmieder RE, Sundgaard-Riise K, Nunez BD, Amodeo C. disparate cardiovascular findings in men and women with essential hypertension. Ann Int Med. 1987;107:158–161. doi: 10.7326/0003-4819-107-2-158. [DOI] [PubMed] [Google Scholar]
- 7.Hall JE, Granger JP, Smith MJ, Premen AJ. Role of renal hemodynamics and arterial pressure in aldosterone “Escape”. Hypertension. 1984;6:I183–I192. doi: 10.1161/01.hyp.6.2_pt_2.i183. [DOI] [PubMed] [Google Scholar]
- 8.Dustan HP, Valdes G, Bravo EL, Tarazi RC. excessive sodium retention as a characteristic of salt-sensitive hypertension. Am J Med Sci. 1986;292:67–74. doi: 10.1097/00000441-198608000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Messerli FH, DeCarvalho JGR, Christie B, Frohlich ED. essential hypertension in black and white subjects-hemodynamic findings and fluid volume state. Am J Med. 1979;67:27–31. doi: 10.1016/0002-9343(79)90065-2. [DOI] [PubMed] [Google Scholar]
- 10.Frohlich ED, Messerli FH, Dunn FG, Oigman W, Ventura HO, Sundgaard-Riise K. Greater renal vascular involvement in the black patient with essential hypertension. a comparison of systemic and renal hemodynamics in black and white patients. Mineral Electrolyte Metab. 1984;10:173–177. [PubMed] [Google Scholar]
- 11.Entwisle G, Apostolides AY, Hebel JR, Henderson MM. Target organ damage in black hypertensives. Circulation. 1977;55:792–796. doi: 10.1161/01.cir.55.5.792. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan NM. Clinical Hypertension. Williams & Wilkins; Philadelphia: 1986. Hypertension in the Population at Large. [Google Scholar]
- 13.McClellan W, Tuttle E, Issa A. Racial differences in the incidence of hypertensive end-stage renal disease (ESRD) are not entirely explained by differences in the prevalence of hypertension. Am J Kidney Dis. 1988;12:285–290. doi: 10.1016/s0272-6386(88)80221-x. [DOI] [PubMed] [Google Scholar]
- 14.Whittle JC, Whelton PK, Seidler AJ, Klag MJ. Does racial variation in risk factors explain black-white differences in the incidence of hypertensive end-stage renal disease? Arch Intern Med. 1991;151:1359–1364. [PubMed] [Google Scholar]
- 15.Shulman NB, Ford CE, Hall WD, Blaufox MD, Simon D, Langford HG, Schneider KA. prognostic value of serum creatinine and effect of treatment of hypertension on renal failure: Results from the Hypertension Detection and Follow-up Program. Hypertension and Renal Function. 1989;13:I80–I93. doi: 10.1161/01.hyp.13.5_suppl.i80. [DOI] [PubMed] [Google Scholar]
- 16.Breckenridge A, Dollery CT, Parry EHO. Prognosis of treated hypertension: Changes in life expectancy and causes of death between 1952 and 1967. Q J Med. 1970;39:411–429. [PubMed] [Google Scholar]
- 17.Davidov M, Mroczek W, Gavrilovich L, Finnerty F. Long-term follow-up of aggressive medical therapy of accelerated hypertension with azotemia. Angiology. 1976;26:396–407. [Google Scholar]
- 18.Mroczek WJ, Davidov M, Gabrilovich L, Finnerty FA. The value of aggressive therapy in the hypertensive patient with azotemia. Circulation. 1969;40:893–904. doi: 10.1161/01.cir.40.6.893. [DOI] [PubMed] [Google Scholar]
- 19.Luke RG. Essential Hypertension: A renal disease? A review and update of the evidence. Hypertension. 1993;21:380–390. doi: 10.1161/01.hyp.21.3.380. [DOI] [PubMed] [Google Scholar]
- 20.Brazy PC, Stead WW, Fitzwilliam JF. Progression of renal insufficiency: Role of blood pressure. Kidney Int. 1989;35:670–674. doi: 10.1038/ki.1989.37. [DOI] [PubMed] [Google Scholar]
- 21.Wolff FW, Lindeman RD. Effects of treatment in hypertension: Results of a controlled study. J Chron Dis. 1966;19:227–240. doi: 10.1016/0021-9681(66)90128-7. [DOI] [PubMed] [Google Scholar]
- 22.Klahr S, Levey AS, Caggiula AW, Hunsicker L, Kusek JW, Striker G, the MDRD Study Group The effects of dietary protein restriction and blood pressure control on the progression of chronic renal disease. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 23.Toto RD, Mitchell H, Smith R, Lee HC, McIntire D, Pettinger W. Strict blood pressure control and progression of renal disease in hypertensive nephrosclerosis. Kidney Int. 1995;48:851–859. doi: 10.1038/ki.1995.361. [DOI] [PubMed] [Google Scholar]
- 24.Hebert LS, Kusek JW, Greene T, Agodoa LY, Jones CA, Levey AS, Breyer JA, Faubert PF, Rolin HA, Wang S-R. Effects of blood pressure control on progressive renal disease in blacks and whites. Hypertension. 1997;30:428–435. doi: 10.1161/01.hyp.30.3.428. [DOI] [PubMed] [Google Scholar]
- 25.National High Blood Pressure Education Program Working Group . NHBPEP Working Group Report. National Institutes of Health, Department of Health and Human Services, Public Health Service; 1990. [Google Scholar]
- 26.Wright JT, Jr, Kusek JW, Toto RD, Lee JY, Agodoa LY, Kirk KA, Randall OS, Glassock R. Design and baseline characteristics of participants in the African American Study of Kidney Disease and Hypertension (AASK) Pilot Study. Control Clin Trials. 1996;17(Suppl 4):3S–16S. doi: 10.1016/s0197-2456(96)00081-5. [DOI] [PubMed] [Google Scholar]
- 27.Phillips R, Brittain K, Charleston J, Johnson F, Kusek J, Richardson A, Thompson B. Recruitment in the African American Study of Kidney Disease and Hypertension. J Am Soc Nephrol. 1998;9:159A. [Google Scholar]
- 28.Anderson S, Rennke HG, Brenner BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest. 1986;77:1993–2000. doi: 10.1172/JCI112528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dworkin LD, Parker M, Freiner HF. Nifedipine decreases glomerular injury in rats with remnant kidneys by inhibiting glomerular hypertrophy (Abstract) Kidney Int. 1989;35:427. [Google Scholar]
- 30.Lewis E, Hunsicker L, Bain R, Rohde R, the collaborative study group The effect of angiotensin converting enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329L:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 31.Maschio G, Alberti D, Janin G, Locatelli F, Manne JFE, Motolese M, Ponticelli C, Ritz E, Zucchelli P, the Angiotensin Converting Enzyme Inhibition in Progressive Renal Insufficiency Study Group Effect of the Angiotensin Converting enzyme inhibitor Benazepril on the progression of Chronic renal insufficiency. N Engl J Med. 1996;334:939–944. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 32.Bauer JH, Reams GP, Hewett J, Klachko D. A randomized, double-blind, placebo-controlled trial to evaluate the effect of enalapril in patients with clinical diabetic nephropathy. Am J Kidney Dis. 1992;20:443–457. doi: 10.1016/s0272-6386(12)70256-1. [DOI] [PubMed] [Google Scholar]
- 33.Slataper R, Vicknair N, Sadler R, Bakris GL. Comparative effects of different anti-hypertensive treatments of progression of diabetic renal disease. Arch Intern Med. 1993;153:973–980. [PubMed] [Google Scholar]
- 34.Bakris GL, Barnhill BW, Sadler R. Treatment of arterial hypertension in diabetic man: importance of therapeutic selection. Kidney Int. 1992;41:912–919. doi: 10.1038/ki.1992.139. [DOI] [PubMed] [Google Scholar]
- 35.Epstein M, Oster JRL. Beta-blockers and the kidney. Mineral Electrolyte Metab. 1982;8:237. [PubMed] [Google Scholar]
- 36.Bauer JH, Reams GP. Renal protection in essential hypertension: How do angiotensin converting enzyme inhibitors compare with calcium antagonists. J Am Soc Nephrol. 1990;1:580–587. [PubMed] [Google Scholar]
- 37.DeCesaris R, Ranieri G, Filitti V, Andriani A, Vincenzo Bonfantino M. Effects of atenolol and enalapril on kidney function in hypertensive diabetic patients. J Cardiovasc Pharmacol. 1993;22:208–214. doi: 10.1097/00005344-199308000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Levey AS, Beck GJ, Bosch JP, Caggiula AW, Greene T, Hunsicker LG, Kusek JW, Klahr S, the MDRD Study Group Short term effects of protein intake, blood pressure and anti-hypertensive therapy on GFR in the Modification of Diet in Renal Disease (MDRD) Study. J Am Soc Nephrol. 1996;7:2097–2109. [Google Scholar]
- 39.Hansen HP, Rossing P, Tarnow L, Nielsen FS, Jensen B, Parving HH. Elevated GFR after withdrawal of chronic anti-hypertensive treatment in diabetic nephropathy (Abstract) J Am Soc Nephrol. 1994;5:378. [Google Scholar]
- 40.Apperloo AJ, deZeeuw D, van Essen G, de Jong P. The initial GFR decline on anti-hypertensive treatment in long term interaction studies is reversible after withdrawal of treatment (Abstract) J Am Soc Nephrol. 1994;5:324. [Google Scholar]
- 41.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 42.Diggle PJ, Liang K, Zeger SL. Analysis of Longitudinal Data. Clarendon Press; Oxford, UK: 1994. [Google Scholar]
- 43.Lan RKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 44.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 45.Cox DR, Oakes D. Analysis of Survival Data. Chapman and Hall; New York: 1984. [Google Scholar]
- 46.Schluchter MD, Greene T, Beck GJ. Analysis of change in the presence of informative censoring: Application to a longitudinal clinical trial of progressive renal disease. Stat Med. 2001;20:989–1007. doi: 10.1002/sim.720. [DOI] [PubMed] [Google Scholar]
- 47.Follmann D, Wu M. An approximate generalized linear model with random effects for informative missing data. Biometrics. 1995;51:151–168. [PubMed] [Google Scholar]
- 48.Greene T. A model for a proportional treatment effect on disease progression. Biometrics. 2001;57:354–360. doi: 10.1111/j.0006-341x.2001.00354.x. [DOI] [PubMed] [Google Scholar]
- 49.Rahman M, Douglas JG, Wright JT. Pathophysiology and treatment implications of hypertension in the African-American population. Endocrin Metab Clin N Am. 1997;26:125–144. doi: 10.1016/s0889-8529(05)70237-1. [DOI] [PubMed] [Google Scholar]
- 50.Rostand SG, Brown G, Kirk KA, Rutsky EA, Dustan HP. Renal insufficiency in treated essential hypertension. N Engl J Med. 1989;320:684–688. doi: 10.1056/NEJM198903163201102. [DOI] [PubMed] [Google Scholar]
- 51.Walker WG, Neaton JD, Cutler JA, Neuwirth R, Cohen JD. Renal function change in hypertensive members of the Multile Risk Factor Intervention Trial. Racial and treatment effects. The MRFIT Research Group. JAMA. 1992;268:3085–3091. [PubMed] [Google Scholar]
- 52.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 53.Peterson J, Adler S, Burkart J, Greene T, Hebert L, Hunsicker L, King A, Klahr S, Massry S, Seifter J, the Modification of Diet in Renal Disease Study Group Blood pressure control, proteinuria, and the progression of renal disease: The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123:754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
- 54.Hansson L. Anti-hypertensive treatment: Does the J-curve exist? Cardiovasc Drugs Ther. 2000;14:367–372. doi: 10.1023/a:1007804030510. [DOI] [PubMed] [Google Scholar]
- 55.Ruggenenti P, Perna A, Benini R, Remuzzi G. Effects of dihydropyridine calcium channel blockers, angiotensin-converting enzyme inhibition, and blood pressure control on chronic, nondiabetic nephropathies. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN) J Am Soc Nephrol. 1998;9:2096–2101. doi: 10.1681/ASN.V9112096. [DOI] [PubMed] [Google Scholar]
- 56.Greene T, Lai J, Levey AS. Interpretation of clinical studies of renal disease. In: Neilson EG, Couser WG, editors. Immunologic Renal Disease. 2nd Lippincoll-Raven Publishers; Philadelphia, PA: 2001. pp. 887–914. [Google Scholar]