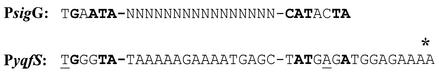Abstract
The temporal and spatial expression of the yqfS gene of Bacillus subtilis, which encodes a type IV apurinic/apyrimidinic endonuclease, was studied. A reporter gene fusion to the yqfS opening reading frame revealed that this gene is not transcribed during vegetative growth but is transcribed during the last steps of the sporulation process and is localized to the developing forespore compartment. In agreement with these results, yqfS mRNAs were mainly detected by both Northern blotting and reverse transcription-PCR, during the last steps of sporulation. The expression pattern of the yqfS-lacZ fusion suggested that yqfS may be an additional member of the EσG regulon. A primer extension product mapped the transcriptional start site of yqfS, 54 to 55 bp upstream of translation start codon of yqfS. Such an extension product was obtained from RNA samples of sporulating cells but not from those of vegetatively growing cells. Inspection of the nucleotide sequence lying upstream of the in vivo-mapped transcriptional yqfS start site revealed the presence of a sequence with good homology to promoters preceding genes of the σG regulon. Although yqfS expression was temporally regulated, neither oxidative damage (after either treatment with paraquat or hydrogen peroxide) nor mitomycin C treatment induced the transcription of this gene.
Endogenous and environmental factors such as reactive oxygen species, UV light, and chemical carcinogens alter the chemical structure of DNA bases, producing lesions that are substrates for a myriad of DNA glycosylases of the base excision repair (BER) pathway (27). The apurinic/apyrimidinic (AP) sites generated not only by the action of DNA glycosylases but also by the spontaneous depurination and depyrimidination of DNA (29, 30) are inherently toxic and highly mutagenic and thus should be rapidly processed and eliminated (31). The first catalytic event during the repair of AP sites is carried out by AP endonucleases, which cleave the DNA backbone immediately 5′ of an AP site, generating a 5′ deoxyribose-phosphate group and a 3′ deoxyribose-hydroxyl group. AP endonucleases have been classified into two families, namely, ExoIII and type IV AP endonucleases (3, 13), and these enzymes have been conserved across the species of the three domains of life (23).
Dormant spores of Bacillus subtilis are more resistant than their vegetatively growing counterparts to several chemical substances, including acids, bases, alkylating agents, and oxidizing agents (reviewed in references 40, 41, and 58). The existence of core coats, the low permeability of spores to hydrophilic compounds, and the protection of spore DNA from damage by its saturation with α/β-type small acid-soluble proteins (SASPs) account for this resistance (reviewed in references 40, 56, and 58). It has been demonstrated that α/β-type SASPs slow DNA depurination-depyrimidination, as well as hydroxyl radical-induced DNA backbone cleavage, thus contributing to spore resistance to heat and oxidizing agents (reviewed in references 40 and 58). α/β-type SASPs bind to spore DNA and are in part responsible of the strong resistance of B. subtilis spores to UV light (reviewed in references 40, 41, and 58); however, these DNA-binding proteins do not confer protection to DNA against base alkylation (55).
The genome of B. subtilis (26) possesses genes that potentially encode ExoIII and type IV AP endonucleases, namely, exoA and yqfS, whose products show a high level of homology to ExoIII and type IV AP endonucleases, respectively. Although the enzymology of B. subtilis ExoA has been studied in detail (53), nothing has been reported regarding the mechanisms that control its expression during growth and sporulation of B. subtilis.
The expression of DNA repair systems in the gram-positive spore-forming bacterium B. subtilis has been shown to be differentially regulated during growth and differentiation (4, 11, 32, 34), as well as during spore germination and outgrowth (54). DNA lesions acquired during unpredictable periods of B. subtilis spores dormancy must be necessarily corrected during germination by spore-specific expressed DNA repair systems (reviewed in references 40 and 58). The best example studied thus far is the correction of the UV-C induced spore photoproduct (5-thyminil-5,6-dihydrothymine) through both the specific spore photoproduct lyase protein (SplB) and the general excision-repair system (UVR) (reviewed in references 40 and 41). However, during unpredicted periods of spore dormancy B. subtilis spores could potentially accumulate, in addition to spore photoproduct (SP), different types of DNA lesions, such as strand brakes, cyclobutane pyrimidine dimers (CPDs), chemically altered bases, and AP sites that could affect essential functions such as transcription and replication during germination (40). Although the expression of splB in the forespore compartment by σG RNA polymerase has been widely substantiated (44, 45), few data exist in the literature concerning the expression of other specific or general DNA repair systems in the forespore compartment.
As mentioned above, in the genome of B. subtilis exists an open reading frame (ORF), yqfS, whose predicted product shows 53% homology with the type IV AP endonuclease of Escherichia coli. We describe here the expression of the cloned yqfS gene of B. subtilis from an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible promoter in E. coli. Our results demonstrate that a His6-YqfS purified enzyme is able to process the cleavage of abasic sites in the DNA. In addition, our results demonstrated that the expression of yqfS is forespore specific but was not induced by the stress imposed by superoxide radicals, by hydrogen peroxide, or by the DNA-damaging agent mitomycin C.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
B. subtilis and E. coli strains used in the present study are shown in Table 1. Plasmids used in this work are listed in Table 1. Media used were Difco sporulation medium (DSM) (52) and Luria-Bertani (LB) medium (38). When appropriate, antibiotics were added to the medium at the following final concentrations: chloramphenicol, 3 μg/ml; ampicillin, 50 μg/ml; and kanamycin, 10 μg/ml. Liquid cultures were shaken at 250 rpm at 37°C. Cultures on solid medium were grown at 37°C. The optical density (OD) of liquid cultures was monitored with a Pharmacia Ultrospec 2000 spectrophotometer set at 600 nm.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype and/or phenotypea | Source (reference) |
|---|---|---|
| Strains | ||
| B. subtilis | ||
| 168 | trpC2 | Laboratory stock |
| WN118 | sigGΔ1 trpC2 | Wayne Nicholson |
| PERM317 | trpC2 yqfS-lacZ; Cmr | This study |
| PERM336 | sigGΔ1 trpC2 yqfS-lacZ | This study |
| YB3000 | metB5 trpC2 xin- 1 sigB amyE (deleted for spβ) pCCR202 (recA-lacZ at amyE); Cmr | R. E. Yasbin |
| E. coli | ||
| SURE | e14−(McrA−) Δ(mcrCB-hsdSMR-mrr)171 endA1 supE44 thi-1 gyrA96 relA1 lac recB recJ sbcC umuC::Tn5 (Kanr) uvrC [F′ proAB laclq lacZΔM15 Tn10 (Tetr)] | Stratagene |
| PERM162 | E. coli SURE, pUC18; Ampr Tcr | This study |
| PERM253 | E. coli SURE, pPERM253; Ampr Tcr | This study |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac/F′ proAB lacIqZΔ M15 Tn10 (Tetr) | Stratagene |
| PERM267 | E. coli XL1-Blue, pPERM253; Ampr | This study |
| XL10-Gold Kan | Tetr Δ(mcrA) 183, Δ(mcrCB-hsdSMR-mrr); 173 endA1 sup E44 thi-1 recA1 gyrA96 relA1 lacHte [F′ proAB lacIqZΔM15 Tn10 (Tetr) Tn5 (Kanr) Amy] | Stratagene |
| PERM348 | E. coli XL-Gold, pPERM348, Ampr | This study |
| Plasmids | ||
| pJF751 | Integrational lacZ fusion vector; Cmr | W. Nicholson (17) |
| pUC18 | Multisite E. coli cloning vector | Laboratory stock (63) |
| pPERM253 | yqfS gene cloned in pUC18 | This study |
| pPERM267 | 514-pb EcoRI/NaeI fragment of yqfS from pPERM253 cloned in pJF751 | This study |
| pPERM348 | yqfS ORF cloned into the BamHI site of PQE-30 | This study |
Cmr, chloramphenicol resistant; Ampr, ampicillin resistant; Tcr, tetracycline resistant.
Genetic and molecular biology techniques.
Preparation of competent E. coli or B. subtilis cells and their transformation with DNA was performed as described elsewhere (5, 49). Extraction of chromosomal DNA from B. subtilis was carried out according to the protocol of Cutting and Vander Horn (12). Small-scale preparation of plasmid DNA from E. coli cells, enzymatic manipulations, and agarose gel electrophoresis were performed by standard techniques (49). Large-scale preparation and purification of plasmid DNA was accomplished by using commercial ion-exchange columns according to the instructions of the supplier (Qiagen, Inc., Valencia, Calif.). Nucleic acid sequencing by dideoxynucleotide chain termination (50) was performed with the Thermo Sequenase radiolabeled terminator cycle sequencing kit (U.S. Biochemical Corporation, Cleveland, Ohio). Sequencing products were analyzed by autoradiography after electrophoresis through a 6% polyacrylamide sequencing gel. Alternatively, DNA plasmids purified through Qiagen columns were processed for sequencing in a Perkin-Elmer (Norwalk, Conn.) model 377A automated DNA sequencer.
Cloning of yqfS and construction and integration of a yqfS-lacZ gene fusion.
The complete yqfS gene was amplified by PCR with genomic DNA from B. subtilis 168 as a template and the oligonucleotide primers 5′-GGGAATTCGCCGAAGAAGGTTAAGCC-3′ (forward) and 5′-CGGGATCCGGCCGTTGAAGTAGCGAACC-3′ (reverse). The primers were designed to insert EcoRI and BamHI sites (underlined). Amplification was performed on 0.1 μg of chromosomal DNA by using an MJ Research (Watertown, Mass.) Minicycler with Vent DNA polymerase (New England Biolabs, Beverly, Mass.) according to the manufacturer's recommendations. The 1,181-bp PCR fragment extending from 110 bp upstream of the yqfS start codon through 157 bp downstream of the yqfS stop codon was digested with SmaI and BamHI and ligated into pUC18 to generate pPERM253. pPERM253 was replicated in E. coli XL1-Blue, and the cloned yqfS gene was sequenced on both strands.
Construction of an in-frame translational yqfS-lacZ fusion was performed in the integrative plasmid pJF751 (17) by inserting a 472-bp EcoRI-NaeI fragment from plamid pPERM253 into pJF751 previously digested with EcoRI and SmaI. The resulting construction, containing the yqfS-lacZ fusion and designated pPERM317, was propagated into E. coli XL1-Blue. Plasmid pPERM317 was introduced by transformation into competent cells of B. subtilis 168, and transformants were selected on solid DSM containing chloramphenicol.
Purification of His6-YqfS and substrates for AP endonuclease activity.
E. coli PERM348 containing plasmid pPERM348 (Table 1) was grown in 50 ml of LB medium, supplemented with ampicillin (100 μg/ml), at 37 oC to an OD at 600 nm (OD600) of 0.5. Expression of yqfS was induced during 4 h at 37°C by the addition of IPTG to 0.5 mM. Cells were collected by centrifugation and washed two times with 10 ml of 50 mM Tris-HCl (pH 7.5)-300 mM NaCl (buffer A). The cells were disrupted in 10 ml of the same buffer containing lysozyme (10 mg/ml) for 30 min at 37°C. The cell homogenate was subjected to centrifugation to eliminate undisrupted cells and cell debris, and the supernatant was applied to a 5-ml nickel-nitrilotriacetic acid-agarose column previously equilibrated with buffer A. The column was washed with 50 ml of buffer A containing 10 mM imidazole plus 50 ml of buffer A containing 20 mM imidazole, and the protein bound to the resin was eluted with 15 ml of buffer A containing 100 mM imidazole; 2-ml fractions were collected during this last step. Aliquots (15 μl) of the cell homogenate, the flowthrough, and the bound fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Two types of substrates were prepared to assay AP endonuclease activity of His6-YqfS, namely, pBluescript (Stratagene), which was partially depurinated after a previously described protocol (28) and a 5′-end-radiolabeled double-stranded 19-mer nucleotide containing a single abasic site (20).
The endonuclease activity of His6-YqfS against pBluescript containing AP sites (AP-pB) was determined in a mixture reaction of 25 μl containing 600 ng of purified His6-YqfS and 100 ng of substrate in 50 mM Tris-HCl (pH 7.5) containing 1 mM dithiothreitol. The reactions were incubated at 37°C for 30 min and analyzed by electrophoresis on a 1% agarose gel stained with ethidium bromide.
Endonuclease activity against the double-stranded radiolabeled 19-mer containing a single AP site was performed in a total volume of 15 μl containing 50 mM Tris-HCl (pH 7.5), 1 mM dithiothreitol, and 500 nmol of unlabeled and 10 nmol of double-stranded radiolabeled 19-mer containing a single AP site. Different amounts of His6-YqfS were added to the mixture reactions and incubated for 30 min at 37°C. The reactions were separated on a 20% denaturing acrylamide gel and then subjected to autoradiography.
Cell growth and enzymatic assays.
B. subtilis strains carrying the yqfS-lacZ fusion were grown and allowed to sporulate in liquid DSM. Samples of 1.5 ml were collected during vegetative growth and throughout sporulation. Cells were washed with 0.1 M Tris-HCl (pH 7.5) and processed for determination of β-galactosidase (42) and glucose dehydrogenase (GDH) activities (19, 42). The β-galactosidase activities were determined in cell extracts obtained from mother cells and forespore fractions prepared according to a previously described protocol (36, 44).
Northern blot and primer extension experiments.
The total RNA for both Northern blotting experiments and mapping of the 5′end of yqfS was isolated as previously described (35). Northern blots were performed with RNA samples isolated from strains B. subtilis 168 and WN118 (sigG mutant). RNA samples (20 μg) were separated by electrophoresis through 1% agarose-formamide gel and transferred to a high-bound nylon membrane. The membrane containing the transferred RNA was hybridized at 70°C with a 1,181-pb EcoRI-BamHI fragment from pPERM253 containing the entire yqfS sequence. The probe was labeled by random priming with [α-32P]dCTP by using the Rediprime II DNA labeling system according to the instructions of the provider (Amersham Biosciences, Buckinghamshire, England). Detection of hybrids was performed by autoradiography exposing the membrane to Kodak X-Omat films.
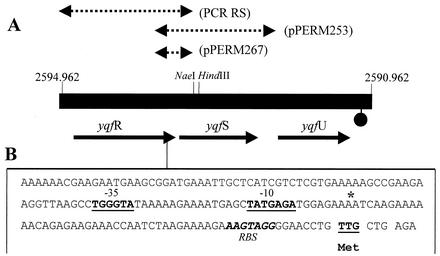
The 5′ end of yqfS was mapped by primer extension (37) of yqfS transcripts produced during sporulation. To this end, total RNA was isolated from vegetative and sporulating cells of B. subtilis PERM317. In order to obtain the maximum amount of yqfS transcripts during sporulation, we monitored the expression of β-galactosidase activity directed by the yqfS-lacZ fusion in this strain. The total RNA (40 μg from each sample) was hybridized with the 20-mer oligonucleotide 5′-CGGCGCGTATTTTGCGGTGC-3′, which was complementary to the yqfS mRNA from nucleotides 106 to 124 downstream from the putative yqfS translational start codon. The oligonucleotide was labeled on its 5′ end with [γ-32P]ATP and T4 polynucleotide kinase. The primer was extended with Moloney murine leukemia virus reverse transcriptase, and the extended products were separated by electrophoresis through a 6% polyacrylamide DNA sequencing gel. The position of the extended products was determined by running a sequencing reaction generated with the same 20-base primer and a 1,978-bp PCR product (PCR RS) extending from 247 bp upstream of the yqfR start codon to 416 bp downstream of the start codon of yqfS (Fig. 1).
FIG. 1.
yqfS region of the B. subtilis chromosome and DNA sequences lying upstream of the yqfS ORF. (A) Genetic organization of the yqfS locus between indicated coordinates of the B. subtilis chromosome (filled box). Dashed lines above the ORFs (arrows) show the DNA fragments cloned into the indicated plasmids. Downstream of yqfU ORF a putative transcriptional terminator is shown (stem-loop structure). (B) Sequence of the intergenic region between yqfR and yqfS. The in vivo-mapped transcriptional start site of yqfS is indicated by an asterisk immediately downstream of the −10 and −35 sequences that might function as a promoter for RNA polymerase-σG. RBS (putative ribosome-binding site).
RT-PCR experiments.
Total RNA from vegetative or sporulating B. subtilis 168 cells, grown in DSM, was isolated by using the TRI reagent (Molecular Research Center, Inc.). Reverse transcription-PCRs (RT-PCRs) were performed with the RNA samples and a Master Amp RT-PCR kit (Epicentre Technologies) according to the instructions of the provider. The primers used for RT-PCRs were 5′-CCTGTTGCTGAGAATAGGC-3′ (forward) and 5′-CGGCGCGTATTTTGCGGTGG-3′ (reverse) to generate a 132-bp RT-PCR product extending from 4 bp upstream from the start codon of yqfS to 128 bp downstream of this point (Fig. 1). As a control, in each experiment, the absence of chromosomal DNA in the RNA samples was assessed by mounting PCRs with Vent DNA polymerase (New England Biolabs) and the set of primers described above.
RESULTS
Cloning of yqfS.
The existence of a type IV AP endonuclease in the genome of B. subtilis was investigated by using the primary structure of E. coli Nfo (51) as a query to search against the database of National Center for Biotechnology Information with a Gapped BLAST program (2). As described in Materials and Methods, this approach was used to retrieve a gene termed yqfS from the genome of B. subtilis (26). Analysis of the yqfS primary structure revealed an ORF of 891 bp with enough information for the synthesis of a predicted protein of 31 kDa. Amino acid alignments showed that YqfS possesses homologies of 53, 52, and 32% with E. coli Nfo (51), Saccharomyces cerevisiae Apn1 (46), and Thermotoga maritima endonuclease IV (20), respectively.
Purification and enzymatic activity of YqfS.
The His6-YqfS protein synthesized in E. coli was purified to homogeneity by metal chelate affinity chromatography, yielding a 36-kDa protein (data not shown).
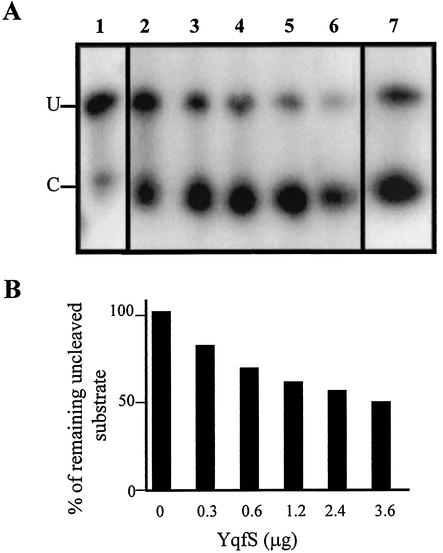
To corroborate the predicted AP endonuclease activity of YqfS, two enzymatic assays were performed. First, the His6-YqfS pure enzyme was incubated with a partially depurinated plasmid DNA as a substrate (AP-pB). The results presented in Fig. 2 reveal the conversion of the closed covalently circular depurinated plasmid (CCC) to the open circular form (OC) due to single-strand breaks performed by the His6-YqfS purified protein (lane 3). As shown in Fig. 2 (lane 4), the nondepurinated plasmid (U-pB) was not a substrate for the His-tagged YqfS protein. Controls shown in Fig. 2 revealed that neither the untreated nor the depurinated plasmid were converted to the OC form in the absence of the His6-YqfS protein (lanes 1 to 2). Second, a 5′-end radiolabeled double-stranded 19-mer nucleotide containing a single AP site was used as a substrate for the YqfS pure protein. Essentially, different amounts of the His6-tagged protein were incubated with 510 nM of this AP substrate. The products of the reaction analyzed on a denaturing polyacrylamide gel revealed that the endonucleolytic activity of YqfS at the AP site was dependent on the concentration of the enzyme used (Fig. 3A, lanes 2 to 6). To better evaluate this conclusion, these results were analyzed by densitometry, thereby corroborating that cleavage of the AP substrate by His6-YqfS is concentration dependent (Fig. 3B). Although the radiolabeled 20-bp-mer was also cleaved by E. coli Nfo (Fig. 3A, lane 7), it was observed that a fraction of the substrate was partially degraded (Fig. 3A, lane 1). The results presented in Fig. 3A (lane 7) also revealed that another fraction of the radiolabeled substrate was inaccessible to the enzyme; such a fraction most probably corresponded to nondepurinated compound. These results demonstrate for the first time that the product encoded by the yqfS gene possesses activity of AP endonuclease, a result in agreement with its high structural similarity to the family IV AP endonucleases.
FIG. 2.
Endonuclease activity of His6-YqfS against a plasmid containing AP sites. Aliquots (600 ng) of His6-YqfS were incubated with 100 ng of either untreated (U-pB [lane 4]) or AP-containing sites (AP-pB [lane 3]) of pBluescript. Lane 1, AP sites-containing plasmid incubated with 50 mM Tris-HCl (pH 7.5)-300 mM NaCl; lane 2, untreated plasmid incubated with 50 mM Tris-HCl (pH 7.5)-300 mM NaCl. The reactions were incubated at 37°C for 30 min and then analyzed by electrophoresis on a 1% agarose gel stained with ethidium bromide.
FIG. 3.
Endonuclease activity of His6-YqfS against a double-stranded 19-mer containing a single AP site. (A) A total of 510 nmol of 5′-end-radiolabeled double-stranded 19-mer nucleotide containing a single AP site was incubated for 30 min at 37°C with different concentrations of His6-YqfS. The reactions were separated on a 20% denaturing acrylamide gel and then subjected to autoradiography. Lane 1, no enzyme; lanes 2 to 6, 0.3, 0.6, 1.2, 2.4, and 3.6 μg of His6-YqfS, respectively; lane 7, 2 U of E. coli Nfo. Radioactively labeled cleaved (C) and uncleaved (U) strands are as indicated. (B) Densitometry of the experiment shown in panel A; the percentage of uncleaved substrate was plotted as a function of the amount of His6-YqfS added to the reaction.
Expression of a yqfS during growth and sporulation.
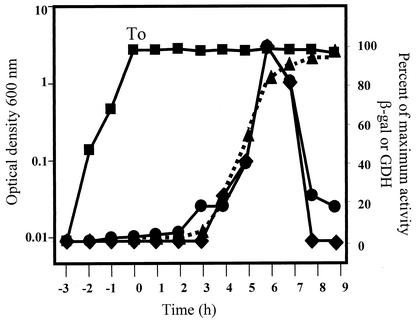
The strain B. subtilis PERM317 harboring a single copy of the yqfS-lacZ fusion was grown in DSM to induce sporulation. Determination of yqfS-directed β-galactosidase activity during growth and sporulation stages revealed a temporal pattern of expression. Although no β-galactosidase activity was detected during vegetative growth, Fig. 4 reveals that enzymatic activity was detectable after T0, reached a maximum during T6 and T7, and then decreased. The expression pattern of the reporter gene (Fig. 4) was similar to that observed for genes whose expression occurs during the last steps of sporulation in the forespore compartment, such as the operon splA-splB (44), gdh (39), and ssp (35, 36) genes. To further investigate this observation, two approaches were followed. First, cell fractioning experiments were performed to investigate whether the expression of the reporter gene occurred inside of the spore. The results of Fig. 4 show that β-galactosidase activity started to accumulate inside of the forespores from sporulation stage T5 and continued to accumulate until at least stage T9.
FIG. 4.
Expression of a yqfS-lacZ translational fusion during growth and sporulation of B. subtilis. B. subtilis PERM317 was grown to sporulation in liquid DSM (▪). Samples were collected at different times and treated with lysozyme, and the extracts were assayed for either β-galactosidase (♦) or GDH (•) activity. The β-galactosidase activity inside of the forespore lysozyme-resistant fraction (▴) was assayed as described in Materials and Methods.
The cell extracts used to determine β-galactosidase activity were also assayed for GDH activity, an enzyme encoded by the stage III, forespore-specific gdh gene (19, 39). The results shown in Fig. 4 revealed that the expression patterns of the yqfS-lacZ fusion and the GDH activity followed essentially identical kinetics, strongly indicating that yqfS gene expression is activated in the forespore compartment during the last steps of sporulation.
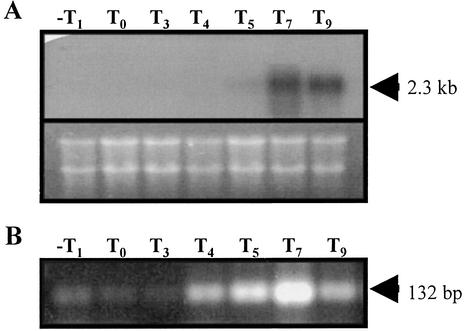
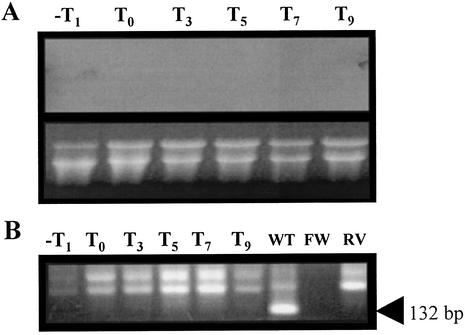
To further support this contention, Northern blot experiments were performed with total RNA isolated from cells of strain B. subtilis PERM168 collected before and after the onset of sporulation. The results (Fig. 5A) indicated that yqfS mRNA appeared as a 2.3-kb band during sporulation stages T5 through T9, observing a major hybridization signal at T7. As shown in Fig. 5A, no signal was detected in the blot with RNA isolated from cells growing exponentially, supporting the conclusion that yqfS expression is sporulation specific. Moreover, RT-PCR experiments resulted in the major amplification of a yqfS product when total RNA isolated from sporulating cells was used as a template. Figure 5B shows that the RT-PCR product of yqfS (132 bp) was more abundant with RNA samples of the step T7 of sporulation.
FIG. 5.
Northern blot (A) and RT-PCR analysis (B) of yqfS transcription during vegetative growth and sporulation. (A) B. subtilis 168 was induced to sporulate in liquid DSM. Total RNA was isolated (35) during the times (in hours) indicated (T0 = end of exponential growth). Then, 20-μg samples were separated on agarose-formaldehyde gels (lower panel) and transferred to nylon membranes. The membrane was hybridized with a 32P-labeled 1,181-bp fragment encompassing the entire yqfS sequence as described in Materials and Methods. (B) RNA samples (1 μg) isolated from a B. subtilis 168 DSM culture at the times indicated (in hours) were processed for RT-PCR analysis as described in Materials and Methods. The arrowhead shows the size of the expected RT-PCR product.
σG dependence of yqfS expression.
The expression of forespore specific genes in B. subtilis is carried out through the sequential action of two temporally expressed RNA polymerases containing either σF or σG factors (21, 43). However, as shown above, the expression pattern of the yqfS-lacZ fusion was very similar to the σG-dependent gdh gene, suggesting that yqfS is under the control of σG-containing RNA polymerase. This notion was directly tested by two different approaches. First, the yqfS-lacZ fusion was introduced by transformation into competent cells of B. subtilis WN126 harboring a deletion of the σG gene, an spo mutant in which sporulation is arrested during stage III (24, 60). The resulting strain, B. subtilis PERM336, grown in DSM expressed very low levels of yqfS-directed β-galactosidase activity during both vegetative- and stationary-growth phases (data not shown). Consistent with this result, the levels of GDH in this strain were almost zero (data not shown).
In a second approach, Northern blot experiments were performed with RNA isolated from vegetative and stationary cells of B. subtilis sigGΔ1 grown in liquid DSM. The results shown in Fig. 6A revealed the lack of yqfS mRNAs in this sigG mutant genetic background, since no hybridization signal was detected during both exponential-growth-phase and stationary-growth-phase cells. Such a result was also confirmed by RT-PCR experiments, which failed to amplify the 132-bp yqfS fragment from RNA samples isolated before and after the onset of sporulation (Fig. 6B). Taken together, these results are consistent with yqfS expression being dependent on σG RNA polymerase.
FIG. 6.
Northern blot (A) and RT-PCR analysis (B) of yqfS transcription during vegetative growth and sporulation of B. subtilis sigGΔ1 (strain WN118). (A) B. subtilis WN118 was grown in liquid DSM. Total RNA was isolated (35) during the times indicated (in hours). Samples (20 μg) were separated on agarose-formaldehyde gels (lower panel) and transferred to nylon membranes. The membrane was hybridized with a 32P-labeled 1,181-bp fragment encompassing the entire yqfS sequence as described in Materials and Methods. (B) RNA samples (1 μg) isolated at the times indicated (in hours) from a B. subtilis sigGΔ1 DSM culture were processed for RT-PCR analysis as described in Materials and Methods. For the wild type (WT), the RNA was isolated from B. subtilis 168 (Fig. 5); FW was obtained with the forward primer in the absence of RNA, and RV was obtained with the reverse primer in the absence of RNA. The arrowhead shows the size of the expected RT-PCR product.
Mapping the transcriptional start site of yqfS.
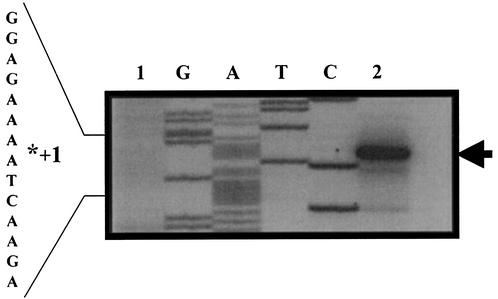
The genetic organization of the yqfS locus reveals that this gene is flanked upstream by yqfR, which encodes a putative RNA helicase, and downstream by yqfU, which encodes a protein of unknown function (Fig. 1). The existence of only one potential transcriptional terminator until the end of yqfU suggests that the three genes could be cotranscribed as a polycistronic message. To investigate this possibility, primer extension analysis was performed to map the 5′ ends of the mRNAs originating from upstream from the yqfS coding sequence. Experiments were carried out with total RNA isolated from B. subtilis PERM317 harboring the yqfS-lacZ fusion. Cells used to isolate RNA were harvested during both vegetative growth and the T7 sporulation stage, the time of maximum expression of the yqfS-lacZ fusion. The results shown in Fig. 7 (lane 2) revealed the synthesis of a major extension product located 54 to 55 bp upstream of translation start codon of yqfS. Such an extension product was obtained only in experiments performed with RNA isolated from sporulating cells but not with RNA of vegetatively growing cells (Fig. 7, lane 1). Inspection of the nucleotide sequences lying upstream of the in vivo mapped transcriptional yqfS start site revealed the existence of sequences with good homology to promoters preceding genes of the σG regulon (Fig. 8) (21, 43). A higher level of homology was found in the −35 region, which possessed three of the four absolutely conserved bases present on sigG promoters (Fig. 8). On the other hand, the −10 region conserved three of the four absolutely conserved residues observed in such σG promoters. However, it was found that the −10 and −35 regions were separated by 16 bp instead of the reported 17 to 18 bp for the σG consensus sequence (Fig. 8).
FIG. 7.
Primer extension analysis for mapping the transcriptional start site of yqfS. Total RNA was isolated (34) from either vegetative (lane 1) or sporulating (stage T7; lane 2) B. subtilis PERM317 cells grown in DSM. Primer extension was performed as described in Materials and Methods. The asterisk indicates the position of the primer extension product in the DNA sequence lying upstream of yqfS (see Fig. 1). The 5′ end of the yqfS transcript was determined by running a DNA sequencing ladder generated with the same primer (lanes G, A, T, and C) and was labeled with an arrowhead.
FIG. 8.
Comparison of the consensus EσG (19) promoter sequence (top line) with the putative promoter sequence lying upstream of yqfS (bottom line). Absolutely conserved (boldface) or highly conserved (underlined) bases in EσG-type promoters (21, 43). The position of the mapped transcriptional start site of yqfS is indicated with an asterisk.
Induction of the yqfS-lacZ fusion by oxidative stress or during the SOS response.
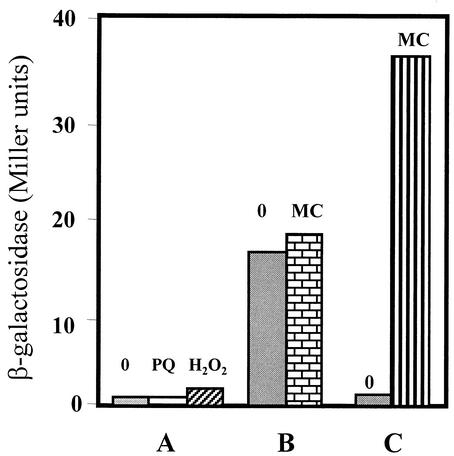
In E. coli, the expression of the type IV AP endonuclease nfo gene is induced by generators of superoxide radicals, such as paraquat (8). On the other hand, B. subtilis responds to H2O2 stress displaying an adaptive response that induces the expression of genes such as katA (catalase), ahpCF (alkyl hydroperoxide reductase), mrgA, and the hemA operon (1, 4, 7, 9, 10, 14). We therefore investigated whether the yqfS gene in B. subtilis is also induced by the oxidative stress imposed by either superoxide radicals or hydrogen peroxide. To this end, the strain B. subtilis PERM317 containing the yqfS-lacZ fusion, integrated into the yqfS locus, was grown in LB medium to the mid-exponential phase and treated with paraquat (10 μM) or hydrogen peroxide (200 μM). The results (Fig. 9A) revealed that, at the concentrations tested, neither paraquat nor H2O2 was capable of inducing the expression of the yqfS-lacZ fusion.
FIG. 9.
Lack of induction of a yqfS-lacZ fusion by paraquat, H2O2, or mitomycin. B. subtilis PERM317 was grown to an OD600 of 0.5 in either minimal Spizizen medium (A) or LB medium (B). The culture made in minimal Spizizen medium was divided into three subcultures; one (labeled “0”) was left untreated, and the other two were treated with either paraquat (PQ; 10 μM) or H2O2 (200 μM). The LB culture was treated in the same manner except that mitomycin C (MC; 0.5 μg/ml) was added to the culture. (C) B. subtilis YB3000 was grown in LB medium to an OD600 of 0.5; at this point, the culture was equally divided, and mitomycin C (0.5 μg/ml) was added to one of the subcultures. In all cases, the β-galactosidase activity was determined with cell samples collected 2 h after the addition of the inducers.
Several B. subtilis genes involved in DNA repair, such as uvr components and recA, have been shown to be inducible not only by DNA damage but also by the physiological state of competence (32, 34, 48). These genes (din) are part of a global response which in B. subtilis is called the SOS response (33). In order to determine whether the type IV AP-endonuclease gene of B. subtilis is a component of the B. subtilis SOS regulon, the strain containing the yqfS-lacZ fusion was grown to exponential phase and then treated with mitomycin C to a final concentration of 0.5 μg/ml. As shown in Fig. 9B, mitomycin C induced the β-galactosidase levels of the strain B. subtilis PERM317 only 1.2 times above the levels expressed by the untreated control. In contrast with this result, when B. subtilis YB3000 containing a recA-lacZ fusion was treated with mitomycin (Fig. 9C), the levels of β-galactosidase activity increased 35 times.
DISCUSSION
B. subtilis has been studied extensively as a paradigm for bacterial differentiation and development. Spores produced by this organism prevent or dramatically slow the DNA damage inflicted by oxidative stress, UV light, heat and desiccation (reviewed in references 40 and 58). However, during long periods of dormancy spores accumulate potentially lethal and mutagenic DNA damage such as SP, strand brakes, CPDs, chemically altered bases, and AP sites that could affect transcription and replication during germination (40, 56). Therefore, it is of interest to determine how the many DNA repair systems present are regulated by B. subtilis, especially in relation to the sporulation and germination processes.
Thus, the yqfS ORF was cloned, and the product of this gene was isolated and tested for its enzymatic activity. The results presented in Fig. 2 and 3 clearly indicate that this protein has AP endonuclease activity. Having established the nature of the product of the yqfS gene, we wanted to determine the mechanism(s) that control the expression of this gene. Our data demonstrate that there is temporal and spatial expression of the yqfS gene. Specifically, β-galactosidase activity for a yqfS-LacZ reveals that this gene is not apparently transcribed during vegetative growth but is transcribed during stages of the sporulation process (Fig. 4). Northern blot and RT-PCR experiments (Fig. 5) confirmed a major abundance of yqfS messengers during stages of the sporulation process of the strain B. subtilis PERM317. These results suggested that yqfS expression is temporally activated and confined to the forespore compartment in accordance with a pattern similar to that described for stage III, forespore-specific genes (57). This suggestion was further supported not only by cell fractionation experiments, which demonstrated that yqfS expression occurs inside of the spore, but also by the observation that the kinetics of GDH synthesis, a stage III, forespore-specific marker, are indistinguishable from those observed for the yqfS-lacZ fusion (Fig. 4). These results strongly support the idea that the synthesis of the YqfS protein occurs during the last stages of the sporulation process and is packaged in the spore.
The forespore-specific expression of the yqfS-lacZ fusion during the last steps of B. subtilis sporulation suggested that the transcription of yqfS is carried out by RNA polymerase containing the σG factor (Fig. 4). However, gene expression inside of the forespore occurs by the sequential action of two RNA polymerases containing either the σF or σG factors (21, 25). Therefore, we could not rule out a possible transcription of yqfS by RNA polymerase σF. This point was addressed by measuring the levels of expression of the yqfS-lacZ fusion introduced into a B. subtilis strain lacking the sigG gene (Table 1). The results showed that yqfS-directed β-galactosidase activity is almost null in this genetic background, as is the synthesis of GDH activity (data not shown). In agreement with this observation, both Northern blot and RT-PCR experiments performed with total RNA isolated during vegetative and stationary growth of the strain B. subtilis sigGΔ1 demonstrated the absence of yqfS messengers in this mutant strain (Fig. 6). Taken collectively, these results strongly suggest that yqfS expression occurs inside of the spore by the action of σG-containing RNA polymerase.
Forespore-specific expressed genes such as sspA-E, splA-splB, gdh, ger, and spoVA, among others, are representative of the σG regulon (15, 16, 19, 21, 25, 44, 45, 57). Experimental evidence has demonstrated that these genes possess specific promoters that are exclusively transcribed by σG containing RNA polymerase (15, 39, 43, 44, 47). The results described above suggest that yqfS might be a new member of this regulon. This conclusion was strongly supported by the in vivo mapping of the transcriptional start site of yqfS (Fig. 7). A major extension product initiating 54 to 55 bp upstream of the putative yqfS start codon was amplified from RNA samples isolated from sporulating but not from vegetatively growing cells (Fig. 7). Inspection of the sequences preceding the yqfS transcriptional start site revealed the existence of a promoter with homology to the consensus sequence of σG promoters (21, 43). Although the −10 region of the putative yqfS promoter shows a low level of homology, the −35 region almost perfectly matched the consensus of σG promoters (Fig. 8). One possible problem with the designation of this putative σG promoter is the spacing between the −35 and −10 regions. However, as mentioned above, our data support the hypothesis that the yqfS gene is transcribed by a σG-containing RNA polymerase.
The yqfS region in the B. subtilis chromosome shows the existence of a set of three genes located in the same orientation, in the following order: yqfR, yqfS, and yqfU (Fig. 1). The lack of putative transcriptional terminators downstream of yqfR and yqfS suggests that the three genes are transcribed as a polycistronic unit. However, the primer extension experiments described above, together with the identification of a 2.3-kb yqfS messenger, indicate that yqfS is cotranscribed with yqfU as a bicistronic mRNA from the putative yqfS promoter just described.
Expression of the two major AP endonucleases is differentially regulated in E. coli. Whereas exoIII is constitutively expressed, the nfo gene is inducible by oxidative stress. Chemical compounds such as paraquat and menadione, which generate superoxide radicals, induce a 10- to 20-fold increase in the level of Nfo (8). The lack of induction in the levels of expression of the yqfS-lacZ fusion after the treatment of B. subtilis PERM317 with paraquat (Fig. 9) revealed that in B. subtilis the yqfS gene is not regulated by the oxidative stress imposed by superoxide radicals.
In B. subtilis the adaptive response to H2O2 stress is subjected to negative regulation by the repressor PerR, a Fur homolog (6). Treatment of B. subtilis PERM317 with H2O2 did not change the levels of expression of the yqfS-lacZ fusion (Fig. 9), suggesting that yqfS is not regulated by PerR. Consistent with these results, no cis-acting DNA sequences similar to those present in perR boxes (22) were observed around the putative promoter of yqfS.
Analysis of the upstream regions of yqfS also revealed the absence of dinR-like boxes (11, 62). This observation is in agreement with the lack of induction of the yqfS-lacZ fusion after the treatment of B. subtilis PERM317 with the DNA-damaging agent mitomycin (Fig. 9).
Taking all of these results together, we conclude that although in E. coli the expression of nfo is linked to the oxidative stress generated by superoxide radicals (8), in B. subtilis the regulation of yqfS expression occurs in a temporal and forespore-specific manner and appears to be part of the σG regulon. In addition, the lack of induction of β-galactosidase in the yqfS-lacZ fusion strains after treatment by either hydrogen peroxide or the DNA-damaging agent mitomycin revealed that yqfS in not under the control of the PerR or SOS regulons.
Despite the existence of spore mechanisms that prevent or alter DNA insults, potentially lethal and mutagenic damage accumulates in DNA during long-term storage of spores in the laboratory (40, 58) and during the exposure of these spores to environmental stresses, particularly solar radiation (40, 41, 59, 61). Interestingly, artificial and solar UV radiation induce the formation of SP, CPDs, and strand breaks but not of AP sites in B. subtilis spore DNA (59). It remains to be investigated whether AP sites are generated during germination of B. subtilis spores either spontaneously or promoted by oxidative stress or through the action of DNA glycosylases during the elimination of chemically modified bases (18). Moreover, depending on their chemical structure, single-strand breaks generated on spore DNA could be processed as well by YqfS during germination, since it has been well established that type IV AP endonucleases are able to remove phosphoglycoaldehyde, phosphate, deoxyribose-5-phosphate, and 4-hydroxy-2-pentenal from the 3′ terminus of duplex DNA (18). Therefore, as an obligatory step for the correction of the different types of DNA damage processed by the BER pathway, YqfS may play an important role in the repair of DNA damage inflicted on B. subtilis during either spore dormancy or germination.
In conclusion, we provide here for the first time evidence that an important component of the BER system of B. subtilis, namely, the yqfS gene, is specifically expressed inside of the spores during the final developmental stages. Thus, together with the SplB, UVR, and Rec systems, YqfS could be part of the DNA repair proteins that increase the survival potential of B. subtilis spores.
Acknowledgments
This work was supported by grant 31767-N from the Consejo Nacional de Ciencia y Tecnología (CONACYT) of México to M.P.-R. N.U.-E. and J.M.S.-P. were supported by a Doctoral fellowships from CONACYT. R.E.Y. was supported by grant MCB-9975140 from the National Science Foundation.
We thank J. J. García-Soto for critical review of the manuscript and Ada A. Sandoval for technical assistance.
REFERENCES
- 1.Antelman, H., S. Engelmann, R. Schmid, and M. Hecker. 1996. General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon. J. Bacteriol. 178:6571-6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barzilay, G., and L. D. Hickson. 1995. Structure and function of apurinic/apyrimidinic endonucleases. Bioessays 17:713-719. [DOI] [PubMed] [Google Scholar]
- 4.Bol, D. K., and R. E. Yasbin. 1994. Analysis of the dual regulatory mechanisms controlling expression of the vegetative catalase gene of Bacillus subtilis. J. Bacteriol. 176:6744-6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boylan, R. J., N. H. Mendelson, D. Brooks, and F. E. Young. 1972. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J. Bacteriol. 110:281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bsat, N., and J. D. Helmann. 1999. Interaction of Bacillus subtilis Fur (ferric uptake repressor) with the dnB operator in vitro and in vivo. J. Bacteriol. 181:4299-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bsat, N., A. Herbig, L. Casillas-Martínez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologs: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189-198. [DOI] [PubMed] [Google Scholar]
- 8.Chan, E., and B. Weiss. 1987. Endonuclease IV of Escherichia coli is induced by paraquat, DNA damage by oxygen-derived species. Proc. Natl. Acad. Sci. USA 84:3189-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, L., and J. D. Helmann. 1995. Bacillus subtilis MrgA is a Dps (PexB) homologue: evidence for metalloregulation of a stress-oxidative gene. Mol. Microbiol. 18:295-300. [DOI] [PubMed] [Google Scholar]
- 10.Chen, L., L. Keramati, and J. D. Helmann. 1995. Coordinate regulation of Bacillus subtilis coordinate stress genes by hydrogen peroxide and metal ions. Proc. Natl. Acad. Sci. USA 92:8190-8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheo, D. L., K. W. Bayles, and R. E. Yasbin. 1991. Cloning and characterization of DNA damage-inducible promoter regions from Bacillus subtilis. J. Bacteriol. 173:1696-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cutting, S. M., and P. B. Vander Horn. 1990. Genetic analysis, p. 27-74. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Sussex, England.
- 13.Demple, B., and L. Harrison. 1994. Repair of oxidative damage to DNA: enzymology and biology. Annu. Rev. Biochem. 63:915-948. [DOI] [PubMed] [Google Scholar]
- 14.Dowds, B. C. A. 1994. The oxidative stress response in Bacillus subtilis. FEMS Microbiol. Lett. 124:255-264. [DOI] [PubMed] [Google Scholar]
- 15.Fajardo-Cavazos, P., F. Tovar-Rojo, and P. Setlow. 1991. Effect of promoter mutations and upstream deletions on the expression of genes coding for small, acid-soluble spore proteins of Bacillus subtilis. J. Bacteriol. 173:2011-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feavers, I. M., Foulkes, B. Setlow, D. Sun, W. Nicholson. P. Setlow, and A. Moir. 1990. The regulation of transcription of the gerA spore germination operon of Bacillus subtilis. Mol. Microbiol. 4:275-282. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari, E., S. M. H. Haward, and J. A. Hoch. 1985. Effect of sporulation mutations on subtilisin expression, assayed using a subtilisin-β-galactosidase gene fusion, p. 180-184. In J. A. Hoch and P. Setlow (ed.), Molecular biology of microbial differentiation. American Society for Microbiology, Washington, D.C.
- 18.Friedberg, E. C., G. C. Walker, and W. Siede. 1995. DNA repair and mutagenesis. American Society for Microbiology, Washington, D.C.
- 19.Fujita, Y., R. Ramaley, and E. Freese. 1977. Location and properties of glucose dehydrogenase in sporulating cell and spores of Bacillus subtilis. J. Bacteriol. 132:282-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas, B. J., M. Sandigursky, J. A. Tainer, W. A. Franklin, and R. P. Cunningham. 1999. Purification and characterization of Thermotoga maritima endonuclease IV, a thermostable apurinic/apyrimidinic endonuclease and 3′-repair diesterase. J. Bacteriol. 181:2834-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haldenwang, W. 1995. The sigma factors of Bacillus subtilis. Microbiol. Rev. 59:1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbig, A. F., and J. D. Helmann. 2001. Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA. Mol. Microbiol. 41:849-859. [DOI] [PubMed] [Google Scholar]
- 23.Hosfield, D. J., Y. Guan, B. J. Haas, R. P. Cunningham, and J. A. Tainer. 1999. Structure of the DNA repair enzyme endonuclease IV and its DNA complex: double nucleotide flipping at abasic sites and three-metal-ion catalysis. Cell 98:397-408. [DOI] [PubMed] [Google Scholar]
- 24.Karmazyn-Campelli, C., C. Bonamy, B. Savelli, and P. Stragier. 1989. Tandem genes enconding sigma factors for consecutive steps of development in Bacillus subtilis. Genes Dev. 3:150-157. [DOI] [PubMed] [Google Scholar]
- 25.Kroos, L., and S. Cutting. 1994. Intercellular and intercompartmental communication during Bacillus subtilis sporulation, p. 155-180. In P. J. Piggot, C. P. Moran, Jr., and P. Youngman (ed.), Regulation of bacterial differentiation. American Society for Microbiology, Washington, D.C.
- 26.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V., Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Conerton, A. Danchin, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 27.Krokan, E. H., R. Standal, and G. Slupphaug. 1997. DNA glycosylases in the base excision repair of DNA. Biochem. J. 325:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin, J. D., A. W. Johnson, and B. Demple. 1988. Homogeneous Escherichia coli endonuclease IV: characterization of an enzyme that recognizes oxidative damage in DNA. J. Biol. Chem. 263:8066-8071. [PubMed] [Google Scholar]
- 29.Lindahl, T., and A. Andersson. 1972. Rate of chain breakage of apurinic sites in double-stranded DNA. Biochemistry 11:3618-3623. [DOI] [PubMed] [Google Scholar]
- 30.Lindahl, T. 1993. Instability and decay of the primary structure of DNA. Nature 362:709-715. [DOI] [PubMed] [Google Scholar]
- 31.Loeb, L. A., and B. D. Preston. 1986. Mutagenesis by apurinic/apyrimidinic sites. Annu. Rev. Genet. 20:201-230. [DOI] [PubMed] [Google Scholar]
- 32.Love, P. E., M. S. Lyle, and R. E. Yasbin. 1985. DNA damage inducible (din) loci are transcriptionally activated in competent Bacillus subtilis. Proc. Natl. Acad. Sci. USA 82:6201-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Love, P. E., and R. E. Yasbin. 1984. Genetic characterization of the inducible SOS-like system of Bacillus subtilis. J. Bacteriol. 160:910-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lovett, C. M., Jr., P. E. Love, and R. E. Yasbin. 1989. Competence specific induction of the Bacillus subtilis RecA protein analog: evidence for dual regulation of a recombination protein. J. Bacteriol. 171:2318-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mason, J. M., P. Fajardo-Cavazos, and P. Setlow. 1988. Levels of mRNAs which code for small, acid soluble spore proteins and their lacZ gene fusions in sporulating cells of Bacillus subtilis. Nucleic Acids Res. 16:6567-6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mason, J. M., R. H. Hackett, and P. Setlow. 1988. Regulation of expression of genes coding for small, acid-soluble proteins of Bacillus subtilis spores: studies using lacZ gene fusions. J. Bacteriol. 170:239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKnight, S. L., and R. Kingsbury. 1982. Transcription control signals of a eukaryotic protein-coding gene. Science 217:316-324. [DOI] [PubMed] [Google Scholar]
- 38.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N. Y.
- 39.Nakatani, Y., W. L. Nicholson, K. D. Nietzke, P. Setlow, and E. Freese. 1989. Sigma-G RNA polymerasa controls forespore specific expression of the glucose dehydrogenase operon in Bacillus subtilis. Nucleic Acids Res. 17:999-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicholson, W. L., N. Munakata, G. Horneck, H. G. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholson, W. L., and P. Fajardo-Cavazos. 1997. DNA repair and the ultraviolet radiation resistance of bacterial spores: from the laboratory to the environment. Rec. Res. Dev. Microbiol. 1:125-140. [Google Scholar]
- 42.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination, and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.). Molecular biological methods for Bacillus. John Wiley & Sons, Sussex, England.
- 43.Nicholson, W. L., D. Sun, B. Setlow, and P. Setlow. 1989. Promoter specificity of σG-containing RNA polymerase from sporulating cells of Bacillus subtilis: identification of a group of forespore-specific promoters. J. Bacteriol. 171:2708-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedraza-Reyes, M., F. Gutiérrez-Corona, and W. L. Nicholson. 1994. Temporal regulation and forespore-specific expression of the spore photoproduct lyase gene by Sigma-G RNA polymerase during Bacillus subtilis sporulation. J. Bacteriol. 176:3983-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pedraza-Reyes, M., F. Gutiérrez-Corona, and W. L. Nicholson. 1997. Spore photoproduct lyase operon (splAB) regulation during Bacillus subtilis sporulation: modulation of splB-lacZ fusion expression by P1 promoter mutations and by an in-frame deletion of splA. Curr. Microbiol. 34:133-137. [DOI] [PubMed] [Google Scholar]
- 46.Ramotar, D. 1997. The apurinic-apyrimidinic endonuclease IV family of DNA repair enzymes. Biochem. Cell. Biol. 75:327-336. [PubMed] [Google Scholar]
- 47.Rather, P. N., and C. P. Moran, Jr. 1988. Compartment-specific transcription in Bacillus subtilis: identification of the promoter for gdh. J. Bacteriol. 170:5086-5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raymond-Denise, A., and N. Guillen. 1992. Expression of the Bacillus subtilis dinR and recA genes after DNA damage and during competence. J. Bacteriol. 174:3171-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 50.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saporito, S. M., and R. P. Cunningham. 1988. Nucleotide sequence of the nfo gene of Escherichia coli K-12. J. Bacteriol. 170:5141-5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schaeffer, P., J. Millet, and J.-P. Aubert. 1965. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. USA 54:704-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shida, T., T. Ogawa, N. Ogasawara, and J. Sekiguchi. 1999. Characterization of Bacillus subtilis exoA protein: a multifunctional DNA-repair enzyme similar to Escherichia coli exonuclease III. Biosci. Biotechnol. Biochem. 9:1528-1534. [DOI] [PubMed] [Google Scholar]
- 54.Setlow, B., and P. Setlow. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Setlow, B., K. J. Tautvydas, and P. Setlow. 1998. Small acid-soluble spore proteins of the α/β-type do not protect the DNA in Bacillus subtilis spores against base alkylation. Appl. Environ. Microbiol. 64:1958-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Setlow, P. 1988. Small acid-soluble spore proteins of Bacillus species: structure, synthesis, genetics, function and degradation. Annu. Rev. Microbiol. 42:319-338. [DOI] [PubMed] [Google Scholar]
- 57.Setlow, P. 1989. Forespore-specific genes of Bacillus subtilis:function and regulation of expression, p. 211-221. In I. Smith, R. Slepecky, and P. Setlow (ed.), Regulation of prokaryotic development: structural and functional analysis of bacterial sporulation and germination. American Society for Microbiology, Washington, D.C.
- 58.Setlow, P. 1995. Mechanisms for the prevention of damage to DNA in spores of Bacillus species. Annu. Rev. Microbiol. 49:29-54. [DOI] [PubMed] [Google Scholar]
- 59.Slieman, T. A., and W. L. Nicholson. 2000. Artificial and solar UV radiation induces strand breaks and cyclobutane pyrimidine dimers in Bacillus subtilis spore DNA. Appl. Environ. Microbiol. 66:199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun, D., P. Stragier, and P. Setlow. 1989. Identification of a new sigma factor which allows RNA polymerase to transcribe the sspE gene and other forespore specific genes during sporulation of Bacillus subtilis. Genes Dev. 3:141-149. [DOI] [PubMed] [Google Scholar]
- 61.Tyrrel, R. M. 1978. Solar dosimetry with repair deficient bacterial spores: action spectra, photoproduct measurements and a comparison with other biological systems. Photochem. Photobiol. 27:571-579. [DOI] [PubMed] [Google Scholar]
- 62.Winterling, K. W., D. Chafin, J. J. Hayes, J. Sun., A. S. Levine, R. E. Yasbin, and R. Woodgate. 1998. The Bacillus subtilis DinR binding site: redefinition of the consensus sequence. J. Bacteriol. 180:2201-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]