Abstract
The influence of anterior pituitary hormones on the gastrointestinal tract of humans and animals has been reported. Hypophysectomy (HYPOX) in the rat causes atrophy of the intestinal mucosa, reduction of gastric secretion and intestinal absorption, and increased susceptibility to infections. To our knowledge, there are no studies on the humoral immune response of the gut-associated lymphoid tissue after HYPOX. We have reported that decreased secretion of vasopressin and oxytocin due to neurointermediate pituitary lobectomy (NIL) diminishes humoral and cell-mediated immune responses. However, no data have been published on whether NIL can affect intestinal immune responses. We analyzed the effects of HYPOX and NIL on bacterial colonization of the intestinal lumen, Peyer's patches, and spleen as well as the serum immunoglobulin G (IgG) and IgM and specific intestinal IgA levels in response to Salmonella enterica serovar Typhimurium oral infection. Results showed the following: (i) Salmonella serovar Typhimurium was eliminated from the intestinal lumen at the same rate in rats that underwent a sham operation, HYPOX, and NIL; (ii) Salmonella serovar Typhimurium colonization of Peyer's patches and spleen was significantly higher in both HYPOX and NIL rats than in sham-operated rats; (iii) serum IgG and IgM and intestinal IgA against surface proteins of Salmonella serovar Typhimurium were significantly lower in HYPOX and NIL rats than in sham-operated rats; and (iv) compared to NIL rats, higher Peyer's patch and spleen bacterial colonization and decreased IgG, IgM, and IgA production were observed in HYPOX rats. We conclude that hormones from each pituitary lobe affect the systemic and gastrointestinal humoral immune responses through different mechanisms.
Multidirectional interactions between nervous, endocrine, and immune systems in health and in the course of inflammatory and infectious diseases have been well established (5). The central nervous system (CNS) signals to the immune system via hormonal and neural pathways, and the immune system signals to the CNS through various cytokines. The CNS regulates the immune system via pituitary hormones, mainly growth hormone (GH) and prolactin (PRL), which are immunostimulatory (6, 7, 8, 37, 44, 45, 46), and the hypothalamic-pituitary-adrenocortical (HPA) axis, which inhibits immune responses and acts as an immunomodulator-immunosuppressor (16, 61, 67, 68). Whereas most information regarding these interactions is related to systemic immune responses (6, 7, 16, 37, 46, 61, 67, 68), much less is known about the interactions between the hypothalamus, the pituitary, and local gastrointestinal immune reactions (9, 43, 49).
Gut-associated lymphoid tissue (GALT) is a large and complex immune apparatus which is anatomically and functionally different from those found elsewhere in the body (41). It contains a special type of plasma cells which produce predominantly immunoglobulin A (IgA) isotype. Mucosal epithelial cells are central players in the regulation of the natural and acquired immunity of the host and also participate in the active transport of polymeric IgA, produced in the mucosal and glandular tissues, to the mucosal surface (9, 43, 49).
Evidence that pituitary hormones play a role in protection against Salmonella enterica serovar Typhimurium infection has been reported (20, 23). In hypophysectomized (HYPOX) rats, the increased susceptibility to intraperitoneal Salmonella serovar Typhimurium infection is restored by GH treatment to normal. In intact rats and mice, GH and PRL enhance the resistance to Salmonella serovar Typhimurium infection by increasing phagocytosis and intracellular destruction of bacteria by peritoneal macrophages. It was also shown that GH and PRL stimulate chemotaxis of peritoneal granulocytes (17, 21, 22, 23, 38). The HPA axis is activated during many bacterial and viral infections, resulting in an increase of circulating glucocorticoids (GCs). The increased secretion of GCs is critical for the survival of the host (67, 68). GCs released by immobilization stress, burn injury, or Salmonella serovar Typhimurium infection increase bacterial translocation from the gastrointestinal tract to the mesenteric lymph nodes (19, 27, 31). It was reported that stress decreases the production and secretion of IgA by the salivary glands (12). The effects of GC administration on GALT (57) and the increased bacterial translocation by the activation of the HPA axis during intestinal infection are consistent with the view that GCs suppress immune reactions (42, 59).
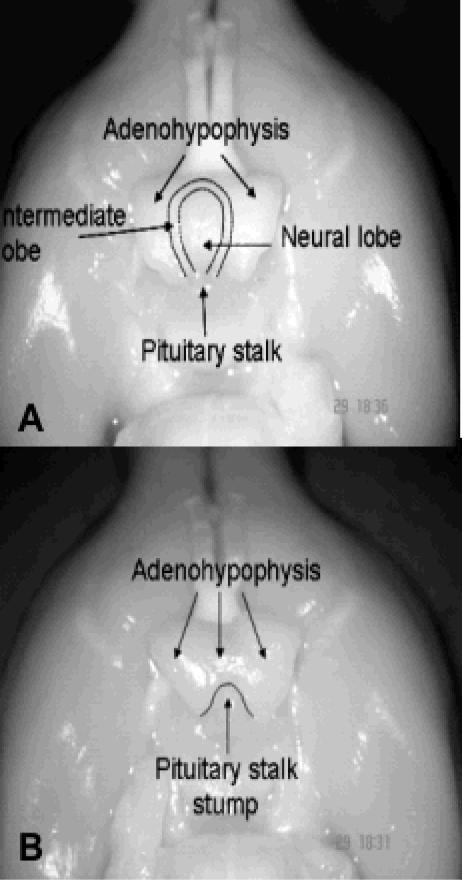
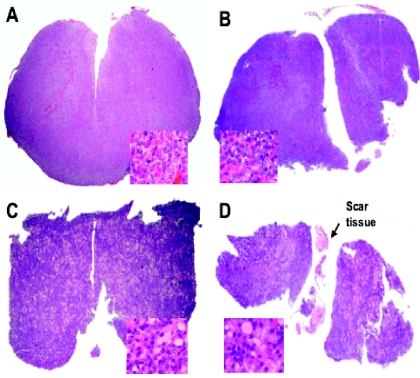
In rats, the pituitary gland is composed of three lobes: the anterior or adenohypophysial lobe, which synthesizes and secretes GH, PRL, adrenocorticotropin, thyrotropin (TSH), follicle-stimulating hormone, and luteinizing hormone; the intermediate lobe, which synthesizes and secretes melanocyte-stimulating hormone (MSH); and the posterior or neural lobe, which stores and releases the hypothalamic hormones vasopressin (AVP) and oxytocin. Anatomically, the anterior lobe is loosely joined to the intermediate lobe, whereas the intermediate and neural lobes are tightly joined to each other, forming a single neurointermediate lobe (Fig. 1A). The anatomical disposition of the anterior and neurointermediate lobes, the long pituitary stalk, and the median eminence permit with relative ease the performance of complete HYPOX or selective surgical separation of the two pituitary lobes without damage to neighboring structures (Fig. 1B) (55). Pituitary thyrotrophs respond to thyroid hormone deficiency with hypertrophy and hyperplasia and an increase in TSH secretion (54). In order to determine if neurointermediate pituitary lobectomy (NIL) causes damage to the anterior pituitary lobe or to the portal vessels of the hypothalamic-pituitary circulatory system, the pituitary histology and TSH plasma levels in groups of animals who had sham operations, thyroidectomy (TX), NIL, and NIL plus TX were evaluated. Histological results showed scar tissue around the gland and small isolated remnants of the intermediate lobe. In the adenohypophysis, no signs of infarct or injury were observed. Compared with the sham operation, NIL did not affect adenohypophysial histology (Fig. 2A and B) and plasma TSH levels. On the other hand, animals that underwent TX and NIL plus TX developed hypertrophy and hyperplasia of thyrotrophs (Fig. 2C and D) and increased TSH plasma levels. These results indicate that NIL did not affect the hypothalamic-adenohypophysial vascular/functional connections (55).
FIG. 1.
Ventral views of brains from intact and NIL rats to show the pituitary stalk, intermediate and neural pituitary lobes (A), and the presence in the NIL rat of the pituitary stalk stump without the intermediate and posterior lobes (B). The pituitaries were lifted to show their dorsal surface with the several pituitary lobes.
FIG. 2.
Slides of adenohypophysial tissue from sham-operated (A), NIL (B), TX (C), and NIL plus TX (D) rats with staining by the hematoxylin-eosin method (×40). No signs of infarction or other histological lesions were found in NIL rats with or without TX, except scar tissue and small isolated remnants from the intermediate lobe. The inset square in each panel shows an amplified view of the pituitary tissues (×1,000). No histological differences between sham-operated (A) and NIL (B) animals were observed. Slides of tissues from NIL rats with or without TX (C and D) showed the cells typical of thyroidectomy.
Recently, we demonstrated that vasopressin from the neural lobe is involved in the control of humoral and cell-mediated immune responses. NIL rats immunized with sheep red blood cells or dinitrochlorobenzene showed decreased immune responses (51). Decreased autoimmune response was also evident in HYPOX and NIL rats with experimentally induced autoimmune encephalomyelitis (EAE) (53). Administration of desmopressin, a synthetic analog of vasopressin, to NIL and HYPOX animals restored and increased the susceptibility to EAE (52). All of these results suggest that anterior and posterior pituitary hormones participate as stimulating factors in the control of systemic and GALT immune responses. In the present work, HYPOX and NIL rats were orally infected with nonlethal doses of Salmonella serovar Typhimurium, and their systemic and intestinal immune responses were investigated.
MATERIALS AND METHODS
Animals.
Male Wistar rats weighing from 290 to 310 g from the Aguascalientes University colony were used. Animals were housed under controlled temperature (22 to 24°C) and light-dark conditions (lights on from 7 am to 7 pm). The diet consisted of Purina rat chow and tap water ad libitum. The diet of HYPOX rats was supplemented with sugar cookies and lettuce. The animals were habituated to our housing conditions for at least 7 days before surgery and were treated according to the Institutional Normative Welfare Standards.
Rats were divided into three groups: animals who underwent sham operations, HYPOX animals, and NIL animals. The groups contained 14 to 15 animals each (7 to 8 animals per cage).
Surgeries. (i) NIL operation.
The employed method for the NIL was the same as that described by Ben-Jonathan and Peters (5) as well as Mena et al. (39). Briefly, the rats were anesthetized with methyl ether, and the trachea was cannulated. Fifteen minutes before anesthesia, 0.06 mg atropine was administered subcutaneously to each rat to prevent excessive secretion in the respiratory tract. Removal of the neurointermediate lobe (posterior and intermediate lobes) of the pituitary was performed under a dissecting microscope through the parapharyngeal approach by gentle aspiration via a bent needle after an undisturbed view had been achieved. The total time of anesthesia did not exceed more than 15 min, and full recovery occurred within 30 to 60 min. After surgery, all animals that had undergone operation were injected with penicillin (Penprocilina, 5,000 IU intramuscularly; Lakeside, México) once daily for 3 days.
(ii) HYPOX and sham operations.
The procedure for HYPOX was the same as for NIL except that the entire pituitary gland was removed, whereas in sham-operated animals, the procedure was terminated when the pituitary capsule was surgically opened and the pituitary gland was directly visualized.
Salmonella serovar Typhimurium strain.
The bacterial strain was ATCC 14028, cultivated in brain heart infusion broth (Difco, Detroit, Mich.) for 18 to 24 h at 37°C under agitation. Bacteria were harvested at the end of the exponential growth phase and used for animal inoculation and for surface protein extraction.
Preparation of surface proteins of Salmonella serovar Typhimurium.
Harvested bacteria were washed once in Tricine {N-[Tris(hydroxymethyl)-methyl] glycine} buffer (pH 7.2) and extracted with buffered 6 M urea. The extract was dialyzed for 3 days against running tap water. The extract components were separated by centrifugation (34,800 × g, 60 min), and then the soluble material was kept at −20°C (26). The protein concentration was determined with a Bradford assay kit (Bio-Rad, D.F. México, Mexico).
Salmonella serovar Typhimurium immunization.
Salmonella serovar Typhimurium inoculations were carried out between 4 and 8 weeks after surgeries. Rats were pretreated orally with 1 ml of 0.1 M sodium bicarbonate solution and then orally infected with 108 CFU of Salmonella serovar Typhimurium through a plastic tube. Rats were monitored twice daily, and the percentage of survivors was recorded (1).
Intestinal colonization.
Intestinal colonization was determined by counting viable Salmonella serovar Typhimurium cells in feces by plating them on culture medium (SS agar; Difco, Detroit, Mich.). Fecal extracts were prepared by vortexing three fecal pellets from each rat in 1 ml of extraction buffer (phosphate-buffered saline [PBS] containing 0.02% azide, 1% bovine serum albumin, 1 mM phenylmethylsulfonyl fluoride, and 5 mM EDTA).
Animal sacrifice.
Nine days after the oral inoculation, rats were exsanguinated by cardiac puncture under ether anesthesia, and the sera were collected and stored at −20°C until IgG and IgM titers were measured by enzyme-linked immunosorbent assay (ELISA). Intestinal fluid was collected by passing 5 ml of PBS containing 0.05% bovine serum albumin and 0.02% sodium azide through the small intestine of each rat. The washout material was centrifuged at 10,000 × g for 20 min at 4°C, and the supernatant was harvested, lyophilized, and stored at −20°C until the measurement of total IgA by ELISA. The sella turcica was examined under a dissecting microscope for pituitary or neurointermediate lobe remnants. Only rats with successful HYPOX and NIL with no damage to the anterior lobe and complete removal of neural and intermediate lobes were included in the study. In HYPOX and NIL rats, a remnant pituitary stalk stump, measuring 0.2 to 0.5 mm, was usually found (Fig. 1).
Persistence of Salmonella serovar Typhimurium in organs.
To determine the persistence of Salmonella serovar Typhimurium in vivo, Peyer's patches and spleens were removed aseptically and spleens were weighed. Single-cell suspensions were harvested and treated with 0.1% Triton X-100, plated onto SS agar, and incubated overnight at 37°C. Colonies were subsequently counted.
ELISA for IgG, IgM, and IgA titers.
The optimal antibody dilutions were determined by checkerboard titration employing pools of sera from the nonimmunized and immunized rats as well as a control positive serum, obtained by repeated immunizations of rats with the bacterial proteins. The specificity of the indirect ELISA was confirmed by immunoblotting using randomized samples of serum and intestinal fluid and the same bacterial proteins. The antibodies in these samples recognized proteins with molecular weights between 200 and 25 kDa (data not shown).
Ninety-six-microwell polystyrene plates were coated with 20 μg of Salmonella serovar Typhimurium surface proteins in 0.1 M carbonate, pH 9.6. The plates were incubated for 18 h at 4°C, washed three times with PBS containing 0.05% Tween 20 (PBS-T), blocked by adding 100 μl of PBS-T with 5% skim milk, then again incubated for 1 h at 37°C, and washed 10 times with PBS-T. One hundred microliters of test serum or intestinal fluid (diluted 1:100 or 1:2 in PBS-T, respectively) were incubated at 4°C for 18 h. After washing the plates 10 times with PBS-T, 100 μl of a 1:10,000 dilution of secondary antibody (horseradish peroxidase-labeled goat anti-rat IgG, anti-rat IgM, or anti-rat IgA) was added to each well. After incubation for 1 h at 37°C, the plates were washed 10 times with PBS-T; then, the substrate solution (0.1% H2O2 plus 0.1% orthophenylenediamine in citrate phosphate buffer) was added (100 μl to each well); and after 15 min the reaction was stopped with 2.5 M sulfuric acid (100 μl). The optical density of each well was determined at 490 nm by using an enzyme immunoassay reader (Bio-Rad).
Statistics.
Data are presented as the mean ± standard deviation (SD). Statistical significances of the differences between experimental groups were determined by analysis of variance, followed by two-tailed Student's t test. Differences of P < 0.05 were considered significant.
RESULTS
Water intake and urine output.
Compared with the sham-operated group, HYPOX and NIL animals had significantly increased water intake and urine output (diabetes insipidus). NIL induced more marked polydipsia and polyuria than HYPOX; the differences were apparent through the entire experimental period (data not shown).
Intestinal colonization.
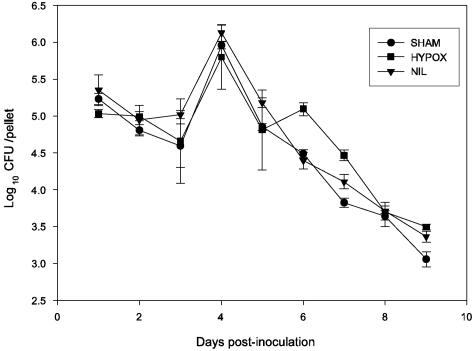
Bacterial number reached the maximum on the fourth day followed by a gradual decrease until the ninth day. No differences were noted in the kinetics of Salmonella elimination in the different groups (P > 0.05) (Fig. 3). No clinical signs of salmonellosis were observed during the course of infection, and there was no mortality.
FIG. 3.
Fecal excretion of Salmonella serovar Typhimurium. Rats were orally inoculated with 108 CFU of serovar Typhimurium, and the number of bacteria present in a pool of fecal pellets collected daily was measured by plating. Data are expressed as means ± SD of results from four to six rats per group. In all groups, the number of bacteria significantly increased by day 4 and gradually decreased by day 9 after inoculation. No statistical differences were observed in daily bacteria numbers between groups. The statistical evaluation was performed with analysis of variance applying the Bonferroni correction.
Bacterial counts in Peyer's patches and spleen.
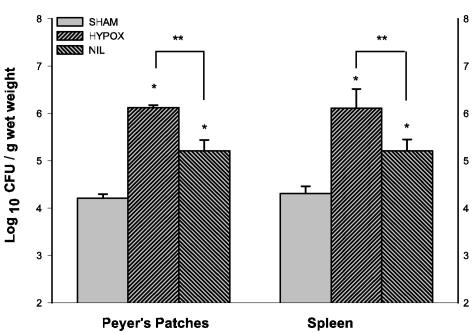
Nine days after inoculation, the number of Salmonella serovar Typhimurium cells in Peyer's patches and spleens of HYPOX and NIL groups was higher than in the sham-operated group (P < 0.001) (Fig. 4). The number of bacteria in HYPOX animals was significantly increased compared to the NIL group (P < 0.01).
FIG. 4.
Persistence of serovar Typhimurium infection in Peyer's patches and spleen. Sham-operated, HYPOX, and NIL groups were orally infected and sacrificed 9 days postinoculation. Tissues were aseptically removed and processed for bacterial quantification. Data are expressed as means ± SD of results from four to six rats per group. In Peyer's patches and spleens, bacterial counts were significantly higher in HYPOX and NIL groups than in the sham-operated group (*, P < 0.001) and significantly higher in the HYPOX group than in the NIL group (**, P < 0.01).
Intestinal IgA.
Nine days after Salmonella serovar Typhimurium inoculation, the titers of specific intestinal IgA response in HYPOX and NIL animals were significantly lower than in the sham-operated group (P < 0.001) (Fig. 5). IgA production was more markedly decreased in HYPOX rats than in NIL rats (P < 0.001).
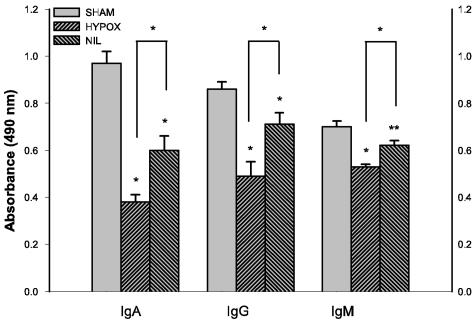
FIG. 5.
Intestinal IgA and serum IgG and IgM response to serovar Typhimurium. Intestinal IgA or serum IgG and IgM antibodies were quantified by ELISA using serovar Typhimurium surface antigens. Serum and gut samples were obtained 9 days postinoculation. The samples were assayed in triplicate, and the titers were expressed as the absorbance at 490 nm. Data are expressed as means ± SD of results from four to six rats per group. The immunoglobulin levels were significantly lower in HYPOX and NIL groups than in the sham-operated group (*, P < 0.001; **, P < 0.01) and significantly lower in the HYPOX group than in the NIL group (*, P < 0.001).
Serum IgG and IgM.
Serum levels of IgG and IgM were significantly lower in the HYPOX and NIL groups than in the sham-operated group (Fig. 5). Compared with NIL rats, lower levels of both immunoglobulins were found in HYPOX animals (P < 0.001) (Fig. 5).
DISCUSSION
The purpose of our work was to examine the effects of HYPOX and NIL on the severity of infection and plasma IgM and IgG and intestinal IgA responses to Salmonella serovar Typhimurium oral inoculation.
Oral doses of 108 CFU of Salmonella serovar Typhimurium did not cause clinical symptoms of salmonellosis; however, the intestinal lumen, the Peyer's patches, and the spleen were colonized by Salmonella serovar Typhimurium, and the development of adaptive immune responses to Salmonella serovar Typhimurium were obvious in the various experimental groups. Compared with sham operations, HYPOX and NIL resulted in a significant increase in bacterial colonization of Peyer's patches and spleens and a decrease of humoral immune responses. A significant difference in immunoglobulin responses was also observed between the HYPOX and NIL groups, with more marked impairment in HYPOX animals. These results indicate that hormones from the individual lobes participate as stimulating factors in the control of systemic and GALT immune responses. The effects of GH and PRL on the immune system have been established, whereas hormones of the neurointermediate lobe are at present subjects of intensive investigation.
Elimination of gram-negative bacteria (including Salmonella serovar Typhimurium) from the intestinal lumen depends on the production of humoral factors, such as lactoferrin, lysozyme, and defensins, by macrophages and other immune cells (2, 58, 60). In our HYPOX and NIL rats, intestinal elimination of Salmonella serovar Typhimurium was similar to that seen in sham-operated animals, indicating that pituitary hormones may not be necessary to resist intestinal Salmonella serovar Typhimurium infection. It is known that HYPOX animals develop an increased susceptibility to intraperitoneal Salmonella serovar Typhimurium infection, and GH and PRL treatments protect the rats against the disease (20, 23). Similarly, PRL increases the protection in normal mice after intraperitoneal inoculation of Salmonella serovar Typhimurium (17, 38). Since the immune responses are PRL and GH dependent and no pituitary hormones are produced in the HYPOX animals, the formation of anti-Salmonella serovar Typhimurium IgG, IgM, and IgA immunoglobulins is difficult to interpret. These results may be partially explained by the gradual increase in plasma PRL levels observed in the HYPOX animals, which reach values equivalent to 50% of that found in intact animals 7 to 9 weeks after surgery (45; A. Quintanar-Stephano and A. Organista-Esparza, unpublished). Although the source of this nonpituitary PRL is not known, one possibility is from the T lymphocytes. This hypothesis is supported by the demonstration that T lymphocytes in cell culture are able to synthesize and secrete PRL with biological activity (18, 62). Further studies are needed to prove this hypothesis.
After luminal infection, Salmonella serovar Typhimurium colonizes Peyer's patches and mesenteric lymph nodes and subsequently enters the enteric circulation via efferent lymph vessels, causing transient bacteremia and colonization of the spleen and liver. The number of Salmonella serovar Typhimurium cells in the Peyer's patches and the spleen was significantly higher in HYPOX and NIL rats than in intact rats, indicating that the total or partial ablation of the hypophysis increased susceptibility to infection after oral inoculation with Salmonella serovar Typhimurium. These findings demonstrate that the pituitary gland is required for protection against infection by intraperitoneal Salmonella inoculation (20).
The partial or total removal of the pituitary may affect the activity of phagocytes, the principal cells of the innate immunity involved in killing Salmonella serovar Typhimurium (33, 40). It was previously demonstrated that peritoneal macrophages from HYPOX rats have an impaired tumor necrosis factor alpha response to in vitro lipopolysaccharide stimulation (21) and are less effective in killing Salmonella serovar Typhimurium than those derived from rats with intact pituitaries (21).
GH injections enhanced resistance of both intact and HYPOX rats following a challenge with Salmonella serovar Typhimurium (21, 23). The enhanced resistance is correlated with the ability of peritoneal macrophages from these animals to generate toxic oxygen metabolites, such as superoxide anion and hydrogen peroxide (23). In addition, GH activates human monocytes for enhanced reactive oxygen intermediate production in vitro (65, 66).
The HYPOX rats had higher numbers of Salmonella serovar Typhimurium cells in Peyer's patches and spleen than sham-operated and NIL rats, suggesting that the low serum IgG and IgM and intestinal IgA immunoglobulin levels in HYPOX rats may be due to the insufficient immune-stimulating effect of the nonpituitary PRL (45). Further experiments are necessary to confirm this suggestion.
Most of the pituitary hormones directly or indirectly modulate inflammatory/immune responses. For example, adrenocorticotropin increases the secretion of GCs, which in turn stimulates the immune function at physiological doses (16, 42, 56, 59, 69). GH, PRL, TSH, and β-endorphin produced in the anterior pituitary and the AVP released from the posterior pituitary also have immunopotentiating and proinflammatory properties (29, 34, 47). Therefore, the differences between NIL and HYPOX rats may be related to the amount of hormones that regulate the immune response located in the anterior and posterior pituitary.
The fact that HYPOX induced a more marked decrease in the humoral immune responses to Salmonella serovar Typhimurium than NIL suggests that the hormones α-MSH, AVP, and oxytocin from the neurointermediate pituitary lobe may affect adaptive immune responses. The direct anti-inflammatory effects of α-MSH on immunocytes has been described previously (10, 13, 36, 63). Since NIL eliminates the intermediate lobe—the main source of pituitary α-MSH—an increased inflammatory response to Salmonella serovar Typhimurium infection may be expected. However, our results show that α-MSH from the intermediate pituitary lobe is not involved in the immune response to Salmonella serovar Typhimurium infection. Further experiments are required to test this possibility.
The presence of specific vasopressin receptors and the stimulatory effects of vasopressin on various immune cells, including peritoneal macrophages (25, 32), have been described (3, 11, 14, 15, 24, 30, 35, 64). In the present experiments, serum IgG and IgM and intestinal IgA titers in NIL animals were significantly decreased compared with the sham-operated rats but significantly higher than in the HYPOX animals. The cause of these reduced humoral immune responses may be the decreased secretion of the neurointermediate pituitary hormones. However, several studies suggest that lack of AVP may be responsible for the decreased immune responses. In previous experiments, we found that NIL decreased humoral and cell-mediated immune responses: NIL (i) decreased hemagglutination and IgG and IgM responses to sheep red blood cells (48, 51), (ii) decreased contact hypersensitivity to dinitrochlorobenzene (51), and (iii) protected against EAE (53). In order to determine whether AVP plays a role as an immunostimulating factor, we treated NIL animals with desmopressin, a synthetic analog of AVP. The results showed that desmopressin restored the susceptibility of NIL animals to EAE (52). In agreement with these previous findings, the present results suggest that the higher colonization of the Peyer's patches and spleens and the decreased IgG, IgM, and IgA responses to Salmonella serovar Typhimurium may be due to AVP deficiency in the NIL animals.
It was previously described that oxytocin acts on immune cells through the AVP receptors, although it requires a concentration 10 times higher than that of AVP to obtain similar effects on T cells for interferon gamma production (64). More studies are required to analyze the effects of oxytocin on humoral immune responses.
Peyer's patches and the spleen receive catecholaminergic, cholinergic, and peptidergic innervation, as well as important input from the sympathetic and parasympathetic divisions of the autonomic nervous system (4). The abundant innervation of the spleen and Peyer's patches provides the anatomical basis for the interaction between the nervous and immune systems. For example, norepinephrine-containing nerve fibers localized in Peyer's patches modulate the internalization of pathogenic Salmonella choleraesuis into isolated jejunal Peyer's patches (28). However, there are no neural pathways directly connecting the hypothalamus with the Peyer's patches and spleen. Therefore, the observed effects are probably the result of hormonal effects instead of the disruption of neural pathways.
Based on our experiments, it can be concluded that through different mechanisms, hormones from both the anterior and neurointermediate pituitary lobes play an important role in the control of systemic and gastrointestinal immune responses. However, more experiments are needed to establish the interactions between the hypothalamo-neurohypophysial (AVP) and immune systems.
Acknowledgments
We thank Alejandro Organista-Esparza, Humberto González-Velazco, Evangelina Delgado-González, Roberto Ramírez-Ruíz, Jesús Martínez, Pilar Galarza, and Allan Larsen for skillful technical assistance.
Editor: F. C. Fang
REFERENCES
- 1.Ascón, M. A., D. M. Hone, N. Walters, and D. W. Pascual. 1998. Oral immunization with a Salmonella vaccine vector expressing recombinant enterotoxigenic Escherichia coli K99 fimbriae elicits elevated antibody titers for protective immunity. Infect. Immun. 66:5470-5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayabe, T., D. P. Satchell, C. L. Wilson, W. C. Parks, M. E. Selsted, and A. J. Ouellette. 2000. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1:113-118. [DOI] [PubMed] [Google Scholar]
- 3.Bell, J., M. W. Adler, J. I. Greenstein, and L. Y. Liu-Chen. 1993. Identification and characterization of [125I]arginine vasopressin binding sites on human peripheral blood mononuclear cells. Life Sci. 52:95-105. [DOI] [PubMed] [Google Scholar]
- 4.Bellinger, D. L., D. Lorton, C. Lubahn, and D. L. Felten. 2001. Innervation of lymphoid organs—association of nerves with cells of the immune system and their implication in disease, p. 55-111. In R. Ader, D. L. Felten, and N. Cohen (ed.), Psychoneuroimmunology, 3rd ed. Academic Press, San Diego, Calif.
- 5.Ben-Jonathan, N., and L. L. Peters. 1982. Posterior pituitary lobectomy: differential elevation of plasma prolactin and luteinizing hormone in estrous and lactating rats. Endocrinology 110:1861-1865. [DOI] [PubMed] [Google Scholar]
- 6.Berczi, I., and A. Szentivanyi (ed.). 2003. Neuroimmmune biology, vol. 3. The immune-neuroendocrine circuitry. History and progress I, p. 495-536. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 7.Berczi, I., E. Nagy, K. Kovacs, and E. Horvath. 1981. Regulation of humoral immunity in rats by pituitary hormones. Acta Endocrinol. (Copenhagen) 98:506-513. [DOI] [PubMed] [Google Scholar]
- 8.Berczi, I., E. Nagy, S. L. Asa, and K. Kovacs. 1984. The influence of pituitary hormones on adjuvant arthritis. Arthritis Rheum. 27:682-688. [DOI] [PubMed] [Google Scholar]
- 9.Bienenstock, J. 1992. Cellular communication networks. Implications for our understanding of gastrointestinal physiology. Ann. N. Y. Acad. Sci. 664:1-9. [DOI] [PubMed] [Google Scholar]
- 10.Blalock, J. E. 1999. Proopiomelnocortin and the immune-neuroendocrine connection. Ann. N. Y. Acad. Sci. 885:161-172. [DOI] [PubMed] [Google Scholar]
- 11.Block, L. H., R. Locher, W. Tenschert, W. Siegenthaler, T. Hofmann, E. Mettler, and W. Vetter. 1981. 125I-8-L-arginine vasopressin binding to human mononuclear phagocytes. J. Clin. Investig. 68:374-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosch, J. A., C. Ring, E. J. de Geus, E. C. Veerman, and A. V. Amerongen. 2002. Stress and secretory immunity. Int. Rev. Neurobiol. 52:213-253. [DOI] [PubMed] [Google Scholar]
- 13.Catania, A., and J. M. Lipton. 1993. α-Melanocyte stimulating hormone in the modulation of host reactions. Endocr. Rev. 14:564-573. [DOI] [PubMed] [Google Scholar]
- 14.Chikanza, I. C., and A. S. Grossman. 1998. Hypothalamic-pituitary-mediated immunomodulation: arginine vasopressin is a neuroendocrine immune mediator. Br. J. Rheumatol. 37:131-136. [DOI] [PubMed] [Google Scholar]
- 15.Chikanza, I. C., P. Petrou, and G. Chrousos. 2000. Perturbations of arginine vasopressin secretion during inflammatory stress. Pathophysiologic implications. Ann. N. Y. Acad. Sci. 917:825-834. [DOI] [PubMed] [Google Scholar]
- 16.Chrousos, G. P. 1995. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 332:1351-1362. [DOI] [PubMed] [Google Scholar]
- 17.Di Carlo, R., R. Meli, M. Galdiero, I. Unzo, C. Bentivoglio, and C. R. Carratelli. 1993. Prolactin protection against lethal effects of Salmonella typhimurium. Life Sci. 53:981-989. [DOI] [PubMed] [Google Scholar]
- 18.Draca, S. 1995. Prolactin as an immunoreactive agent. Immunol. Cell Biol. 73:481-483. [DOI] [PubMed] [Google Scholar]
- 19.Dunn, A. J., T. Ando, R. F. Brown, and R. D. Berg. 2003. HPA axis activation and neurochemical responses to bacterial translocation from the gastrointestinal tract. Ann. N. Y. Acad. Sci. 992:21-29. [DOI] [PubMed] [Google Scholar]
- 20.Edwards, C. K., III, L. M. Yunger, R. M. Lorence, R. Dantzer, and K. W. Kelley. 1991. The pituitary gland is required for protection against lethal effects of Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 88:2274-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards, C. K., III, R. M. Lorence, D. M. Dunham, S. Arkins, L. M. Yunger, J. A. Greager, R. J. Walter, R. Dantzer, and K. W. Kelley. 1991. Hypophysectomy inhibits the synthesis of tumor necrosis factor alpha by rat macrophages: partial restoration by exogenous growth hormone or interferon gamma. Endocrinology 128:989-996. [DOI] [PubMed] [Google Scholar]
- 22.Edwards, C. K., III, S. Arkins, L. M. Yunger, A. Blum, R. Dantzer, and K. W. Kelley. 1992. The macrophage-activating properties of growth hormone. Cell. Mol. Neurobiol. 12:499-510. [DOI] [PubMed] [Google Scholar]
- 23.Edwards, C. K., III, S. M. Ghiasuddin, L. M. Yunger, R. M. Lorence, S. Arkins, R. Dantzer, and K. W. Kelley. 1992. In vivo administration of recombinant growth hormone or gamma interferon activities macrophages: enhanced resistance to experimental Salmonella typhimurium infection is correlated with generation of reactive oxygen intermediates. Infect. Immun. 60:2514-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elands, J., A. Resnik, and E. R. De Kloet. 1990. Neurohypophyseal hormone receptors in the rat thymus, spleen, and lymphocytes. Endocrinology 126:2703-2710. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Repollet, E., S. Opava-Stitzer, S. Tiffany, and A. Schwartz. 1983. Effects of endogenous antidiuretic hormone (ADH) on macrophage phagocytosis. J. Histochem. Cytochem. 31:956-959. [DOI] [PubMed] [Google Scholar]
- 26.Foulaki, K., W. Gruber, and S. Schlecht. 1989. Isolation and immunological characterization of a 55-kilodalton surface protein from Salmonella typhimurium. Infect. Immun. 57:1399-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuzuka, K., C. K. Edwards III, M. Clare-Salzer, E. M. Copeland III, L. L. Moldawer, and D. W. Mozingo. 2000. Glucocorticoid and Fas ligand induced mucosal lymphocyte apoptosis after burn injury. J. Trauma 49:710-716. [DOI] [PubMed] [Google Scholar]
- 28.Green, B. T., M. Lyte, A. Kulkarni-Narla, and D. R. Brown. 2003. Neuromodulation of enteropathogen internalization in Peyer's patches from porcine jejunum. J. Neuroimmunol. 141:74-78. [DOI] [PubMed] [Google Scholar]
- 29.Heijnen, C. J., A. Kavelaars, and R. E. Ballieux. 1991. Beta-endorphin: cytokine and neuropeptide. Immunol. Rev. 119:41-63. [DOI] [PubMed] [Google Scholar]
- 30.Hu, S. B., Z. S. Zhao, C. Yhap, A. Grinberg, S. P. Huang, H. Westphal, and P. Gold. 2003. Vasopressin receptor 1a-mediated negative regulation of B cell receptor signaling. J. Neuroimmunol. 135:72-81. [DOI] [PubMed] [Google Scholar]
- 31.Jones, W. G., II, J. P. Minei, R. P. Richardson, T. J. Fahey III, S. E. Calvano, A. C. Anyonacci, and G. T. Shires III. 1990. Pathophysiologic glucocorticoid elevations promote bacterial translocation after thermal injury. Infect. Immun. 58:3257-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khegai, I. I., M. A. Gulyaeva, N. A. Popova, L. A. Zakharova, and L. N. Ivanova. 2003. Immune system in vasopressin-deficient rats during ontogeny. Bull. Exp. Biol. Med. 136:448-450. [DOI] [PubMed] [Google Scholar]
- 33.Kirby, A. C., U. Yrlid, and M. J. Wick. 2002. The innate immune response differs in primary and secondary salmonella infection. J. Immunol. 169:4450-4459. [DOI] [PubMed] [Google Scholar]
- 34.Klein, J. R. 2003. Physiological relevance of thyroid stimulating hormone and thyroid stimulating hormone receptor in tissues other than the thyroid. Autoimmunity 36:417-421. [DOI] [PubMed] [Google Scholar]
- 35.Locher, R., W. Vetter, and L. H. Block. 1983. Interactions between 8-L-arginine vasopressin and prostaglandin E2 in human mononuclear phagocytes. J. Clin. Investig. 71:884-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luger, T. A., T. E. Scholzen, T. Brzoska, and M. Bohm. 2003. New insights into the functions of alpha-MSH and related peptides in the immune system. Ann. N. Y. Acad. Sci. 994:133-140. [DOI] [PubMed] [Google Scholar]
- 37.Madden, K. S., and D. L. Felten. 1995. Experimental basis for neural-immune interactions. Physiol. Rev. 75:77-106. [DOI] [PubMed] [Google Scholar]
- 38.Meli, R., G. M. Raso, C. Bentivoglio, I. Nuzzo, M. Galdiero, and R. Di Carlo. 1996. Recombinant human prolactin induces protection against Salmonella typhimurium infection in the mouse: role of nitric oxide. Immunopharmacology 34:1-7. [DOI] [PubMed] [Google Scholar]
- 39.Mena, F., D. Aguayo, M. Vigueras, A. Quintanar-Stephano, G. Perera, and T. Morales. 1996. Effect of posterior pituitary lobectomy on in vivo and in vitro secretion of prolactin in lactating rats. Endocrine 5:285-290. [DOI] [PubMed] [Google Scholar]
- 40.Mittrücker, H. W., and S. H. Kaufmann. 2000. Immune response to infection with Salmonella typhimurium in mice. J. Leukoc. Biol. 67:457-463. [DOI] [PubMed] [Google Scholar]
- 41.Mowat, A. M., and J. L. Viney. 1997. The anatomical basis of intestinal immunity. Immunol. Rev. 156:145-166. [DOI] [PubMed] [Google Scholar]
- 42.Munck, A., and A. Naray-Fejes-Toth. 1992. The ups and downs of glucocorticoid physiology. Permissive and suppressive effects revisited. Mol. Cell. Endocrinol. 90:C1-C4. [DOI] [PubMed] [Google Scholar]
- 43.Nagura, H., M. Kimura, M. Kubota, and N. Kimura. 1994. Neuroendocrine-immune interaction in the gastrointestinal tract, p. 253-265. In I. Berczi and J. Szelenyi (ed.), Advances in psychoneuroimmunology. Plenum, New York, N.Y.
- 44.Nagy, E., and I. Berczi. 1981. Prolactin and contact sensitivity. Allergy 36:429-431. [DOI] [PubMed] [Google Scholar]
- 45.Nagy, E., and I. Berczi. 1991. Hypophysectomized rats depend on residual prolactin for survival. Endocrinology 128:2776-2784. [DOI] [PubMed] [Google Scholar]
- 46.Nagy, E., I. Berczi, and H. G. Friesen. 1983. Regulation of immunity in rats by lactogenic and growth hormones. Acta Endocrinol. (Copenhagen) 102:351-357. [DOI] [PubMed] [Google Scholar]
- 47.Navolotskaya, F. V., N. V. Malkova, T. A. Zargarova, T. N. Lepikhova, S. B. Krasnova, and V. M. Lipkin. 2002. Effect of synthetic beta-endorphin-like peptide immunorphin on human T lymphocytes. Biochemistry (Moscow) 67:357-363. [DOI] [PubMed] [Google Scholar]
- 48.Organista-Esparza, A., M. Tinajero-Ruelas, M. Medina-Fernández, I. O. Sánchez-Herrera, and A. Quintanar-Stephano. 2003. Effects of neurointermediate pituitary lobectomy and hypophysectomy on humoral immune response in the Wistar rat, p. 96. In Mexican Society of Physiological Sciences (ed.), XLVI National Congress of Physiological Sciences, Aguascalientes, México.
- 49.Ottaway, C. A. 1991. Neuroimmunomodulation in the intestinal mucosa. Gastroenterol. Clin. N. Am. 20:511-529. [PubMed] [Google Scholar]
- 50.Pascual, D. W., and K. L. Bost. 2005. Neuropeptides for mucosal immunity, p. 737-748. In J. Mestecky, M. E. Lamm, W. Strober, J. Bienenstock, J. R. McGhee, and L. Mayer (ed.), Mucosal immunology, 3rd ed. Elsevier, Amsterdam, The Netherlands.
- 51.Quintanar-Stephano, A., K. Kovacs, and I. Berczi. 2004. Effects of neurointermediate pituitary lobectomy on humoral and cell-mediated immune responses in the rat. Neuroimmunomodulation 11:233-239. [DOI] [PubMed] [Google Scholar]
- 52.Quintanar-Stephano, A., A. Organista-Esparza, M. Tinajero-Ruelas, R. Chavira-Ramírez, M. Medina-Fernández, I. O. Sánchez-Herrera, I. Berczi, and K. Kovacs. 2004. Effects of neurointermediate pituitary lobectomy and desmopressin on experimental autoimmune encephalomyelitis in rats. Presented at Experimental Biology 2004 FASEB Meeting, Washington, D.C.
- 53.Quintanar-Stephano, A., R. Chavira-Ramírez, K. Kovacs, and I. Berczi. 2005. Neurointermediate pituitary lobectomy decreases the incidence and severity of experimental autoimmune encephalomyelitis in Lewis rats. J. Endocrinol. 184:51-58. [DOI] [PubMed] [Google Scholar]
- 54.Quintanar-Stephano, A., and C. Valverde. 1997. Mitogenic effects of thyroxine and TRH on the thyrotrophs and somatotrophs of the anterior pituitary gland in thyroidectomized rats. J. Endocrinol. 154:149-153. [DOI] [PubMed] [Google Scholar]
- 55.Quintanar-Stephano, A., I. Villalpando-Fierro, P. Damián-Matsumura, M. Tinajero-Ruelas, G. Lucero-Moreno, E. Martínez-Carrillo, and K. Kovacs. 2001. Effects of protracted neurointermediate pituitary lobectomy on hormone secretion on number of each type of endocrine cell in the anterior pituitary lobe of rats. Endocr. Pathol. 12(Suppl.):235-237. [Google Scholar]
- 56.Reichlin, S. 1993. Neuroendocrine-immune interactions. N. Engl. J. Med. 329:1246-1252. [DOI] [PubMed] [Google Scholar]
- 57.Roy, M. J., and T. J. Walsh. 1992. Histopathologic and immunohistochemical changes in gut-associated lymphoid tissues after treatment of rabbits with dexamethasone. Lab. Investig. 66:437-443. [PubMed] [Google Scholar]
- 58.Salzman, N. H., D. Ghosh, K. M. Huttner, Y. Paterson, and C. L. Bevins. 2003. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422:522-526. [DOI] [PubMed] [Google Scholar]
- 59.Sapolsky, R. M., L. M. Romero, and A. U. Munck. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory and preparative actions. Endocr. Rev. 21:55-89. [DOI] [PubMed] [Google Scholar]
- 60.Selsted, M. E., S. L. Miller, A. H. Henschen, and A. J. Ouellette. 1992. Enteric defensins: antibiotic peptide components of intestinal host defense. J. Cell Biol. 118:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sternberg, E. M. 2001. Neuroendocrine regulation of autoimmune/inflammatory disease. J. Endocrinol. 169:429-435. [DOI] [PubMed] [Google Scholar]
- 62.Stevens, A., D. W. Ray, J. Worthington, and J. R. Davis. 2001. Polymorphisms of the human prolactin gene—implications for production of lymphocyte prolactin and systemic lupus erythematosus. Lupus 10:676-683. [DOI] [PubMed] [Google Scholar]
- 63.Taylor, A. W. 2003. Modulation of regulatory T cell immunity by the neuropeptide alpha-melanocyte stimulating hormone. Cell Mol. Biol. 49:143-149. [PubMed] [Google Scholar]
- 64.Torres, B. A., and H. M. Johnson. 1997. Neuroendocrine peptide hormone regulation of immunity. Chem. Immunol. 69:155-184. [DOI] [PubMed] [Google Scholar]
- 65.Warwick-Davies, J., D. B. Lowrie, and P. J. Cole. 1995. Growth hormone is a human macrophage activating factor. Priming of human monocytes for enhanced release of H2O2. J. Immunol. 154:1909-1918. [PubMed] [Google Scholar]
- 66.Warwick-Davies, J., D. B. Lowrie, and P. J. Cole. 1995. Growth hormone activation of human monocytes for superoxide production but not tumor necrosis factor production, cell adherence, or action against Mycobacterium tuberculosis. Infect. Immun. 63:4312-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Webster, J. I., and E. M. Sternberg. 2004. Role of hypothalamic-pituitary-adrenal axis, glucocorticoids and glucocorticoid receptors in toxic sequelae of exposure to bacterial and viral products. J. Endocrinol. 181:207-221. [DOI] [PubMed] [Google Scholar]
- 68.Webster, J. I., L. Tonelli, and E. M. Sternberg. 2002. Neuroendocrine regulation of immunity. Annu. Rev. Immunol. 20:125-163. [DOI] [PubMed] [Google Scholar]
- 69.Wiegers, G. J., and J. M. H. M. Reul. 1998. Induction of cytokine receptors by glucocorticoids: functional and pathological significance. Trends Pharmacol. Sci. 19:317-321. [DOI] [PubMed] [Google Scholar]