Abstract
Enterotoxigenic Escherichia coli (ETEC) and enteropathogenic E. coli (EPEC) are common causes of diarrhea in children in developing countries. Dual infections with both pathogens have been noted fairly frequently in studies of diarrhea around the world. In previous laboratory work, we noted that cholera toxin and forskolin markedly potentiated EPEC-induced ATP release from the host cell, and this potentiated release was found to be mediated by the cystic fibrosis transmembrane conductance regulator. In this study, we examined whether the ETEC heat-labile toxin (LT) or the heat-stable toxin (STa, also known as ST) potentiated EPEC-induced ATP release. We found that crude ETEC culture filtrates, as well as purified ETEC toxins, did potentiate EPEC-induced ATP release in cultured T84 cells. Coinfection of T84 cells with live ETEC plus EPEC bacteria also resulted in enhanced ATP release compared to EPEC alone. In Ussing chamber studies of chloride secretion, adenine nucleotides released from the host by EPEC also significantly enhanced the chloride secretory responses that were triggered by crude ETEC filtrates, purified STa, and the peptide hormone guanylin. In addition, adenosine and LT had additive or synergistic effects in inducing vacuole formation in T84 cells. Therefore, ETEC toxins and EPEC-induced damage to the host cell both enhance the virulence of the other type of E. coli. Our in vitro data demonstrate a molecular basis for a microbial interaction, which could result in increased severity of disease in vivo in individuals who are coinfected with ETEC and EPEC.
Along with rotavirus, enterotoxigenic Escherichia coli (ETEC) and enteropathogenic E. coli (EPEC) are among the most common causes of diarrhea in children in developing countries (29). Dual infections with both pathogens have been noted fairly frequently in studies of diarrhea around the world for many years (41, 42). More recent studies of diarrhea etiology have begun to describe multiple infections carefully, and evidence is emerging that patients with multiple pathogens are likely to experience more-severe diarrheal disease (2, 8, 24, 35, 40, 59).
One might expect dual infections with EPEC and ETEC to occur occasionally by chance alone, especially in poverty-stricken areas with poor hygiene. However, studies of diarrhea etiology in areas with good sanitation also noted dual infections with EPEC and ETEC. The tendency of EPEC and ETEC to occur in multiple infections has been noted in epidemiology surveys in developed countries such as northern Italy (8), Israel (24), and Yugoslavia prior to the Balkan war (13). EPEC and ETEC coinfection was also found in an investigation of a hospital outbreak in Durban, South Africa (1). Several of the children in the latter outbreak had a triple infection with EPEC, ETEC, and rotavirus.
Recent work from our laboratory suggested a molecular mechanism by which EPEC and ETEC might interact. Specifically, we found that cholera toxin (CT) and forskolin enhance EPEC-induced ATP release from the host cell (15) without any enhancement of EPEC-mediated cell killing. In a subsequent study, we determined that cholera toxin and forskolin enhance cystic fibrosis transmembrane conductance regulator (CFTR)-dependent release of ATP from the host cell (14). CT and forskolin raise cyclic AMP (cAMP) levels in the host cell, activate cAMP-dependent protein kinase, and result in the phosphorylation and activation of CFTR (28, 45). Since the ETEC heat-labile toxin (LT) and ETEC heat-stable toxin STa (also called ST) also act by elevating cyclic nucleotides in the host cell, we suspected that ETEC toxins or ETEC infection might enhance EPEC-induced ATP release. In addition, we wished to determine if the chloride secretory responses triggered by adenine nucleotides in epithelial tissues were additive with those triggered by ETEC toxins.
MATERIALS AND METHODS
Bacterial strains used.
Four well-studied classic human EPEC strains were used in this investigation: E2348/69 (serotype O127:H6), B171-8 (O111:NM), JCP88 (O119:B14) and E851/71 (O142:H6), as described in several publications (4, 23, 37, 46, 47). Enterotoxigenic E. coli strain H10407 (serotype O78: H11), which produces both LT-I and the heat-stable enterotoxin (STa), was used as a prototype ETEC strain and was a gift from James Fleckenstein, University of Tennessee (26). A genetically altered version of strain H10407 was also provided by James Fleckenstein, who had constructed a deletion of the gene encoding the A subunit of LT, eltA. James Fleckenstein designated the LT-deleted strain JF570, but in this report we have referred to it as H10407ΔLT.
Materials.
The following reagents were obtained from Sigma-Aldrich Chemicals (St. Louis, Mo.): α,β-methylene-ADP, creatine kinase, phosphocreatine, forskolin, adenosine, AMP, ADP, type III collagen, E. coli heat-stable toxin, carbachol, and phosphatidylinositol-specific phospholipase C (PI-PLC, from Bacillus cereus). Guanylin was purchased from Bachem Bioscience, Inc. (King of Prussia, Pa.), and cholera toxin was from List Biological Laboratories (Campbell, Calif.). U73122, an inhibitor of PI-PLC, was from Biomol (Plymouth Meeting, Pa.). Purified LT-II toxins were endotoxin free and were gifts from Terry Connell, Department of Microbiology, University at Buffalo.
Preparation of sterile filtrates of overnight cultures of ETEC strains.
Strain H10407 or H10407ΔLT were grown overnight in Casamino Acids-yeast extract medium (CAYE) supplemented with trace minerals. CAYE medium was 2% Casamino Acids, 0.6% yeast extract, 43 mM NaCl, 38 mM K2HPO4, 0.25% glucose, and 0.1% trace minerals as previously described (55). Cultures were at 37°C with shaking at 300 rpm. The overnight cultures were centrifuged at 3,000 × g for 10 min to pellet the bulk of the bacteria, and then the supernatant was filtered through a 0.45-μm syringe-tip filter. Aliquots of this sterile filtrate were made and frozen at −70°C and used for subsequent experiments up to 2 months after preparation.
Ussing chamber studies.
Ussing chamber studies of secretion were performed on T84 cell monolayers grown in Snap-Well inserts (Corning Costar, Corning, NY). Caco-2 cells were also used for some Ussing chamber studies and gave similar results, but these results are not shown. The Snap-Well inserts, which had a 0.4-μm pore size, were coated with 32 μg collagen per well by the application of 0.16 ml of 0.2-mg/ml type III collagen (Sigma; dissolved in warm 0.2 M acetic acid) to the Snap-Well and allowing it to dry in the tissue culture hood under UV light.
T84 cells were seeded onto the Snap-Well inserts at ∼1.2 × 106 cells per well and allowed to grow to confluence for 7 to 9 days. At this time, the monolayers had transepithelial electrical resistances (TER) of 400 to 1,000 Ω · cm2.
A Snap-Well insert containing a monolayer of T84 cells was placed in the plexiglass “slider” and inserted into the Ussing chamber (Physiologic Instruments, San Diego, CA) at 37°C and continuously short circuited by a four-electrode, automatic voltage clamp apparatus which measured short-circuit current (Isc) and TER; chamber fluid resistance was automatically subtracted. The voltage clamp apparatus used was from Physiologic Instruments, model VCC MC6. Transepithelial resistance was determined by passing 10 s of 10-mV current pulses through the tissues. Short-circuit current was measured by passing sufficient current through the tissues via Ag-AgCl electrodes to reduce the spontaneous transepithelial potential to 0. The composition of the tissue bathing solution was (in micromoles): 140 Na+, 124 Cl−, 21 HCO3−, 5.4 K+, 2.4 HPO42−, 0.6 H2PO4−, 1.2 Mg2+, 1.2 Ca2+, and 10 glucose. The pH of this solution was 7.4 when gassed with a mixture of 95% O2 to 5% CO2. To help maintain adhesion of the monolayer to the Snap-Well insert, the apical side of the chamber was filled with 5 ml of this bath solution, and the basolateral compartment was filled with 4 ml of bath solution. The chambers were bubbled slowly with a 95% O2 to 5% CO2 gas mixture.
Ussing chamber current and resistance data were collected in digital form on a Dell PC computer using Acquire & Analyze 2.0 software that accompanied the instrument. Raw Isc values were converted to microamperes per square centimeters by dividing by the area of the monolayer (1.13 cm2).
ATP release.
ATP release was measured as previously described (14, 15) by preparing sterile filtrates of supernatant medium collected in the presence of an ATP-regenerating system and a nucleotidase inhibitor (α,β-methylene-ADP) and assaying for ATP with a luciferase luminescence assay kit (Roche Applied Science, Indianapolis, Ind.). The ATP-regenerating system is included to trap released ATP and prevent its degradation by nucleotidases. Some experiments (Fig. 1 and 2B) were done using cells grown in 96-well plates with 0.2 ml of medium per well; therefore, absolute ATP levels were lower than in other experiments performed in 48-well plates.
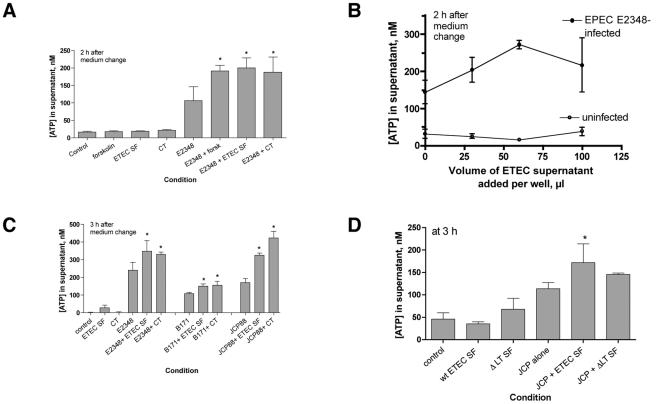
FIG. 1.
Effect of ETEC culture filtrates on EPEC-induced ATP release from host cells. Culture filtrates were prepared from overnight cultures of ETEC strain H10407 or H10407ΔLT (D) in CAYE medium as described in Materials and Methods. ETEC sterile filtrates were applied to T84 cells in 96-well plates for 18 to 20 h (60 μl per well unless otherwise indicated). The medium was changed to serum-free medium without antibiotics, and cells were infected with EPEC at an MOI of 100:1. After 45 min to allow EPEC adherence, the medium was changed again to include an ATP-regenerating system (creatine kinase plus phosphocreatine) and the nucleotidase inhibitor α,β-methylene-ADP; incubation was continued for 2 to 3 h as shown on the figures. Then, the plates were swirled to allow mixing to occur; aliquots were collected, subjected to filtration via a 0.45-μm filter to remove bacteria or detached host cells, and assayed for ATP. Abbreviations: forsk, 20 μM forskolin added the day of the experiment; ETEC SF, ETEC sterile filtrate; CT, 200-ng/ml cholera toxin; E2348, EPEC strain E2348/69; B171, EPEC strain B171-8; JCP, EPEC strain JCP88; wt, wild type; ΔLT SF, sterile filtrate prepared from strain H10407ΔLT.
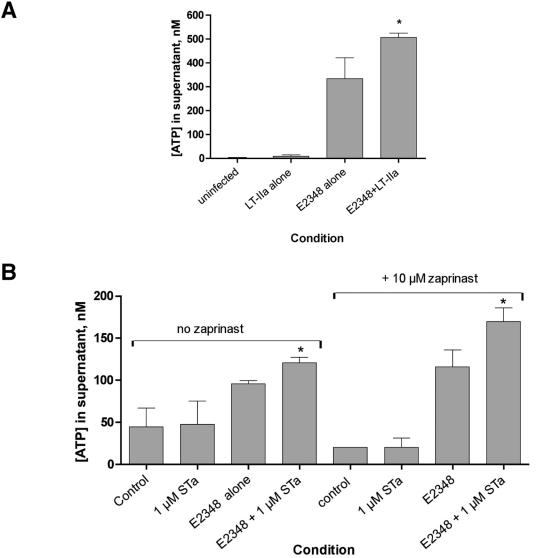
FIG. 2.
Effect of purified E. coli enterotoxins on EPEC-induced ATP release. T84 cells were grown in 48-well (A) or 96-well (B) plates. (A) Purified, pyrogen-free LT-IIa toxin at 720 ng/ml was applied overnight; on the next day, the medium was changed as described in the legend to Fig. 1 and in Materials and Methods. After EPEC infection, supernatant medium was collected 2 h after the medium change. (B) Purified STa was applied to some wells just before infection with EPEC strain E2348/69, and then STa was added again to the same 1 μM concentration after the medium change. The phosphodiesterase inhibitor Zaprinast (10 μM) was added to some wells after the medium change (B, right). Supernatants were collected 3 h after the medium change shown in panel B.
Vacuole formation studies.
T84 cells were grown to near confluence on Permanox plastic Lab-Tek chamber slides (Nunc-Intermed, Napierville, Ill.). Cells were treated with toxins or adenosine or inhibitors, fixed, stained with Giemsa stain as previously described (16), and then photographed at ×250 magnification without oil. Latex beads (2 μm in size; Sigma) were used for size determination.
Data analysis and presentation.
All error bars shown in graphs and error values reported in the text are standard deviations (SDs). Significance was tested by one-way analysis of variance with the Tukey-Kramer posttest for multiple comparisons, using InStat software for Macintosh from GraphPad software (San Diego, Calif.). Graphs were prepared using Prism 4.0 software, also from GraphPad. Asterisks shown on graphs indicate a P value of <0.05.
RESULTS
In initial experiments, we tested whether a crude sterile filtrate of ETEC culture medium could potentiate EPEC-induced ATP release. As with cholera toxin and forskolin, ETEC sterile filtrate alone did not trigger any ATP release from T84 cells (Fig. 1A, left). However, the ETEC sterile filtrate did potentiate EPEC-induced ATP release, and the magnitude of the potentiation was similar to that observed with full doses of cholera toxin and forskolin (Fig. 1A). When tested on T84 cell wells containing 200 μl of medium, the enhancing effects of ETEC sterile filtrate peaked when about 60 μl of filtrate was applied per well and then diminished if a larger volume was applied (Fig. 1B). The enhancing effect of ETEC sterile filtrate was observed with all wild-type EPEC strains tested (E2348/69, B171-8, and JCP88) (Fig. 1C), as well as EPEC strain E851/71 (Fig. 3). When a sterile filtrate was prepared from strain H10407ΔLT, the enhancing effect on ATP release appeared to be less than that of the wild-type ETEC filtrate (Fig. 1D), suggesting that the heat-labile toxin is an important, biologically active component of the sterile filtrate in filtrates of strain H10407.
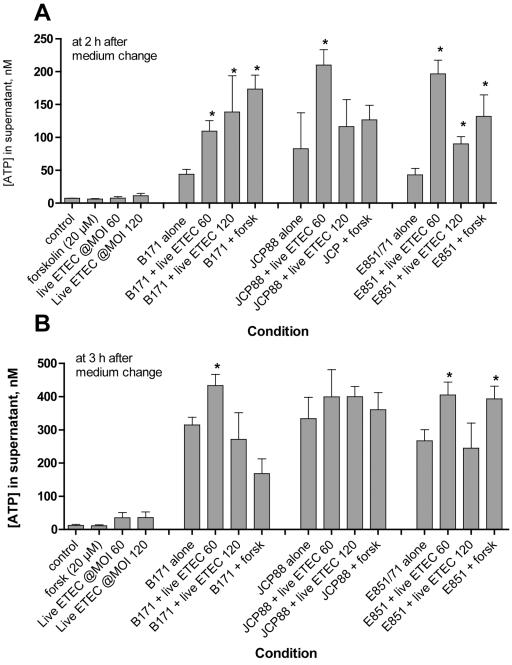
FIG. 3.
Effect of infection with live ETEC on EPEC-induced ATP release from T84 cells. ETEC strain H10407 was grown overnight in CAYE medium, and then T84 cells were infected with 2.5 μl or 5 μl of overnight culture, resulting in an ETEC MOI of 60 or 120, respectively. Overnight cultures of the EPEC strains, in contrast, were subcultured for 2 h in serum-free Dulbecco's modified Eagle's medium and then used to infect T84 cells in 48-well plates. The MOIs were 140, 260, and 120 for EPEC strains B171-8, JCP88, and E851/71, respectively. In this experimental design, therefore, the ETEC had a 2-h “head start” relative to the EPEC strains. Supernatants were collected at 2 and 3 h for the ATP assay as shown in the graphs.
Figure 2A shows that purified heat-labile toxin, in this case LT-IIa, also potentiated EPEC-induced ATP release from host cells in a manner similar to that of the crude sterile filtrate. When purified STa toxin was tested by this assay, it was also able to enhance EPEC-induced ATP release (Fig. 2B); this enhancement was of even greater magnitude when an inhibitor of cyclic GMP phosphodiesterase, Zaprinast, was included in the culture medium (Fig. 2B, right). Therefore, purified cholera toxin, a culture filtrate containing LT-I, purified LT-IIa, and purified STa were all able to potentiate EPEC-induced ATP release from T84 cells in this assay (Fig. 1 and 2).
The increased EPEC-induced ATP release by CT, LT, ETEC sterile filtrates, and STa toxin was not due to an increase in EPEC adherence to toxin-treated cells. EPEC adherence was the same in toxin-treated as in control T84 cells, as assessed by a visual adherence assay and by a quantitative adherence assay (measured as total cell-associated bacteria) (data not shown).
In the experiments shown in Fig. 1 and 2A, we applied ETEC sterile filtrates or purified LT or CT to the monolayers overnight to allow time for the full intoxication of cells before challenge with EPEC infection. We also wished to test whether infection with live ETEC bacteria would potentiate EPEC-induced ATP release. Figure 3 shows the result of one such experiment in which live ETEC bacteria were allowed to infect the T84 monolayer for 2 h before the cells were infected with the indicated EPEC strain. As shown in Fig. 3, potentiation of ATP release in this dual infection was best observed at early times after EPEC infection and was most obvious when the ETEC infection was at a low multiplicity of infection (MOI) of 60:1. At later times or when a high ETEC MOI was used, ETEC potentiation of EPEC-induced ATP release was less prominent or was not observed at all (Fig. 3B and data not shown).
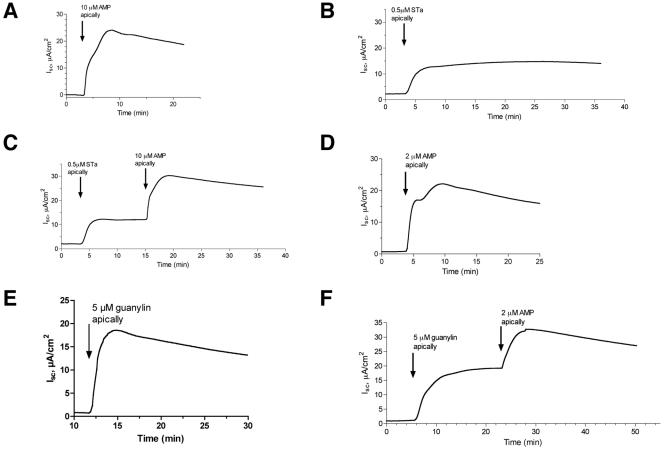
The results of Fig. 1 to 3 indicated that ETEC toxins could potentiate one type of cellular damage caused by EPEC, namely, ATP release. We also wondered if the interaction might act in the other direction, i.e., whether nucleotides released from the host by EPEC might enhance the effects of the ETEC toxins on secretory response in intestinal tissues studied in the Ussing chamber. Figures 4 and 5 show the results of those studies, in which short-circuit current reflects chloride secretion in T84 cell monolayers (20). Figure 4A shows that 10 μM AMP produced an Isc response that was brisk in onset, sustained in duration, and about as large as or even larger than that produced by the ETEC culture filtrate (Fig. 4B), purified STa toxin (Fig. 5B), or guanylin (Fig. 5E). To our knowledge, ours is the first study to directly compare the secretory effects of adenine nucleotides in a head-to-head manner to those of the better-studied ETEC toxins and guanylin. Although we used AMP as the test nucleotide in the tracings shown in Fig. 4 and 5, AMP is in fact converted to adenosine by ecto-5′-nucleotidase (CD73) on the apical surface of the monolayer, as previously described by others and by our own laboratory (15, 51).
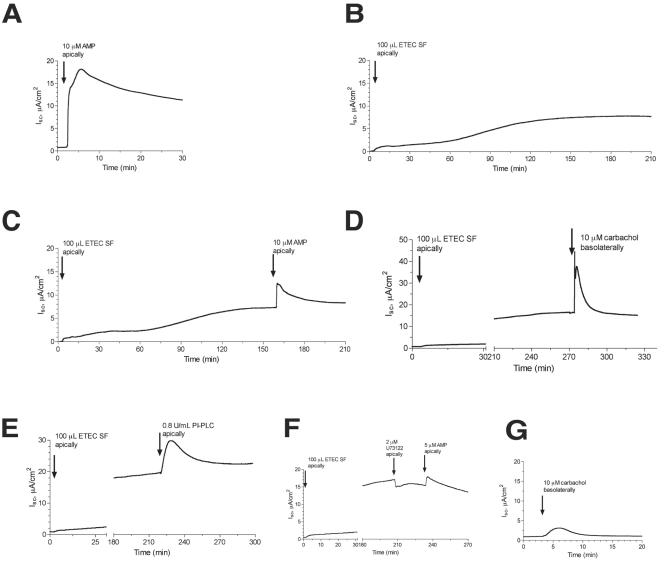
FIG. 4.
Additive effects of AMP and ETEC toxins on short-circuit current in T84 cell monolayers in the Ussing chamber. T84 cells were grown on collagen-coated Snap-Well culture inserts as described in Materials and Methods for 7 to 9 days until they reached confluence and a high transepithelial resistance. AMP, ETEC toxins, and PI-PLC were applied on the apical (i.e., mucosal or luminal) side of the monolayer, while carbachol was applied on the basolateral side. U73122 is an inhibitor of PI-PLC. Raw data (current, voltage, and resistance) were collected in a digital file and then imported into GraphPad Prism for the creation and labeling of the graphs shown. All experiments shown were repeated at least three times, and representative tracings are shown. Using this cell line, preparation, and electrode configuration, a positive short-circuit current (shown as an upward deflection on the graph) represents chloride secretion toward the apical side of the tissue.
FIG. 5.
Additive effects of AMP and STa toxin and guanylin on short-circuit current in T84 cell monolayers in the Ussing chamber. T84 cells were grown on collagen-coated Snap-Well culture inserts, as described in Materials and Methods and in the legend to Fig. 4. (A to C) Secretory effect of 10 μM AMP alone, STa alone, and STa followed by AMP. (D to F) Parallel series of tracings, except that the AMP concentration was reduced to 2 μM and guanylin was used instead of STa.
Having shown that adenine nucleotides can trigger a strong short-circuit current on their own, we next tested if the effects of AMP were additive with those of the potent ETEC toxins (Fig. 4 and 5). Since the ETEC toxins, especially the LTs, take some time to intoxicate the host cell and develop a secretory response, they were applied first. When the secretion was fully developed and had reached a plateau, AMP was added and the tracing was continued. In all cases, we noted that addition of AMP triggered an increase in Isc, even when added to tissues already intoxicated with ETEC sterile filtrate (Fig. 4C). In experiments similar to those shown in Fig. 4C, the addition of 5 or 10 μM AMP increased the peak Isc by 43.6% ± 19.8% above that observed with the ETEC sterile filtrate alone (data are means ± SD of five separate experiments using matched tissues). To determine the mechanism of the interaction between AMP and ETEC sterile filtrate, we tested the additive effects of 10 μM AMP in the presence of inhibitors and compared it to the additive effects of carbachol and other activators of secretory signaling. Figure 4D shows that the effect of carbachol, a muscarinic agonist, resembled that of AMP when carbachol was added with the ETEC sterile filtrate; in this case, carbachol was added to the basolateral side of the tissue because muscarinic receptors are localized there in polarized T84 monolayers. Likewise, the addition of purified PI-PLC to the apical side of the tissue mimicked the effect of AMP addition (Fig. 4E). In contrast, addition of 2 μM U73122, a PI-PLC inhibitor, nearly abolished the additive effects of AMP in this tissue model (Fig. 4F). We conclude that the additive effects of AMP added after ETEC filtrate are most likely due to its ability to raise intracellular calcium via activation of PI-PLC, rather than by further increasing intracellular cAMP levels (21, 38).
In Fig. 5, the effects of AMP added along with maximal concentrations of STa (Fig. 5C) or guanylin (Fig. 5F) were tested. Once again, the combination of AMP plus STa, or AMP plus guanylin, produced a short-circuit current larger than that observed with any of the agonists alone. In experiments similar to those shown in Fig. 5C and 5F, AMP plus STa increased the Isc by 100% ± 44% compared to STa alone, and AMP plus guanylin increased the Isc to a value of 73% ± 68% above that from guanylin alone (mean ± SD for three experiments with STa and three experiments with guanylin). If AMP was added first, subsequent addition of guanylin or STa still resulted in an additive secretory response, showing that the order of addition was not critically important (data not shown). Also, the additive interaction was observed with concentrations of AMP as low as 2 μM (Fig. 5D and 5F), concentrations shown to accumulate in response to EPEC infection in a tissue culture model (15).
In the experiments shown in Fig. 4 and 5, the combination of two secretory stimuli produced an Isc that was generally less than the arithmetic sum of the two agonists applied separately but greater than either agonist alone. In other words, the effects of AMP plus ETEC toxins were not supra-additive or truly synergistic compared to each agonist studied separately, but they did show an enhancing interaction. The lack of a synergistic response between LT toxin and AMP, for example (Fig. 4C), is not surprising, since LT acts via cyclic AMP and adenosine receptors are coupled to secretion at least in part via cyclic AMP (52). The results of Fig. 4 and 5, however, do demonstrate that adenine nucleotides released from host cells by EPEC infection can increase intestinal secretion even in the presence of potent E. coli enterotoxins and the hormone guanylin.
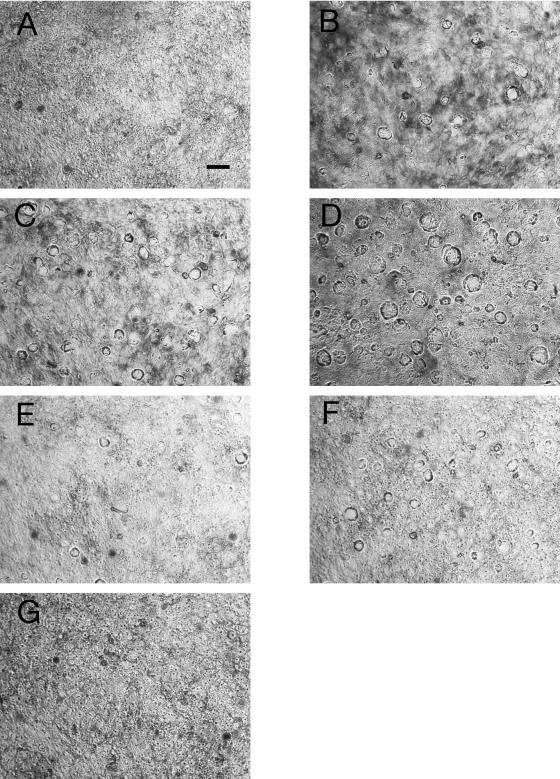
While performing the experiments shown in Fig. 1 to 4, some of us (S.S.C. and T.M.N.) often noted the appearance of large vacuoles in T84 cells treated with cholera toxin or ETEC culture filtrate containing LT-I. We investigated this morphological change by fixing, staining, and photographing the cells (Fig. 6). Figure 6A shows that normal control T84 cells do not contain visible vacuoles, a finding confirmed at higher magnifications (not shown). Adenosine (20 μM) triggers the formation of vacuoles after 3 h of incubation (Fig. 6B), as did 25 ng of CT/ml, a submaximal toxin dose (Fig. 6C). The combination of adenosine plus CT triggered formation of very large vacuoles throughout the monolayer (Fig. 6D), with vacuole size often exceeding 30 μm in diameter. AMP, ADP, and ATP triggered vacuole formation in a manner similar to that of adenosine, and vacuole formation was also observed in Caco-2 cells treated with CT or adenosine. In contrast, COS-7 cells showed no vacuole formation with these treatments (photographs not shown). Semiquantitative image analysis revealed that T84 cells treated with adenosine plus CT showed both a greater number of vacuoles and an increase in vacuole size compared to T84 cells treated with adenosine alone or CT alone. Cells treated with adenosine, CT, ETEC sterile filtrate, or combinations of toxin plus adenosine still showed a high TER in the Ussing chamber (Fig. 4 and 5 and tracings not shown), indicating that monolayer and tight junction integrity was maintained despite the dramatic morphological changes.
FIG. 6.
Additive effects of adenosine and cyclic AMP-elevating toxins on vacuole formation in T84 cells. T84 cells were grown to confluence on Permanox plastic Lab-Tek chamber slides and then treated with cholera toxin, ETEC culture filtrates, adenosine, or combinations of stimuli. Vacuoles were allowed to form for 3 or 16 h. Slides were fixed, stained with Giemsa, and photographed at a magnification of ×200 magnification for all panels. The size bar shown in panel A represents 30 μm, as determined with latex sizing beads. (A) Control T84 cells; (B) cells treated with 20 μM adenosine for 3 h; (C) cells treated with 25-ng/ml CT for 3 h; (D) cells treated with 20 μM adenosine and 25-ng/ml CT for 3 h, showing enhanced formation of vacuoles; (E) cells treated with 100 μl of a sterile filtrate of culture supernatant of ETEC strain H10407 for 19 h; (F) cells treated with 100 μl of ETEC sterile filtrate for 19 h with 20 μM adenosine added for the last 3 h; (G) cells treated for 19 h with a sterile filtrate of ETEC mutant H10407ΔLT.
Similarly, the combination of ETEC culture filtrate plus adenosine resulted in greater vacuole formation than ETEC sterile filtrate alone (Fig. 6, compare panels E and F). T84 cells treated with a filtrate from H10407ΔLT did not form visible vacuoles (Fig. 6G), indicating that it is the LT-I and not STa that is triggering vacuole formation.
Adenosine-induced vacuole formation was readily reversible within 24 h of removal of the adenosine, whereas CT-induced vacuolization persisted at least 24 h after a medium change to remove unbound toxin (data not shown). The giant vacuoles we observed in T84 cells (Fig. 6) closely resemble those reported by Barkla et al. in the LIM1863 colon carcinoma cells in terms of vacuole size, time of onset (1 to 3 h), and CT concentrations needed (10 to 100 ng/ml) (3). Using electron microscopy, Barkla et al. observed that CT triggered the formation of small vesicles at the cell apex that then ballooned to enormous size, which they felt was due to secretion of fluid into the vesicle lumen.
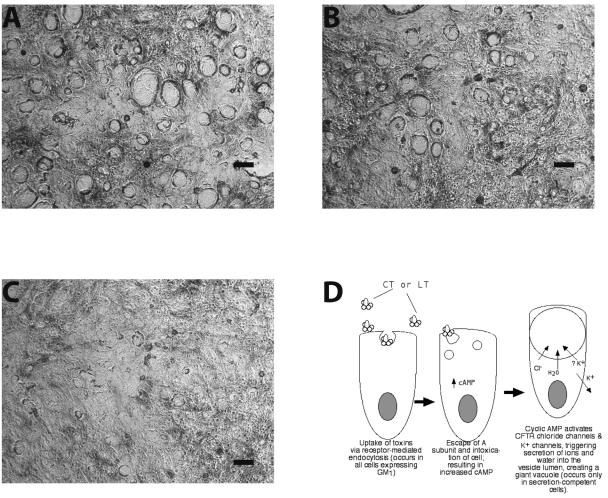
To determine if the theory proposed by Barkla et al. applied to the formation of large vacuoles in T84 cells, we investigated whether CT-induced vacuole production was blocked by inhibitors of the CFTR or other ion channels. Compared to cells treated with 50 ng of CT/ml alone (Fig. 7A), cells treated with CT plus 10 μM CFTRinh-172 showed a smaller average vesicle size (Fig. 7B). CFTRinh-172 is a cell-permeant thiazolidinone that is a potent and specific inhibitor of the CFTR chloride channel, which is localized to the apical membrane (39). Panel 6C shows that 100 μM glyburide (also called glibenclamide) inhibited CT-induced vacuole formation even more strongly than CFTRinh-172. Glyburide is a sulfonylurea drug that blocks one type of K+ efflux channel (7), as well as chloride efflux via the CFTR (33, 49, 56). Potassium channels maintain a negative intracellular potential inside the cell and therefore provide a driving force for continued secretion of chloride (32). The results of the inhibitor experiments suggest that the giant vacuoles observed in response to CT are generated by secretion of ions and fluid into the vesicle lumen (Fig. 7D). Adenosine receptors are also linked to elevation of cyclic AMP in the host cell, so adenosine would also stimulate fluid secretion into vesicles (Fig. 6B, D, and F).
FIG. 7.
Effect of ion channel inhibitors on cholera toxin-induced vacuole formation. T84 cells were grown as described in the legend to Fig. 6, treated with CT at 50 ng/ml for 3 h, fixed, stained, and photographed as before. Size bars, 30 μm. (A) Cells treated with CT alone; (B) cells treated with CT plus 10 μM CFTRinh-172, a thiazolidinone CFTR inhibitor; (C) cells treated with CT plus 100 μM glyburide, a sulfonylurea which is a CFTR and potassium channel inhibitor; (D) sketch depicting the proposed mechanism for the formation of giant vacuoles in T84 cells in response to CT and LT (adenosine receptors are omitted from the diagram for the sake of simplicity).
DISCUSSION
The work presented here shows that ETEC and EPEC bacteria mutually interact in a way that intensifies the pathogenic potential of both pathogens. In regard to ATP release from host cells, ETEC toxins and live ETEC bacteria enhance EPEC-induced ATP release in a supra-additive or synergistic fashion (Fig. 1 to 3). We have recently shown that the enhanced ATP release observed in response to cholera toxin and forskolin is via activation of an ATP efflux pathway mediated by the CFTR (14). CFTR-dependent ATP release in epithelial tissues had been previously described (5, 34, 54), but its activation in response to any microbial infection or toxin had not been previously noted. We believe that the enhancement of EPEC-induced ATP release by the LT and STa enterotoxins, as shown in Fig. 1 to 3, is via the same CFTR-dependent pathway.
In regard to chloride secretion, adenine nucleotides and adenosine released by EPEC infection act additively with ETEC toxins (Fig. 4 and 5); these additive effects are particularly strong with the heat-stable enterotoxin STa (Fig. 5A to C) and its endogenous analog guanylin (Fig. 5D to F). This second type of EPEC-ETEC interaction also involves CFTR but instead affects CFTR's chloride channel function rather than its ability to export ATP. The CFTR is a major chloride channel in the small and large intestines, and its channel activity is regulated by cyclic nucleotides, as well as by intracellular calcium and protein kinase C (PKC). Cholera toxin, LT, and STa all produce diarrhea by triggering CFTR chloride channel activity (9, 25, 27). In addition, adenine nucleotides on epithelial surfaces are broken down to adenosine, act on adenosine receptors, and trigger chloride secretion via CFTR (12, 15, 52, 53). Therefore, the EPEC-ETEC interactions described here, with regard to ATP release (Fig. 1 to 3) and chloride secretion in the Ussing chamber (Fig. 4), all involve different aspects of the functions of CFTR. As shown in Fig. 5 and 6, the interaction between adenosine and toxins in triggering vacuole formation may also involve the CFTR, as well as potassium and other ion channels.
In previous studies we noted another, separate interaction between EPEC and the STa enterotoxin that did not directly involve CFTR. We showed that early in EPEC infection, host cells become hypersensitive to STa, as manifested by an increase in cyclic GMP accumulation to the toxin (17). This hypersensitivity is due to activation of host PKC by EPEC (17), followed by PKC-mediated phosphorylation of guanylyl cyclase C (GC-C), the receptor for STa (18, 19). PKC-mediated phosphorylation of GC-C occurs at residue Ser1029 at the intracellular C-terminal domain of GC-C (57) and results in an increased number of GC-C molecules being expressed at the cell surface, as well as in increased guanylyl cyclase activity (19).
Therefore, including these earlier studies from our laboratory, we have now noted four different ways that EPEC and ETEC bacteria can interact. First, EPEC infection sensitizes cells to the effects of STa (17). Second, ETEC toxins synergistically increase EPEC-induced ATP release from host cells (Fig. 1 to 3). Third, adenine nucleotides act additively with ETEC toxins to trigger chloride secretion in intestinal tissues (Fig. 4 and 5). Fourth, adenosine and adenine nucleotides act additively or synergistically with CT and LT to induce vacuole formation in T84 cells (Fig. 6).
In the case of ETEC-EPEC interaction in vacuole formation, we cannot at present assert that vacuole formation directly causes disease or pathology induced by ETEC or EPEC infection. Vacuole formation was not noted in histological examination of the small intestine in naturally occurring cholera in humans (10) or in experimental cholera in dogs (22), although the colon was not studied in detail in those reports. In colonic cells, vacuole formation seems to be a distinctive morphological response to CT and LT along the lines of the CHO cell stretch response and Y1 adrenal cell rounding, which have been used as bioassays for the presence of these toxins. Since CT and LT are potent mucosal adjuvants, however, it is possible that enhanced vesicle and/or vacuole formation is a mechanism involved in the increased antigen presentation to immune cells in response to these toxins (11, 36, 43, 44, 50). Whether vacuole formation is viewed as pathological damage caused by the microbe or a host defense strategy, it is clear that there is an enhancing interaction between ETEC LT toxin and CT on the one hand and adenine nucleotides and adenosine on the other.
In all of these ways, in vitro studies of cultured cells indicate that EPEC and ETEC can mutually enhance the virulence of the other.
The importance of microbial interactions in disease in humans and animals has been a subject of increased interest recently; in many cases, the molecular bases for such interactions are coming to light (6). As mentioned in the introduction, dual infections involving EPEC and ETEC have frequently been noted in studies of diarrhea in children, but these were not recognized as examples of a microbial interaction because until now, no molecular mechanism had been identified for such an EPEC-ETEC interaction. Examples of possible EPEC-ETEC interactions have also been seen in animals. Wada and colleagues reported on a naturally occurring outbreak of unusually severe diarrhea among piglets, which was determined to be due to dual infection with EPEC (“attaching and effacing E. coli”) and ETEC (58). Other examples of dual infections with EPEC and another pathogen have also been reported. For example, Schauer et al. noted an outbreak of enterocolitis in rabbits due to the combination of rabbit EPEC and an intracellular pathogen, Lawsonia intracellularis (48). In a study of turkeys, Guy et al. found that dual infection with an avian EPEC and turkey coronavirus produced a much higher mortality rate than with either microbe singly (30).
If EPEC and ETEC mutually enhance each other's virulence, one might wonder why E. coli strains have not emerged with the genetic characteristics of both, given the ability of E. coli for horizontal exchange of genetic material. One answer may be that it is difficult for bacteria to express both the type II secretion system (used by Vibrio cholerae and ETEC for the secretion of CT and LT) and the type III secretion system (used by EPEC, EHEC, Salmonella, and others for secretion of effectors). However, another answer may be that such EPEC-ETEC hybrid strains are already starting to emerge. Hedberg et al. described a foodborne diarrheal outbreak due to an E. coli strain not fitting into the usual categories for diarrheagenic E. coli (31). This strain, serotype O39:NM, had the characteristics of an atypical EPEC but also expressed the enteroaggregative heat-stable toxin EAST1, which has the same mechanism of action as STa. In many countries, microbiology laboratories (including many state, provincial, and national reference laboratories) no longer test for EPEC or ETEC in fecal specimens. Therefore, EPEC-ETEC “hybrids” and strains similar to that described by Hedberg could already exist or could emerge without detection or recognition. Based on the results described here, etiologic and epidemiologic studies of diarrhea should be alert for dual infections with EPEC and ETEC, since our work and clinical observations would indicate that these dual infections could result in more-severe disease (1, 2).
Acknowledgments
We thank the NIAID, National Institutes of Health, for supporting this work via grant R21 AI 54892 (to J.K.C. and M.E.D.).
We thank Terry D. Connell, Department of Microbiology and Immunology, University at Buffalo, for providing purified LT-IIa and LT-IIb, and James Fleckenstein, University of Tennessee at Memphis, for providing ETEC strains H10407 and H10407ΔLT. We thank Alan S. Verkman, University of California at San Francisco, for providing CFTRinh-172.
Editor: J. D. Clements
REFERENCES
- 1.Adhikari, M., Y. Coovadi, and J. Hewitt. 1985. Enteropathogenic Escherichia coli (EPEC) and enterotoxigenic (ETEC) related diarrhoeal disease in a neonatal unit. Ann. Trop. Paediatr. 5:19-22. [DOI] [PubMed] [Google Scholar]
- 2.Albert, M., A. Faruque, S. Faruque, R. Sack, and D. Mahalanabis. 1999. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangaldesh. J. Clin. Microbiol. 37:3458-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barkla, D. H., R. H. Whitehead, and I. P. Hayward. 1992. Effects of cholera toxin on human colon carcinoma cell lines. Pathology 24:296-301. [DOI] [PubMed] [Google Scholar]
- 4.Bieber, D., S. Ramer, C.-Y. Wu, W. Murray, T. Tobe, R. Fernandez, and G. Schoolnik. 1998. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280:2114-2118. [DOI] [PubMed] [Google Scholar]
- 5.Braunstein, G. M., R. M. Roman, J. P. Clancy, B. A. Kudlow, A. L. Taylor, V. G. Shylonsky, B. Jovov, K. Peter, T. Jilling, I. I. Ismailov, D. J. Benos, L. M. Schwiebert, J. G. Fitz, and E. M. Schwiebert. 2001. Cystic fibrosis transmembrane conductance regulator facilitates ATP release by stimulating a separate ATP release channel for autocrine control of cell volume regulation. J. Biol. Chem. 276:6621-6630. [DOI] [PubMed] [Google Scholar]
- 6.Brogden, K., J. Guthmiller, and C. Taylor. 2005. Human polymicrobial infections. Lancet 365:253-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryan, J., and L. Aguilar-Bryan. 1999. Sulfonylurea receptors: ABC transporters that regulate ATP-sensitive K(+) channels. Biochim. Biophys. Acta 1461:285-303. [DOI] [PubMed] [Google Scholar]
- 8.Cevenini, R., O. Varoli, F. Rumpianesi, R. Mazzaracchio, A. Nanetti, and M. La Placa. 1985. A two-year longitudinal study on the etiology of acute diarrhea in young children in northern Italy. Microbiologica 8:51-58. [PubMed] [Google Scholar]
- 9.Chao, A., F. de Sauvage, Y.-J. Dong, J. Wagner, D. Goeddel, and P. Gardner. 1994. Activation of intestinal CFTR Cl-channel by heat-stable enterotoxin and guanylin via cAMP-dependent protein kinase. EMBO J. 13:1065-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, H.-C., V. Reyes, and J. Fresh. 1971. An electron microscopic study of the small intestine in human cholera. Virchows Arch. B Cell Pathol. 7:236-259. [DOI] [PubMed] [Google Scholar]
- 11.Cheng, E., L. Cardenas-Freytag, and J. D. Clements. 1999. The role of cAMP in mucosal adjuvanticity of Escherichia coli heat-labile enterotoxin (LT). Vaccine 18:38-49. [DOI] [PubMed] [Google Scholar]
- 12.Clancy, J. P., F. E. Ruiz, and E. J. Sorscher. 1999. Adenosine and its nucleotides activate wild-type and R117H CFTR through an A2B receptor-coupled pathway. Am. J. Physiol. 276:C361-C369. [DOI] [PubMed] [Google Scholar]
- 13.Cobeljic, M., D. Mel, L. Arsic, B. Sokolovski, B. Nikolovski, E. Sopovski, M. Kulazov, and S. Kalenic. 1989. The association of enterotoxigenic and enteropathogenic Escherichia coli and other enteric pathogens with childhood diarrhoea in Yugoslavia. Epidemiol. Infect. 103:53-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crane, J. K., T. M. Naeher, S. S, Choudhari, and E. M. Giroux. 2005. Two pathways for ATP release from host cells in enteropathogenic Escherichia coli infection. Am. J. Physiol. Gastrointest. Liver Physiol. 289:G407-G417. [DOI] [PubMed] [Google Scholar]
- 15.Crane, J., R. Olson, H. Jones, and M. Duffey. 2002. Release of ATP during host cell killing by enteropathogenic E. coli and its role as a secretory mediator. Am. J. Physiol. Gastrointest. Liver Physiol. 282:G74-G86. [DOI] [PubMed] [Google Scholar]
- 16.Crane, J. K., S. Majumdar, and D. P. Pickhardt. 1999. Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect. Immun. 67:2575-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crane, J. K., and J. S. Oh. 1997. Activation of host cell protein kinase C by enteropathogenic Escherichia coli. Infect. Immun. 65:3277-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crane, J. K., and K. L. Shanks. 1996. Phosphorylation and activation of the intestinal guanylyl cyclase receptor for Escherichia coli heat-stable toxin by protein kinase C. Mol. Cell. Biochem. 165:111-120. [DOI] [PubMed] [Google Scholar]
- 19.Crane, J. K., M. S. Wehner, E. J. Bolen, J. J. Sando, J. Linden, R. L. Guerrant, and C. L. Sears. 1992. Regulation of intestinal guanylate cyclase by the heat-stable enterotoxin of E. coli (STa) and protein kinase C. Infect. Immun. 60:5004-5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dharmsathaphorn, K., J. A. McRoberts, K. G. Mandel, L. D. Tisdae, and H. Masui. 1984. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am. J. Physiol. 246:G204-G208. [DOI] [PubMed] [Google Scholar]
- 21.Dharmsathaphorn, K., and S. J. Pandol. 1986. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J. Clin. Investig. 77:348-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott, H., C. Carpenter, R. Sack, and J. Yardley. 1970. Small bowel morphology in experimental canine cholera. Lab. Investig. 22:112-120. [PubMed] [Google Scholar]
- 23.Elliott, S. J., L. A. Wainwright, T. K. McDaniel, K. G. Jarvis, Y.-K. Deng, L.-C. Lai, B. P. McNamara, M. S. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus for enterocyte effacement (LEE) from enteropathogenic E. coli E2348/69. Mol. Microbiol. 28:1-4. [DOI] [PubMed] [Google Scholar]
- 24.Eshel, G., More, A., E. Gluskin, J. Karpuch, E. Azizi, G. Mundel, and M. Aladjem. 1990. Acute gastoenteritis due to double infection with enteropathogenic Escherichia coli or Salmonella and another pathogen. Isr. J. Med. Sci. 26:316-318. [PubMed] [Google Scholar]
- 25.Field, M., L. Graf, Jr., W. Laird, and P. Smith. 1978. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc. Natl. Acad. Sci. USA 75:2800-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleckenstein, J. M., D. J. Kopecko, R. L. Warren, and E. A. Elsinghorst. 1996. Molecular characterization of the tia invasion locus from enterotoxigenic Escherichia coli. Infect. Immun. 64:2256-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabriel, S. E., K. N. Brigman, B. H. Koller, R. C. Boucher, and M. J. Stutts. 1994. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science 266:107-109. [DOI] [PubMed] [Google Scholar]
- 28.Gadsby, D., and A. Nairn. 1999. Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiol. Rev. 79(Suppl. 1):S77-S107. [DOI] [PubMed] [Google Scholar]
- 29.Guerrant, R. L. 1985. Microbial toxins and diarrhoeal diseases: introduction and overview, p. 1-13. In D. Evered and J. Whelan (ed.), Microbial toxins and diarrhoeal disease. Ciba symposium 112. Pitman, London, United Kingdom. [DOI] [PubMed]
- 30.Guy, J., L. Smith, J. Breslin, J. Vaillancourt, and H. Barnes. 2000. High mortality and growth depression experimentally produced in young turkeys by dual infection with enteropathogenic Escherichia coli and turkey coronavirus. Avian Dis. 44:105-113. [PubMed] [Google Scholar]
- 31.Hedberg, C. W., S. J. Savarino, J. M. Besser, C. J. Paulus, V. M. Thelen, L. J. Myers, D. N. Cameron, T. J. Barrett, J. B. Kaper, M. T. Osterholm, W. Boyer, F. Kairis, L. Gabriel, J. Soler, L. Gyswyt, S. Bray, R. Carlson, C. Hooker, A. Fasano, K. Jarvis, T. McDaniel, and N. Tornieporth. 1997. An outbreak of foodborne illness caused by Escherichia coli O39-NM, an agent not fitting into the existing scheme for classifying diarrheogenic E. coli. J. Infect. Dis. 176:1625-1628. [DOI] [PubMed] [Google Scholar]
- 32.Hoque, K., V. Rajendran, and H. Binder. 2005. Zinc inhibits cAMP-stimulated Cl secretion via basolateral K-channel blockade in rat ileum. Am. J. Physiol. Gastrointest. Liver Physiol. 288:G956-G963. [DOI] [PubMed] [Google Scholar]
- 33.Hwang, T. H., J. S. Jung, H. R. Bae, I. Yun, and S. H. Lee. 1994. Stimulation of Cl− secretion by AlF4− and vanadate in T84 cells. J. Korean Med. Sci. 9:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang, Q., D. Mak, S. Dividas, E. Schweibert, A. Bragin, Y. Zhang, W. Skach, W. Guggino, J. Foskett, and J. Engelhardt. 1998. Cystic fibrosis transmembrane conductance regulator-associated ATP release is controlled by a chloride sensor. J. Cell Biol. 143:645-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keskimäki, M., L. Mattila, H. Peltola, and A. Siitonen. 2000. Prevalence of diarrheagenic Escherichia coli in Finns with or without diarrhea on a round-the-world trip. J. Clin. Microbiol. 38:4425-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lencer, W. I., T. R. Hirst, and R. K. Holmes. 1999. Membrane traffic and the cellular uptake of cholera toxin. Biochim. Biophys. Acta 1450:177-190. [DOI] [PubMed] [Google Scholar]
- 37.Levine, M., D. Nalin, R. Hornick, E. Bergquist, D. Waterman, C. Young, S. Sotman, and B. Rowe. 1978. Escherichia coli strains that cause diarrhea but do not produce heat-labile or heat-stable enterotoxins and are not invasive. Lancet i:1119-1122. [DOI] [PubMed] [Google Scholar]
- 38.Levine, S. A., M. Donowitz, A. J. M. Watson, G. W. G. Sharp, J. K. Crane, and C. S. Weikel. 1991. Characterization of the synergistic interaction of Escherichia coli heat-stable toxin and carbachol. Am. J. Physiol. Gastrointest. Liver Physiol. 261:G592-G601. [DOI] [PubMed] [Google Scholar]
- 39.Ma, T., J. Thiagarajah, H. Yang, N. Sonawane, C. Folli, L. Galietta, and A. Verkman. 2002. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera-toxin induced intestinal fluid secretion. J. Clin. Investig. 110:1651-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masoumi, J., M. Anwar, and S. Bokhari. 1995. Clinical features of infantile diarrhea associated with single or multiple pathogens. J. Pak. Med. Assoc. 45:266-269. [PubMed] [Google Scholar]
- 41.Mathewson, J., R. Oberhelman, H. DuPont, F. de la Cabada, and E. Vasquez-Garibay. 1987. Enteroadherent Escherichia coli as a cause of diarrhea among children in Mexico. J. Clin. Microbiol. 25:1917-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moyenuddin, M., K. Rahman, and D. Sack. 1987. The aetiology of diarrhoea in children at an urban hospital in Bangladesh. Trans. R. Soc. Trop. Med. Hyg. 81:299-302. [DOI] [PubMed] [Google Scholar]
- 43.Nawar, H. F., S. Arce, M. W. Russell, and T. D. Connell. 2005. Mucosal adjuvant properties of mutant LT-IIa and LT-IIb enterotoxins that exhibit altered ganglioside-binding activities. Infect. Immun. 73:1330-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrovska, L., L. Lopes, C. P. Simmons, M. Pizza, G. Dougan, and B. M. Chain. 2003. Modulation of dendritic cell endocytosis and antigen processing pathways by Escherichia coli heat-labile enterotoxin and mutant derivatives. Vaccine 21:1445-1454. [DOI] [PubMed] [Google Scholar]
- 45.Picciotto, M., J. Cohn, G. Bertuzzi, P. Greengard, and A. Nairn. 1992. Phosphorylation of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 267:12742-12752. [PubMed] [Google Scholar]
- 46.Rothbaum, R., A. McAdams, R. Giannella, and J. Partin. 1982. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology 83:441-454. [PubMed] [Google Scholar]
- 47.Rothbaum, R., J. Partin, K. Saalfield, and A. McAdams. 1983. An ultrastructural study of enteropathogenic Escherichia coli infection in human infants. Ultrastruct. Pathol. 4:291-304. [DOI] [PubMed] [Google Scholar]
- 48.Schauer, D. B., S. N. McCathey, B. M. Daft, S. S. Jha, L. E. Tatterson, N. S. Taylor, and J. G. Fox. 1998. Proliferative enterocolitis associated with dual infection with enteropathogenic Escherichia coli and Lawsonia intracellularis in rabbits. J. Clin. Microbiol. 36:1700-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schultz, B., A. Singh, D. Devor, and R. Bridges. 1999. Pharmacology of CFTR chloride channel activity. Physiol. Rev. 79(Suppl.):S109-S144. [DOI] [PubMed] [Google Scholar]
- 50.Shreedhar, V. K., B. L. Kelsall, and M. R. Neutra. 2003. Cholera toxin induces migration of dendritic cells from the subepithelial dome region to T- and B-cell areas of Peyer's patches. Infect. Immun. 71:504-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strohmeier, G. R., W. I. Lencer, T. W. Patapoff, L. F. Thompson, S. L. Carlson, S. J. Moe, D. K. Carnes, R. J. Mrsny, and J. L. Madara. 1997. Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J. Clin. Investig. 99:2588-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strohmeier, G. R., S. M. Reppert, W. I. Lencer, and J. L. Madara. 1995. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J. Biol. Chem. 270:2387-2394. [DOI] [PubMed] [Google Scholar]
- 53.Stutts, M. J., E. R. Lazarowski, A. M. Paradiso, and R. C. Boucher. 1995. Activation of CFTR Cl− conductance in polarized T84 cells by luminal extracellular ATP. Am. J. Physiol. 268:C425-C433. [DOI] [PubMed] [Google Scholar]
- 54.Sugita, M., Y. Yue, and J. Foskett. 1998. CFTR Cl− channel and CFTR-associated ATP channel: distinct pores regulated by common gates. EMBO J. 17:898-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tauschek, M., R. Gorrell, R. Strugnell, and R. Robins-Browne. 2002. Identification of a protein secretory pathway for the secretion of heat-labile enterotoxin by an enterotoxigenic strain of Escherichia coli. Proc. Natl. Acad. Sci. USA 99:7066-7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thiagarajah, J. R., and A. S. Verkman. 2003. CFTR pharmacology and its role in intestinal fluid secretion. Curr. Opin. Pharmacol. 3:594-599. [DOI] [PubMed] [Google Scholar]
- 57.Wada, A., M. Hasegawa, K. Matsumoto, T. Niidome, Y. Kawano, Y. Hidaka, P. Padilla, H. Kurazfono, Y. Shimonishi, and T. Hirayama. 1996. The significance of Ser1029 of the heat-stable enterotoxin receptor (STaR): relation of STa-mediated guanylyl cyclase activation and signaling by phorbol myristate acetate. FEBS Lett. 384:75-77. [DOI] [PubMed] [Google Scholar]
- 58.Wada, Y., Y. Nakaoka, H. Kondo, M. Nakazawa, and M. Jubo. 1996. Dual infection with attaching and effacing Escherichia coli and enterotoxigenic Escherichia coli in post-weaning pigs. J. Comp. Pathol. 114:93-99. [DOI] [PubMed] [Google Scholar]
- 59.Youssef, M., A. Shurman, M. Bougnoux, M. Rawashdeh, S. Bretagne, and N. Strockbine. 2000. Bacterial, viral and parasitic enteric pathogens associated with acute diarrhea in hospitalized children from northern Jordan. EMS Immunol. Med. Microbiol. 28:257-263. [DOI] [PubMed] [Google Scholar]