Abstract
In this study of human polymorphonuclear leukocytes (PMNs), pretreatment with Treponema denticola major outer sheath protein (Msp) inhibited formyl-methionyl-leucyl-phenylalanine (fMLP)-induced chemotaxis, phagocytosis of immunoglobulin G-coated microspheres, fMLP-stimulated calcium transients, and actin assembly. Msp neither altered oxidative responses to phorbol myristate or fMLP nor induced apoptosis. Msp selectively impairs chemotaxis and phagocytosis by impacting the PMN cytoskeleton.
The polymorphonuclear leukocyte (PMN), or neutrophil, is a key cell of innate immunity in peripheral tissues. Its capacity to migrate and to respond with effective antimicrobial activities contains local infections such as those that exacerbate periodontal diseases (14). Treponema denticola, one of the pathogens of chronic periodontitis (8, 20-22), is often used to investigate the interaction of treponemes with host cells in vitro (17). Yet there is little knowledge of the T. denticola factors that may modulate key antimicrobial pathways and mechanisms in PMNs. The crucial role of the cytoskeleton in migration and endocytosis, combined with previous findings that the major outer sheath protein (Msp) of T. denticola perturbed actin assembly in fibroblasts (1, 15, 27), suggested that Msp may modulate human neutrophil chemotaxis and phagocytosis.
Enriched native Msp from the T. denticola type strain, ATCC 35405 (4), was prepared as previously described (7, 13). An anti-Msp rabbit antiserum was raised against the Msp complex of the prominent ∼190-kDa band that had been excised following preparative polyacrylamide gel electrophoresis of unheated samples and then electroeluted at 4°C for 24 h inside dialysis tubing with a 12,000- to 14,000-molecular-weight cutoff (Spectra/Por), followed by overnight dialysis in 10 mM Tris-HCl, pH 8.0.
The PMNs from 10 ml of blood from healthy volunteers were isolated using one-step Polymorphprep (Accurate Chemical, Westbury, NY) (9) and were used, within 3 h, in suspensions of 106 cells/ml of Hanks' balanced salt solution (HBSS). PMNs in the experimental groups were incubated with enriched Msp in HBSS (final concentration range, 0.0064 to 64 μg/ml) for 30 min at room temperature in a shaking bath. Control cells were exposed to the same volume of Msp-free HBSS. The PMNs were washed twice and then subjected to various functional assays in triplicate. At least three independent experiments were performed with cells from different donors. Comparisons of mean values were analyzed by the two-tailed Student t test. Multiple samples were compared by single-factor analysis of variance.
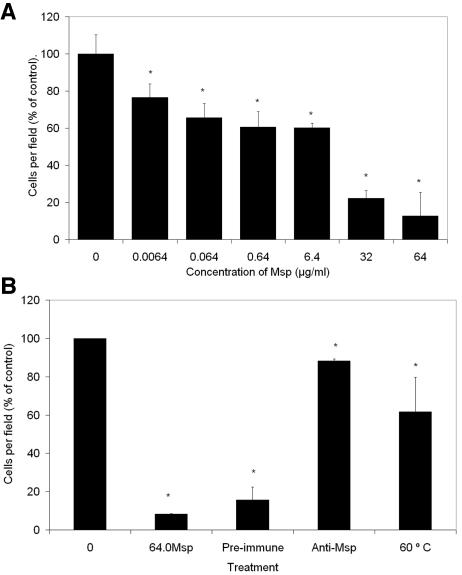
The chemotactic response of PMNs to 10−6 M formyl-methionyl-leucyl-phenylalanine (fMLP) was assayed for 10 min of incubation at 37°C in a modified Boyden chamber (Transwell; pore size, 3.0 μm; Corning, Inc., Corning, NY) by counting the number of PMNs in 10 microscopic fields (magnification, ×40) (24). Pretreatment with Msp diminished the migratory response of these PMNs in a concentration-dependent manner (P < 0.05) (Fig. 1). The chemotaxis experiments were repeated using the highest concentration of Msp (64 μg/ml) which had been heated at 60°C for 30 min or which had been incubated with anti-Msp antibodies or preimmune serum for 1 h at 4°C, followed by absorption by protein A-Sepharose beads (15, 27). Both heating and absorption with anti-Msp antibodies reversed the inhibitory effect (Fig. 1B), indicating that Msp rather than a minor protein or heat-stable contaminant, such as a glycolipid, was the predominant bioactive ingredient. These findings with human PMNs extend a previous observation that Msp (20 μg/ml) impaired chemotaxis of murine PMNs in a Zigmond chamber, in which PMNs migrate laterally from central coordinates on a slide (1).
FIG. 1.
(A) Effect of Msp on neutrophil chemotaxis, assayed using the Boyden chamber with 1 μM fMLP as the chemoattractant. Data are expressed as mean percentages of the control level (taken as 100%) ± standard errors (n = 3). *, P < 0.05. (B) The highest concentration of Msp (64.0 μg/ml) was pretreated with preimmune serum, anti-Msp antiserum, or heat at 60°C for 30 min, and the effect on chemotaxis was compared with that of Msp and Msp-free vehicle (control). Data are expressed as mean percentages of the control level (taken as 100%) ± standard errors (n = 3). *, P < 0.05. Note that the antiserum and 60°C samples were not significantly different from control activated samples but were statistically different from Msp-treated samples. This confirms that the observed effect is a result of the Msp and not a contaminating factor in the bacterial preparation.
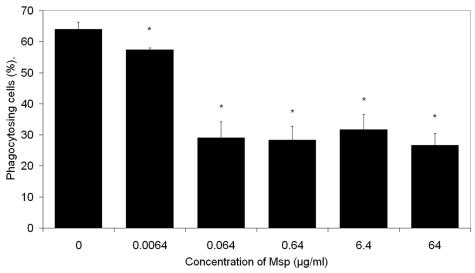
Phagocytosis (37°C, 20 min) was assayed using Nile Red fluorescent FluoSphere immunoglobulin G (IgG)-coated polystyrene microspheres (Molecular Probes, Eugene, OR) as the target particles. The percentage of cells with at least one ingested microsphere was determined by fluorescence microscopy (Texas Red filter set; excitation wavelength, 535 nm; emission wavelength, 575 nm; Eclipse E1000; Nikon, Kawasaki, Japan). The Msp-pretreated PMNs exhibited a 7 to 37% diminished frequency of phagocytosis (P < 0.05). The degree of inhibition reached a plateau for Msp concentrations of 0.064 μg/ml and higher (Fig. 2).
FIG. 2.
Effect of Msp on neutrophil phagocytosis of IgG-coated FluoSphere polystyrene beads. Results were quantified by fluorescence microscopy and are expressed as the mean number of phagocytosing cells × 100/total counted cells (n = 3). *, P < 0.05. Error bars, standard errors.
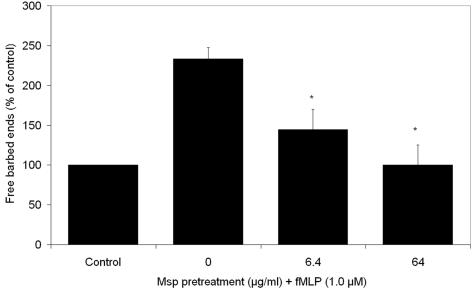
Locomotion and phagocytosis require cytoskeletal reorganization. A pyrene-actin monomer (Cytoskeleton, Denver, CO) incorporation assay with transiently permeabilized PMNs (9) was used to compare the relative number of actin filament free barbed ends in Msp-pretreated and control PMNs stimulated for 2 min with either 1 μM fMLP or fMLP-free buffer. There was a >2-fold rise in the fluorescence intensity of pyrene actin in fMLP-stimulated control PMNs. In contrast, pretreatment with Msp diminished the response to a level comparable to that of PMNs that had not been exposed to fMLP (P < 0.05) (Fig. 3). These data indicate that Msp inhibits incorporation of actin monomers during de novo filament assembly, which is crucial for chemotactic polarization and migration.
FIG. 3.
Effect of Msp on neutrophil actin filament assembly assayed by pyrene actin incorporation. Fluorescence intensity was monitored spectrophotometrically in free barbed ends of actin filaments in Msp-pretreated cells exposed to fMLP and was compared to the fluorescence intensity for control cells that were treated with an Msp-free vehicle and were not stimulated with fMLP. Data are expressed as the mean fluorescence intensity relative to control values ± standard errors (n = 3). *, P < 0.05.
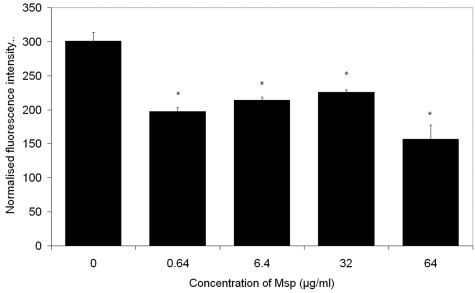
Actin filament assembly is regulated by calcium. Exposure of PMNs to fMLP is known to cause a rapid rise in cytoplasmic Ca2+ levels (2, 5, 23). Confocal photometry was used to compare the intracellular calcium flux of PMNs (106) pretreated with Msp in phosphate-buffered saline (PBS), pH 7.4, for 30 min and control PMNs treated with Msp-free PBS. Cells loaded with Fluo-3/acetoxymethylester (5 μM; Molecular Probes, Eugene, OR) were allowed to attach to bovine serum albumin-coated coverslips for 60 min at 37°C. fMLP (10 nM) was added as the agonist. Fluo-3 emissions were recorded using a confocal laser microscope (40× objective lens; excitation wavelength, 488 nm; emission wavelength, 520 to 530 nm; TCS SL; Leica, Heidelberg, Germany). A time series of images (every 5 s) was collected and analyzed using Leica software. The addition of fMLP to control PMNs caused an immediate and transient (1- to 2-min) increase in normalized cytosolic [Ca2+], which reached its peak amplitude (286% ± 53%) within 25 s. Msp pretreatment significantly diminished the amplitude of the Ca2+ transient (P < 0.05) (Fig. 4).
FIG. 4.
Effect of Msp on fMLP-induced intracellular cytosolic calcium levels. PMNs were loaded with Fluo-3 and allowed to attach for 60 min; then the calcium flux in the cytosol in response to stimulation with 10 nM fMLP was monitored using confocal microscopy. Values are mean amplitudes of calcium transients ± standard errors (n = 3). *, P < 0.05.
For measuring the respiratory burst in response to agonists, the PMNs were exposed to fMLP (10−6 M) or phorbol myristate acetate (10−5 M) (Sigma) in the presence of 1 μM dihydrorhodamine 123 (Sigma), which fluoresces upon oxidation to rhodamine 123 (26). After 25 min, the reaction was stopped by addition of 100 μl PBS; 10,000 cells per sample, gated by relative size and granularity, were analyzed by flow cytometry (excitation wavelength, 488 nm; emission wavelength, 585 nm; FACScan; Becton Dickinson, Franklin Lakes, NJ). Pretreatment with Msp had no effect on the fluorescence intensity of PMNs exposed to either agonist (data not shown).
Apoptosis was measured with the Guava Nexin kit according to its manufacturer's protocol (Guava Technologies Inc., Hayward, CA), using camptothecin (CPT) as a positive control (11). The PMNs (105/test) were incubated with either 64 μg/ml Msp, a control vehicle, or 10 μM CPT (Sigma) for 1, 2, and 3 h. The cells were stained with phycoerythrin-conjugated annexin V, a marker of the early apoptotic pathway, and Nexin 7-amino-actinomycin D, a cell permeant of late-stage apoptosis (16, 25). The cells were analyzed with a Guava personal cytometer. Msp did not induce apoptosis in PMNs compared with vehicle-treated cells, whereas CPT-treated PMNs experienced more-frequent apoptotic events (frequency of apoptosis, 2% ± 0.5% for vehicle-treated PMNs, 4% ± 2.1% for Msp-treated PMNs, and 18% ± 5.3% for CPT-treated PMNs [P < 0.01]). PMNs treated with Msp also showed a lower 7-amino-actinomycin D uptake than PMNs incubated with CPT (2.6% ± 0.7% for vehicle-treated PMNs, 3.8% ± 2.1% for Msp-treated PMNs, and 19.5% ± 4.9% for CPT-treated PMNs [P < 0.01]). These data indicate that Msp induces neither apoptosis nor long-lasting cell membrane permeability in human PMNs.
One of the key functions of cells in innate immunity is their rapid migration (12). Msp impaired human neutrophil chemotaxis and phagocytosis in vitro, probably by perturbing de novo assembly of actin filaments. Calcium is integral to actin filament turnover, which drives membrane lamellipodial extension and resultant locomotion (10). Pretreatment of PMNs with Msp diminished both the cytosolic Ca2+ transient and the detection of actin nucleation sites following exposure of cells to fMLP. These key outcomes impacting the cytoskeleton help explain how Msp may inhibit PMN chemotaxis, perhaps through its impact on cytokinesis. The findings are consistent with previous reports that Msp pretreatment of fibroblasts leads to actin reorganization, diminished calcium store release, and delayed migration (1, 27).
Pretreatment of PMNs with low concentrations of Msp also diminished their capacity to phagocytose IgG-coated microspheres. Inhibition of actin monomer incorporation into filaments would be expected to impair pseudopod formation. Alternatively, Msp could have affected receptor-mediated immunoglobulin engagement analogously to its reduction of the anticipated affinity modulation when the β1 integrins of fibroblasts engage collagen (15). Msp pretreatment did not affect the agonist-stimulated oxidative response. Others have reported that early-growth-phase culture supernatants, phenol extracts, and some lipoproteins of T. denticola can inhibit oxidative responses in PMNs (18, 19). Our results do not conflict with these reports, since the Msp-containing outer sheath does not shed much when T. denticola is growing in fresh medium, and Msp is a nonacylated protein. Notably, human PMNs are able to phagocytose T. denticola and other oral spirochetes. Yet most strains stimulate the oxidative burst only in the presence of serum, and once they are phagocytosed, failure to stimulate the release of lysosomal enzymes is atypical (3). Our findings suggest that Msp is an unlikely source of these inhibitory activities.
Similarly, Msp did not induce significant apoptosis. This finding is consistent with studies of Msp-treated fibroblasts (15, 27) and T. denticola-treated KB epithelial cells (6), target cells that undergo gross cytoskeletal reorganization but remain viable. Enriched Msp is also known to depolarize HeLa cell membranes, presumably by establishing short-lived ion channels (13). Yet the PMNs in this study did not show signs of significant plasma membrane permeability. Consequently, we conclude that native Msp of T. denticola has selective inhibitory effects on actin-dependent functions of human neutrophils in vitro.
Acknowledgments
This study was supported by CIHR grants MGP-5619 (to R.P.E.) and MOP-53136 (to M.G.). B.P.T. was supported by a University of Toronto Open Fellowship.
We thank D. A. Grove for technical assistance.
Editor: J. B. Bliska
REFERENCES
- 1.Amin, M., A. C. S. Ho, J. Y. Lin, A. P. Batista da Silva, M. Glogauer, and R. P. Ellen. 2004. Induction of de novo subcortical actin filament assembly by Treponema denticola major outer sheath protein. Infect. Immun. 72:3650-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson, T., C. Dahlgren, T. Pozzan, O. Stendahl, and P. D. Lew. 1986. Characterization of fMet-Leu-Phe receptor-mediated Ca2+ influx across the plasma membrane of human neutrophils. Mol. Pharmacol. 30:437-443. [PubMed] [Google Scholar]
- 3.Boehringer, H., P. H. Berthold, and N. S. Taichman. 1986. Studies on the interaction of human neutrophils with plaque spirochetes. J. Periodont. Res. 21:195-209. [DOI] [PubMed] [Google Scholar]
- 4.Chan, E. C. S., R. Siboo, T. Keng, N. Psarra, R. Hurley, S. L. Cheng, and I. Iugovaz. 1993. Treponema denticola (ex Brumpt 1925) sp. nov., nom. rev., and identification of new spirochete isolates from periodontal pockets. Int. J. Syst. Bacteriol. 43:196-203. [DOI] [PubMed] [Google Scholar]
- 5.Chen, L. W., and C. R. Jan. 2001. Mechanisms and modulation of formyl-methionyl-leucyl-phenylalanine (fMLP)-induced Ca2+ mobilization in human neutrophils. Int. Immunopharmacol. 1:1341-1349. [DOI] [PubMed] [Google Scholar]
- 6.De Filippo, A. B., R. P. Ellen, and C. A. G. McCulloch. 1995. Induction of cytoskeletal rearrangements and loss of volume regulation in epithelial cells by Treponema denticola. Arch. Oral Biol. 40:199-207. [DOI] [PubMed] [Google Scholar]
- 7.Egli, C., W. K. Leung, K. H. Muller, R. E. Hancock, and B. C. McBride. 1993. Pore-forming properties of the major 53-kilodalton surface antigen from the outer sheath of Treponema denticola. Infect. Immun. 61:1694-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellen, R. P., and V. B. Galimanas. 2005. Spirochetes at the forefront of periodontal infections. Periodontol. 2000 38:13-32. [DOI] [PubMed] [Google Scholar]
- 9.Glogauer, M., J. Hartwig, and T. Stossel. 2000. Two pathways through Cdc42 couple the N-formyl receptor to actin nucleation in permeabilised human neutrophils. J. Cell Biol. 150:785-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janmey, P. A. 1994. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu. Rev. Physiol. 56:169-191. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi, S. D., J. M. Voyich, K. R. Braughton, and F. R. DeLeo. 2003. Down-regulation of proinflammatory capacity during apoptosis in human polymorphonuclear leukocytes. J. Immunol. 170:3357-3368. [DOI] [PubMed] [Google Scholar]
- 12.Lang, K., H. Hatt, B. Niggemann, K. S. Zaenker, and F. Entschladen. 2003. A novel function for chemokines: downregulation of neutrophil migration. Scand. J. Immunol. 57:350-361. [DOI] [PubMed] [Google Scholar]
- 13.Mathers, D. A., W. K. Leung, J. C. Fenno, Y. Hong, and B. C. McBride. 1996. The major surface protein complex of Treponema denticola depolarizes and induces ion channels in HeLa cell membranes. Infect. Immun. 64:2904-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyasaki, K. T. 1991. The neutrophil: mechanisms of controlling periodontal bacteria. J. Periodontol. 62:761-774. [DOI] [PubMed] [Google Scholar]
- 15.Paes Batista da Silva, A., W. Lee, E. Bajenova, C. A. G. McCulloch, and R. P. Ellen. 2004. The major outer sheath protein of Treponema denticola inhibits the binding step of collagen phagocytosis in fibroblasts. Cell. Microbiol. 6:485-498. [DOI] [PubMed] [Google Scholar]
- 16.Rudin, C. M., and C. B. Thompson. 1997. Apoptosis and disease: regulation and clinical relevance of programmed cell death. Annu. Rev. Med. 48:267-281. [DOI] [PubMed] [Google Scholar]
- 17.Sela, M. N. 2001. Role of Treponema denticola in periodontal diseases. Crit. Rev. Oral Biol. Med. 12:399-413. [DOI] [PubMed] [Google Scholar]
- 18.Sela, M. N., A. Bolotin, R. Naor, A. Weinberg, and G. Rosen. 1997. Lipoproteins of Treponema denticola: their effect on human polymorphonuclear neutrophils. J. Periodont. Res. 32:455-466. [DOI] [PubMed] [Google Scholar]
- 19.Sela, M. N., A. Weinberg, R. Borinsky, S. C. Holt, and T. Dishon. 1988. Inhibition of superoxide production in human polymorphonuclear leukocytes by oral treponemal factors. Infect. Immun. 56:589-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simonson, L. G., C. H. Goodman, J. J. Bial, and H. E. Morton. 1988. Quantitative relationship of Treponema denticola to severity of periodontal disease. Infect. Immun. 56:726-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Socransky, S. S., A. D. Haffajee, M. A. Cugini, C. Smith, and R. L. Kent, Jr. 1998. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25:134-144. [DOI] [PubMed] [Google Scholar]
- 22.Socransky, S. S., C. Smith, and A. D. Haffajee. 2002. Subgingival microbial profiles in refractory periodontal disease. J. Clin. Periodontol. 29:260-268. [DOI] [PubMed] [Google Scholar]
- 23.Stendahl, O., K. H. Krause, J. Krischer, P. Jerstrom, J. M. Theler, R. A. Clark, J. L.Carpentier, and D. P. Lew. 1994. Redistribution of intracellular calcium stores during phagocytosis in human neutrophils. Science 265:1439-1441. [DOI] [PubMed] [Google Scholar]
- 24.Van Dyke, T. E., A. A. Reilly, H. Horoszewicz, N.Gagliardi, and R. J. Genco. 1979. A rapid semi-automated procedure for the evaluation of leucocyte locomotion in the micropore filter assay. J. Immunol. Methods 31:271-282. [DOI] [PubMed] [Google Scholar]
- 25.van Heerde, W. L., P. G. de Groot, and C. P. Reutelingsperger. 1995. The complexity of the phospholipid binding protein Annexin V. Thromb. Haemost. 73:172-179. [PubMed] [Google Scholar]
- 26.Walrand, S., S. Valeix, C. Rodriguez, P. Ligot, J. Chassagne, and M. P. Vasson. 2003. Flow cytometry study of polymorphonuclear neutrophil oxidative burst: a comparison of three fluorescent probes. Clin. Chim. Acta 331:103-110. [DOI] [PubMed] [Google Scholar]
- 27.Wang, Q., K. S. Ko, A. Kapus, C. A. G. McCulloch, and R. P. Ellen. 2001. A spirochete surface protein uncouples store-operated calcium channels in fibroblasts: a novel cytotoxic mechanism. J. Biol. Chem. 276:23056-23064. [DOI] [PubMed] [Google Scholar]