Abstract
The innate immune response to Pneumocystis infection is not well understood. In this study, normal C57BL/6 mouse alveolar macrophages were found to respond to Pneumocystis murina organisms through Toll-like receptor 2 (TLR2), leading to the nuclear translocation of NF-κB and the production of proinflammatory cytokine tumor necrosis factor alpha (TNF-α) and chemokine macrophage inflammatory protein 2 (MIP-2). P. murina stimulation of normal alveolar macrophages from C57BL/6 mice resulted in increased TLR2 transcription but not increased TLR4 transcription. In gain-of-function studies with HEK293 cells expressing TLR2 or TLR4, only TLR2 was found to stimulate an NF-κB response to P. murina. TNF-α and MIP-2 production in response to P. murina by mouse alveolar macrophages was inhibited by a monoclonal antibody that specifically blocked the ligand-binding ability of TLR2. Alveolar macrophages from TLR2 knockout (TLR2−/−) mice showed little increase in TNF-α and MIP-2 mRNA levels upon P. murina stimulation. An in vivo study showed that TLR2−/− mice challenged with P. murina had reduced cytokine responses. These results indicate that TLR2 plays a major role in the innate immune response to P. murina.
Pneumocystis is an opportunistic fungal pathogen of immunocompromised hosts. Pneumocystis organisms that infect humans are now called Pneumocystis jiroveci (41) and are a common cause of pneumonia in patients with AIDS (37). Pneumocystis carinii and Pneumocystis murina are those species that infect rats and mice, respectively (17, 41). Although the use of highly active antiretroviral therapy has decreased the incidence of Pneumocystis pneumonia (PcP), the morbidity and mortality rates of PcP remain high (30). Patients who receive long-term immunosuppressive medications or who have genetic immunodeficiency are also susceptible to Pneumocystis infection.
Effective immune responses of the host are required to control PcP. Alveolar macrophages play a critical role in the innate immune response to many microbes. Experimental evidence indicates that alveolar macrophages contribute to the host immune response to Pneumocystis organisms by directly ingesting or killing them and producing inflammatory cytokines and chemokines such as tumor necrosis factor alpha (TNF-α) and macrophage inflammatory protein 2 (MIP-2) (14, 15, 54). The exact mechanism by which Pneumocystis stimulates alveolar macrophages to produce cytokines and chemokines is largely unknown.
Microbes induce immune responses of host cells by interacting with certain cell surface receptors, termed pattern recognition receptors. Toll-like receptors (TLRs) have been identified as important pattern recognition receptors (28, 34) and are a large family with at least 11 members. Each TLR has a specific ligand(s) (44). TLR4 and its coreceptor, MD2, recognize lipopolysaccharides (LPSs) from gram-negative bacteria as well as many other ligands such as glucuronoxylomannan found in the polysaccharide capsule of Cryptococcus neoformans (38) and glycoinositolphospholipids from Trypanosoma cruzi (31). It has been previously reported that impaired recognition through TLR4 is responsible for exacerbated Pneumocystis pneumonia (9). TLR2 recognizes a large variety of ligands including peptidoglycan, lipoprotein, lipopeptide, and zymosan (43). The relationship between TLR2 and Pneumocystis infection has not been studied.
It has previously been reported that TLR2 collaborates with the β-glucan receptor Dectin-1 to mediate the inflammatory response of mouse macrophages to Saccharomyces cerevisiae cell wall component zymosan (12), which is composed primarily of β-glucans and mannans. The cell surface molecules of Pneumocystis organisms include major surface glycoprotein and β-glucan (25, 32, 46), which may be recognized by TLR2 or TLR4. A recent report demonstrated the involvement of the Dectin-1 receptor in nonopsonic phagocytosis of P. murina (40). We hypothesized that Pneumocystis induces the innate immune response of alveolar macrophages by stimulating TLR2 or TLR4. Results of the present study revealed the role of TLR2 in the response of mouse alveolar macrophages to P. murina.
MATERIALS AND METHODS
Mice.
TLR2-deficient (TLR2−/−) mice (C57BL/6 background) were purchased from the Jackson Laboratory (Bar Harbor, ME), and wild-type C57BL/6 mice were obtained from Harlan (Indianapolis, IN). All mice used for this study were female, 6 to 8 weeks of age, and weighed 25 to 30 g. Animal studies were approved by the Indiana University Animal Care and Use Committee. Experiments were carried out under the supervision of veterinarians. Animals were housed in the Indiana University Laboratory Animal Resource Center, which is an American Association of Laboratory Animal Sciences approved facility.
Alveolar macrophage isolation and stimulation.
Alveolar macrophages were isolated by bronchoalveolar lavage. Mice were anesthetized by intramuscular injection of 0.02 ml ketamine cocktail (ketamine hydrochloride [80 mg/ml], acepromazine [1.76 mg/ml], and atropine [0.38 μg/ml]) and then sacrificed by cardiac exsanguination. Lungs were lavaged with 1 ml sterile saline each time through an intratracheal catheter as described previously (22), and a total of 10 ml saline was instilled and recovered from each mouse. The lavage fluid was centrifuged at 300 × g for 10 min to pellet alveolar macrophages. The pelleted cells were resuspended and cultured in a 12-well culture plate at 37°C with 5% CO2 at a concentration of 3 × 105 cells per well in 1 ml RPMI 1640 medium (Sigma Chemical Co., St. Louis, MO) supplemented with 10% fetal bovine serum, 1 mM pyruvate, 1% nonessential amino acids, 14 mM glucose, 17.9 mM NaHCO3, 10 mM HEPES, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. After 2 h of incubation, nonadherent cells were washed off with phosphate-buffered saline (PBS), and the medium was refreshed. P. murina organisms or sterile saline was added to the culture medium, and cells were incubated for another 8 h at 37°C with 5% CO2. For inhibition of the cytokine response to P. murina in alveolar macrophages, monoclonal antibody (MAb) T2.5 against TLR2 (eBioscience, San Diego, CA) was added to the culture medium (50 μg/ml) 30 min prior to stimulation with P. murina organisms.
P. murina organism isolation and quantification.
Pneumocystis organisms were obtained from heavily infected lungs of dexamethasone-immunosuppressed mice and isolated as previously described (3, 33). Pneumocystis-infected mouse lungs were homogenized in ice-cold NKPC buffer (2.68 mM KCl, 1.47 mM KH2PO4, 51.1 mM Na2HPO4, 7.43 mM NaH2PO4, 62 mM NaCl, 0.05 mM CaCl2, 0.05 mM MgCl2) containing 100 mM dithiothreitol. The homogenate was centrifuged at 50 × g for 5 min at room temperature to remove cell debris. Pneumocystis organisms in the supernatant were collected by centrifugation at 10,000 × g for 10 min at 4°C, resuspended in 5 ml of 0.85% NH4Cl-NKPC buffer, and incubated at 37°C for 5 min to lyse erythrocytes. Isolated Pneumocystis organisms were quantified by enumeration of nuclei stained with modified Giemsa stain. After centrifugation (10,000 × g for 5 min at 4°C), Pneumocystis organisms were washed three times in NKPC buffer, resuspended in 1 ml of RPMI 1640 medium supplemented with 10% fetal bovine serum and 7% dimethyl sulfoxide, and then stored at −70°C. Prior to use, cryovials containing the organisms were rapidly thawed in a 37°C water bath, and the organisms were pelleted by centrifugation, washed extensively with sterile saline, and then resuspended in sterile saline at a concentration of 6 × 107 organisms per ml. Pneumocystis preparations were endotoxin, bacterium, and fungus free as determined by Limulus amebocyte lysate test (Cambrex, East Rutherford, NJ) and culture. To minimize the potential effect of contaminating endotoxin, incubations of P. murina organisms with alveolar macrophages were also performed in media containing 1.0 μg/ml polymyxin B to neutralize any residual endotoxin.
In vivo stimulation of mice with P. murina.
Mice were anesthetized by intramuscular injection of 0.02 ml ketamine cocktail as described above and then transtracheally inoculated with 5 × 106 P. murina cells in 50 μl of sterile saline. Control mice were transtracheally inoculated with 50 μl of sterile saline. Eight hours after inoculation, mice were sacrificed, and alveolar macrophages were isolated as described above.
RNA isolation and real-time RT-PCR assay.
Total RNA was isolated from alveolar macrophages using TRIzol reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). RNA concentration and purity were determined by spectrophotometry. cDNA was synthesized from the total RNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) primed by oligo(dT) and random primers; 0.2 μg of total RNA was used for each reaction mixture, with a total reaction mixture volume of 20 μl. The reaction mixtures were incubated at 25°C for 5 min, 42°C for 30 min, and 85°C for 5 min. Two microliters of each cDNA product was used for quantitative PCR analysis. Real-time reverse transcription (RT)-PCR for TLR2, TLR4, TNF-α, and MIP-2 was performed using Assays-on-Demand gene expression kits containing two unlabeled PCR primers and a 6-carboxyfluorescein-labeled Taqman probe (Applied Biosystems, Foster City, CA) on a Smartcycler (Cepheid, Sunnyvale, CA). Ribosomal protein S8 (RPS8) mRNA was assayed in an identical manner with primers RPS8-F (5′-569CCAGCAGGGCAAGCTTCT586-3′) and RPS8-R (5′-650CTTGCCTTCGAGCACATAG632-3′) and a tetrachlorofluorescein-labeled probe (5′-589CCTGTATTGCCTCAAGACCAGG610-3′) as an internal control. The expression level of RPS8 is not affected by Pneumocystis infection. The RPS8 nucleotide sequence was obtained from the GenBank database (accession no. NM_009098). Data were normalized to RPS8 gene expression in each sample and were shown as severalfold increases relative to unstimulated control cells. Means and standard deviations (SD) were calculated from five mice per group.
Quantification of cytokines in culture supernatants by ELISA.
Culture supernatants of alveolar macrophages were harvested after 8 h of culture, centrifuged to remove cellular debris, and stored at −70°C until they were assayed. TNF-α and MIP-2 concentrations in culture supernatants were determined by sandwich enzyme-linked immunosorbent assay (ELISA) as described previously (2). Briefly, Maxisorb plates (Nunc, Naperville, IL) were coated overnight with unlabeled capture antibodies (anti-TNF-α or anti-MIP-2), washed with assay buffer (50 mM Tris [pH 7.0 to 7.5], 0.2% Tween 20), and blocked with 4% bovine serum albumin and 0.01% thimerosal in PBS. Samples or cytokine standards (50 μl) (R&D Systems, Minneapolis, MN) diluted with an equal volume of assay buffer were added, and plates were incubated for 2 h at 37°C. Specific detection antibody (biotinylated anti-TNF-α or anti-MIP-2 antibodies; R&D Systems) was then added, followed by the addition of streptavidin-peroxidase (RDI, Flanders, NJ) and the peroxidase substrate 3,3′,5,5′-tetramethylbenzidine dihydrochloride (Neogen, Lexington, KY). The reaction was stopped with 2 N HCl, and the absorbance at 450 nm (minus the absorbance at 650 nm to correct for optical imperfections in the plate) was determined using a microplate reader (Molecular Dynamics, Sunnyvale, CA). Cytokine concentrations were calculated from serial dilutions of cytokine standards, included in each plate, using the instrument software (SoftPro; Molecular Dynamics).
Detection of NF-κB p65 nuclear translocation by immunofluorescence microscopy.
Lavaged alveolar macrophages were resuspended in RPMI 1640 medium (3 × 105 cells/tube in 0.2 ml medium) and incubated with 1 μg/ml Escherichia coli LPS (Sigma, St. Louis, MO) or purified P. murina (multiplicity of infection [MOI] of 6) at 37°C in 5% CO2 for 1 h. Cells were cytospun onto a microscopic slide, fixed with 4% paraformaldehyde in PBS for 20 min, and permeabilized with 0.1% Triton X-100 in PBS for 10 min at room temperature. The cells were then incubated with 1% bovine serum albumin in PBS for 2 h at room temperature to reduce nonspecific staining followed by rabbit anti-mouse NF-κB p65 polyclonal antibody C20 (1:100 dilution) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 4°C overnight. After washing twice with PBS, the cells were incubated with Alexa Fluor 488-conjugated goat anti-rabbit immunoglobulin G secondary antibody (1:200 dilution) (Molecular Probes, Eugene, OR) for 1 h at room temperature and with 4′,6′-diamidino-2-phenylindole (DAPI) (0.1 μg/ml) (Sigma, St. Louis, MO) to stain nuclei. The cells were washed again five times with PBS and then examined with a fluorescence microscope (BH2-RFCA; Olympus) equipped with a digital camera (DP70; Olympus).
HEK293 cell transfection and stimulation.
TLR2 and TLR4 were transiently expressed in human embryonic kidney (HEK293) cells and then assayed for their responsiveness to P. murina organisms. HEK293 cells were transfected with pFLAG-TLR2 (53), which contains the mouse TLR2 gene; pFLAG-CD14 (53), which contains the mouse CD14 gene; and pBIIX-Luc, which contains a luciferase reporter gene regulated by the NF-κB binding sequence (53). The luciferase gene is expressed only when NF-κB binds to the binding sequence. A separate set of HEK293 cells was transfected with pFLAG-TLR4 (53), which contains the mouse TLR4 gene; pFLAG-CD14; pFLAG-MD2 (53), which contains the mouse MD2 gene; and pBIIX-Luc. CD14 expression enhances the response of HEK293 cells to both TLR2 and TLR4 ligands (8, 24). These plasmids were gifts from D. Zhang (Yale University, New Haven, CT). To normalize for transfection efficiency, the cells were cotransfected with Renilla luciferase reporter plasmid pRL-TK (Promega, Madison, WI). Plasmids were introduced into HEK293 cells by transfection using Transfectin (Bio-Rad, Hercules, CA). Briefly, HEK293 cells were cultured in a 24-well plate at a concentration of 5 × 105 cells per well in 0.5 ml Dulbecco's modified Eagle's medium at 37°C overnight to about 80% confluence. Medium was refreshed just before transfection. The Transfectin mixture was prepared by diluting 1.25 μl of Transfectin in 50 μl of OPTI-MEMI medium (Invitrogen, Carlsbad, CA) to which 1.0 μg of plasmid DNA in 50 μl of OPTI-MEMI was then added after a 5-min incubation at room temperature. The DNA-Transfectin mixture was then added to the cells and mixed gently by rocking the plate back and forth. After 4 h of incubation at 37°C in 5% CO2, the medium was refreshed. Twenty hours later, the cells were stimulated with purified P. murina organisms at an MOI of 0.5, 1, or 5. As controls, some cells were stimulated with the TLR2 ligand Pam3CSK4 (InvivoGen, San Diego, CA) or the TLR4 ligand E. coli LPS (Sigma, St. Louis, MO) at a concentration of 10, 100, 500, or 1,000 ng/ml. After 8 h, cells were lysed and assayed for luciferase activity.
Luciferase assay.
HEK293 cells were assayed for luciferase activity using the Dual-Glo luciferase assay system (Promega, Madison, WI). Cells were washed twice with 1 ml of PBS and lysed in 100 μl of lysis buffer (supplied in the kit). The cell lysate was centrifuged at 12,000 × g for 30 s at room temperature, and 20 μl of the supernatant was mixed with firefly luciferase substrate (beetle luciferine) in a luminometer tube. The light intensity was determined using a Fusion universal microplate analyzer (Packard BioScience, Meriden, CT). The luminescent signal from the firefly luciferase reaction was quenched by the addition of Stop & Glo reagent (supplied in the kit), which also contains Renilla luciferase substrate (coelenterazine). The light intensity derived from the Renilla luciferase activity was determined by the same procedure. The luciferase activity of each sample was normalized to the Renilla luciferase activity. Experimental data were expressed as the severalfold increases over those of unstimulated control cells transfected with the same constructs.
Statistics.
Data were presented as means ± SD of the indicated number of experiments. Differences between groups were determined using the two-tailed Student's t test and were considered statistically significant if the P value was <0.05.
RESULTS
P. murina-induced TNF-α and MIP-2 production in mouse alveolar macrophages.
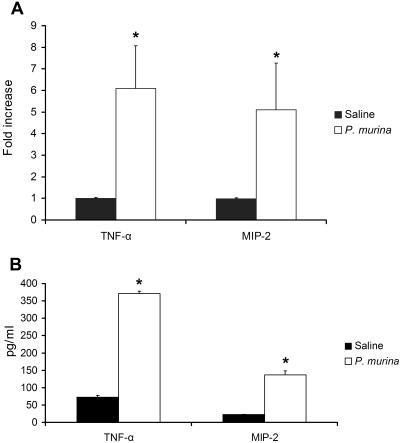
To investigate if P. murina could induce TNF-α and MIP-2 production in mouse alveolar macrophages, 3 × 105 alveolar macrophages from each of five C57BL/6 mice were incubated with P. murina at an MOI of 6 for 8 h; separate aliquots of alveolar macrophages were treated with sterile saline as controls. Total RNA was isolated from treated cells and analyzed by real-time RT-PCR for TNF-α and MIP-2 mRNA levels. Culture supernatants were assayed for TNF-α and MIP-2 concentrations by ELISA. As summarized in Fig. 1A, real-time RT-PCR results showed that TNF-α mRNA from P. murina-stimulated alveolar macrophages was sixfold higher than that of the control in which alveolar macrophages were treated with normal saline (P < 0.05). Similar results were obtained with MIP-2 mRNA levels; P. murina stimulation was found to cause a 5.3-fold increase in the level of MIP-2 mRNA in alveolar macrophages. Protein levels of TNF-α and MIP-2 production were also increased by P. murina stimulation. ELISA results showed that TNF-α and MIP-2 levels in the culture supernatant of P. murina-stimulated alveolar macrophages were fivefold (73.3 ± 4.9 pg/ml versus 370.7 ± 6.8 pg/ml) and sixfold (22.9 ± 0.3 pg/ml versus 136.3 ± 11.9 pg/ml) higher, respectively, than those of controls (Fig. 1B). To exclude the possibility of endotoxin contamination, additional experiments were performed in the presence of the endotoxin-neutralizing agent polymyxin B (1.0 μg/ml), and TNF-α and MIP-2 mRNA expression levels in alveolar macrophages induced by P. murina in the presence of polymyxin B were similar to those shown in the data presented in Fig. 1.
FIG. 1.
Cytokine production in response to P. murina by alveolar macrophages. (A) Alveolar macrophages from C57BL/6 mice were stimulated with P. murina at an MOI of 6 for 8 h and then assessed for TNF-α and MIP-2 mRNA levels by real-time RT-PCR. Controls were aliquots of the same alveolar macrophages stimulated with saline. The mRNA of the RPS8 gene was coamplified with that of TNF-α or MIP-2 to serve as the internal PCR control in each assay. A total of 3 × 105 alveolar macrophages from each mouse were used for each experiment; the cells were cultured in 1 ml/well RPMI 1640 medium in a 12-well plate. The severalfold increase in TNF-α or MIP-2 mRNA levels in P. murina-treated cells is relative to that of the saline-treated control cells, which was set to a value of 1. (B) Culture supernatants of the same P. murina-treated or saline-treated alveolar macrophages were analyzed for TNF-α or MIP-2 protein levels (picograms per milliliter) by ELISA. Bars represent means ± SD for five mice per group (*, P < 0.05 compared with the saline-treated group).
P. murina-activated NF-κB nuclear translocation of mouse alveolar macrophages.
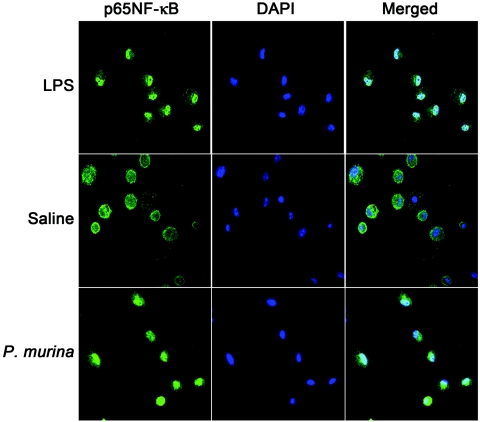
NF-κB is a transcription factor that is critical for the regulation of proinflammatory cytokine expression. To determine if P. murina-induced TNF-α and MIP-2 production correlated with NF-κB activation, P. murina-stimulated alveolar macrophages were examined for NF-κB nuclear translocation. Alveolar macrophages (3 × 105) from C57BL/6 mice were incubated with P. murina at an MOI of 6 for 1 h. As a positive control for NF-κB nuclear translocation, an aliquot of the same alveolar macrophages was treated with 1 μg/ml LPS for 1 h, a concentration known to stimulate NF-κB translocation in human alveolar macrophages (6). Cells were cytospun onto microscopic slides and then reacted with an antibody against p65 of NF-κB, followed by immunofluorescence microscopy. As shown in Fig. 2, nuclear translocation of NF-κB was observed in almost 100% of LPS-treated cells. P. murina stimulation caused NF-κB nuclear translocation in approximately 70% of the cells, whereas very few (<1%) nontreated cells had noticeable NF-κB nuclear translocation.
FIG. 2.
Alveolar macrophage NF-κB nuclear translocation in response to P. murina. Alveolar macrophages (3 × 105) in 0.2 ml RPMI 1640 medium were stimulated with P. murina at an MOI of 6 for 1 h and then examined for NF-κB nuclear translocation by fluorescence microscopy. Positive controls were aliquots of the same alveolar macrophages stimulated with 1 μg/ml LPS, and negative controls were treated with saline. Cells were cytospun onto glass slides, fixed with 4% paraformaldehyde, and then reacted with rabbit anti-mouse NF-κB p65 antibody followed with Alexa Fluor 488-conjugated goat anti-rabbit immunoglobulin G. Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) (0.1 μg/ml). Magnification, ×400.
P. murina stimulation increased TLR2 but not TLR4 expression in macrophages.
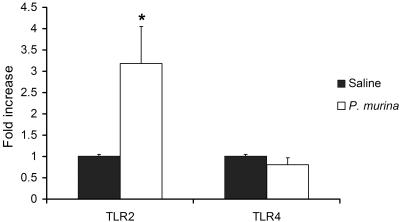
Since NF-κB nuclear translocation is a common result of TLR signaling, P. murina-induced NF-κB activation suggests that TLRs are involved in host responses against P. murina infection. To examine whether P. murina stimulation induces changes in TLR2 and TLR4 expression, 3 × 105 alveolar macrophages from C57BL/6 mice were incubated with P. murina at an MOI of 6 for 8 h. Some cells were treated with sterile saline as a control. TLR2 and TLR4 mRNA levels in treated and nontreated cells were determined by real-time RT-PCR. Figure 3 shows that P. murina stimulation caused a threefold increase in the TLR2 mRNA level in alveolar macrophages (P < 0.05). In contrast, the TLR4 mRNA level was not significantly changed by P. murina stimulation. This result suggests that TLR2 plays a greater role than TLR4 in the response of alveolar macrophages to intact P. murina organisms.
FIG. 3.
Effect of P. murina on the expression of TLR2 and TLR4. Alveolar macrophages from C57BL/6 mice were incubated with P. murina or normal saline (negative control) for 8 h as described in the Fig. 1 legend. Total RNA was isolated from the alveolar macrophages and subjected to Taqman real-time RT-PCR for TLR2 and TLR4 mRNA expression; RPS8 mRNA was coamplified as the internal PCR control. TLR2 and TLR4 real-time PCR results are normalized to those of the RPS8 internal control and are shown as severalfold increases relative to those of saline-treated control cells. Bars are means ± SD of five mice per group (*, P < 0.05 compared with the saline-treated group).
TLR2 expression, but not TLR4 expression, was sufficient to confer responsiveness to P. murina.
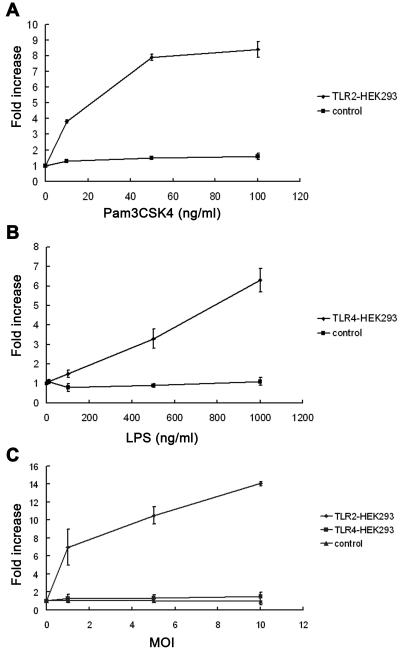
To investigate the roles of TLR2 and TLR4 in the host response to P. murina, HEK293 cells were transiently transfected with plasmids encoding TLR2 or TLR4. HEK293 cells provide a valuable transfection model for this study because they do not express TLRs (18). HEK293 cells were cotransfected with a luciferase reporter plasmid, pBIIX-Luc, on which the expression of the luciferase gene was regulated by NF-κB (53); therefore, NF-κB activation in transfected HEK293 cells is directly proportional to the luciferase activity in the cells. Transfection of HEK293 cells with mouse TLR2 and CD14 conferred responsiveness to the synthetic polypeptide TLR2 ligand Pam3CSK4 (Fig. 4A). Transfection with mouse TLR4, MD2, and CD14 conferred cell responsiveness to the TLR4 ligand E. coli LPS (Fig. 4B). Responses to the appropriate ligands by both TLR2 and TLR4 were dose dependent. Control cells, which were transfected with CD14 only, did not respond to either of these two ligands (Fig. 4A and B). When incubated with P. murina, TLR2-transfected cells showed an MOI-dependent increase in luciferase activity (Fig. 4C). TLR4-tranfected cells and control cells did not respond to P. murina stimulation. These results demonstrate that the NF-κB response to P. murina occurs through TLR2, rather than TLR4, receptor stimulation.
FIG. 4.
Response of TLR2- or TLR4-expressing HEK293 cells to P. murina. HEK293 cells were transiently transfected with plasmids encoding TLR2 and CD14 or with those encoding TLR4, MD2, and CD14. Cells transfected with the plasmid encoding CD14 alone were used as the negative control. All cells were cotransfected with pBIIX-Luc, which contains the firefly luciferase gene, and pRL-TK, which contains the Renilla luciferase gene. Cells were stimulated with the TLR2 ligand Pam3CSK4 (A), the TLR4 ligand E. coli LPS (B), or various numbers of P. murina organisms (C) for 8 h and then subjected to luciferase assay. Data from stimulated cells are shown as severalfold increases in luciferase activity relative to those of unstimulated control cells. Each time point represents means ± SD of results from three different sets of HEK293 cells transfected with the same plasmids.
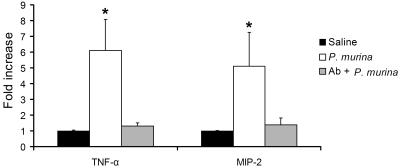
Anti-TLR2 MAb T2.5 decreased the cytokine response of mouse macrophages to P. murina.
T2.5 is a MAb against TLR2 and has been shown to antagonize TLR2-specific activation of mouse and human macrophages both in vitro and in vivo (29). To investigate if TLR2 was necessary for the production of TNF-α and MIP-2 in alveolar macrophages in response to P. murina, MAb T2.5 was added to alveolar macrophage cultures to a final concentration of 50 μg/ml 30 min prior to P. murina stimulation. Compared to the control cells, which were incubated with an equal volume of sterile saline, P. murina-stimulated cells showed a 6-fold increase in TNF-α levels and a 5.5-fold increase in MIP-2 mRNA levels (P < 0.05). Treatment of alveolar macrophages with MAb T2.5 prior to P. murina stimulation abolished the increase in TNF-α and MIP-2 mRNA levels (Fig. 5).
FIG. 5.
Inhibition of P. murina-induced cytokine production by MAb T2.5. Alveolar macrophages from C57BL/6 mice were incubated with MAb T2.5 (Ab) for 30 min and then stimulated with purified P. murina for 8 h. Total RNA isolated from the alveolar macrophages was subjected to real-time RT-PCR analysis for TNF-α and MIP-2 mRNA expression. TNF-α and MIP-2 real-time PCR data are normalized to that of the RPS8 internal control and are shown as severalfold increases relative to those of unstimulated control cells. Bars are means ± SD of five mice per group (*, P < 0.05 compared with the saline group).
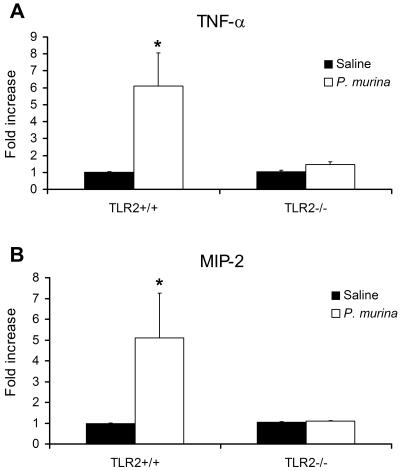
Alveolar macrophages from TLR2−/− mice were defective in cytokine response to P. murina stimulation.
To further confirm the role of TLR2 in the cytokine response of alveolar macrophages to P. murina stimulation, alveolar macrophages from TLR2 knockout (TLR2−/−) mice and wild-type control C57BL/6 mice were incubated with purified P. murina organisms at an MOI of 6 for 8 h and then assessed for TNF-α and MIP-2 mRNA levels by real-time RT-PCR. Cells treated with sterile saline were assayed in a manner identical to that of the control. As shown in Fig. 6A and B, P. murina induced a 6.2-fold increase in TNF-α mRNA expression and a 5.1-fold increase in MIP-2 mRNA expression in alveolar macrophages from wild-type mice. In contrast, alveolar macrophages from TLR2−/− mice showed very little increase in both TNF-α and MIP-2 mRNA levels in response to P. murina stimulation compared to control alveolar macrophages that were stimulated with sterile saline.
FIG. 6.
Diminished TNF-α and MIP-2 mRNA expression in alveolar macrophages from TLR2−/− mice in response to P. murina stimulation. Alveolar macrophages from wild-type C57BL/6 and TLR2−/− mice were stimulated with sterile saline or P. murina for 8 h. Total RNA isolated from the alveolar macrophages was subjected to real-time RT-PCR analysis for TNF-α (A) and MIP-2 (B) mRNA expression. TNF-α and MIP-2 real-time PCR data are normalized to that of the RPS8 internal control and are shown as severalfold increases relative to those of unstimulated control cells. Bars are means ± SD of five mice per group (*, P < 0.05 compared with the saline group).
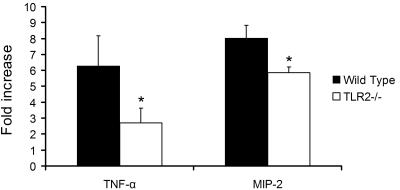
Alveolar macrophages from P. murina-challenged TLR2−/− mice showed lower cytokine responses.
To investigate the role of TLR2 in the inflammatory response to P. murina in vivo, TLR2−/− mice and wild-type control C57BL/6 mice were transtracheally inoculated with 5 × 106 P. murina cells in 50 μl of sterile saline. Mice transtracheally inoculated with 50 μl of sterile saline were used as a control. As shown in Fig. 7, alveolar macrophages from P. murina-challenged wild-type mice showed a 6.3-fold increase in TNF-α mRNA expression and an 8.0-fold increase in MIP-2 mRNA expression compared to expression levels of control mice that were challenged with sterile saline. Alveolar macrophages from P. murina-challenged TLR2−/− mice had only a 2.7-fold increase in TNF-α mRNA expression and a 5.8-fold increase in MIP-2 mRNA expression compared to control TLR2−/− mice that were challenged with sterile saline. The TNF-α and MIP-2 responses of TLR2−/− mice to P. murina were significantly lower than those of wild-type mice (P = 0.01 and 0.03, respectively).
FIG. 7.
Responses of alveolar macrophages to P. murina in vivo. Wild-type and TLR2−/− mice were transtracheally inoculated with 5 × 106 P. murina cells in 50 μl of sterile saline. Control mice were transtracheally inoculated with 50 μl of sterile saline. Eight hours after inoculation, alveolar macrophages were isolated, and total RNA isolated from the alveolar macrophages was subjected to real-time RT-PCR analysis for TNF-α and MIP-2 mRNA expression. TNF-α and MIP-2 real-time PCR data are normalized to that of the RPS8 internal control and are shown as severalfold increases relative to those of control mice that were inoculated with sterile saline. Bars are means ± SD of five mice per group (*, P < 0.05 compared with wild-type mice).
DISCUSSION
As members of the important pattern recognition receptor superfamily, TLRs have been studied by many investigators to determine their roles in immune responses against various microbial pathogens (1, 26, 27, 48). However, the role of TLR2 in Pneumocystis infection is unknown. In this study, we showed that P. murina activates TLR2 in mouse alveolar macrophages, leading to NF-κB nuclear translocation (Fig. 2) and the subsequent induction of TNF-α and MIP-2 production (Fig. 1). The observation that treatment of alveolar macrophages with an anti-TLR2 antibody abrogated TNF-α and MIP-2 production after P. murina stimulation implicates TLR2 in this immune response (Fig. 5). The role of TLR-2 in the innate response against P. murina was further confirmed by the study with TLR2 knockout mice. Alveolar macrophages from these mice fail to respond to P. murina stimulation to produce TNF-α and MIP-2 (Fig. 6).
NF-κB is an important transcription factor of many inflammatory genes, including those that encode cytokines, chemokines, and adhesion molecules (13, 39). Pneumocystis organisms have been shown to activate the NF-κB signaling pathway in both human alveolar macrophages and mouse alveolar epithelial cells (49, 54). In the present study, we showed that in vitro stimulation of mouse alveolar macrophages by P. murina induces similar immune responses, including NF-κB activation and production of TNF-α and MIP-2. Both TNF-α and MIP-2 are induced during Pneumocystis infection in humans and animals (7, 15, 46, 51).
TNF-α and MIP-2 are key factors that initiate inflammatory and immune responses. TNF-α promotes the recruitment of neutrophils, lymphocytes, and monocytes (47). The release of TNF-α by alveolar macrophages in response to Pneumocystis infection has been shown to be critical for the elimination of organisms from the host. Animals challenged with Pneumocystis organisms exhibit substantially impaired clearance of organisms when TNF-α activity is inhibited by neutralizing antibodies or when the TNF-α receptor gene is genetically knocked out (35). A profound increase in TNF-α production by alveolar macrophages is found in AIDS patients with active PcP but not in AIDS patients without PcP (20). TNF-α levels in bronchoalveolar lavage fluid from normal and CD4-depleted mice are increased when the animals are challenged with Pneumocystis organisms (19). Additionally, an in vitro study showed that Pneumocystis organisms can directly stimulate the secretion of TNF-α from rat alveolar macrophages (15).
Many chemokines are involved in the Pneumocystis-induced inflammatory response, including RANTES, monocyte chemotactic protein 1, lymphotactin, MIP-1α, MIP-1β, and MIP-2 (52). MIP-2 is a neutrophil chemoattractant with functions similar to that of interleukin-8 (36). Bronchoalveolar neutrophilia is associated with disease severity and an increased risk of death from PcP in humans (5). However, a recent study showed that neutrophils are not the causative agent of lung damage in mice with Pneumocystis infection (42). MIP-2 is also a potent chemoattractant for T lymphocytes (21). CD8+ lymphocytes have been shown to accumulate in high numbers in the alveoli of Pneumocystis-infected animals (4). The presence of CD8+ T cells in the lung has been shown to cause significant pulmonary inflammation and to contribute to PcP-related respiratory impairment in a CD4+ cell-depleted mouse model of infection (50).
Although how Pneumocystis organisms interact with immune cells is unknown, it is conceivable that they interact with various cell surface receptors. TLR2 has been shown to collaborate with Dectin-1 in the immune response to β-glucan (12), which is a major component of the Pneumocystis cell wall. Pneumocystis β-glucan has been shown to stimulate the release of TNF-α from alveolar macrophages (15). By measuring the mRNA expression levels of TLR2 in alveolar macrophages, we found that TLR2 expression in alveolar macrophages was significantly up-regulated by Pneumocystis stimulation, suggesting a role for TLR2 in the immune response against Pneumocystis infection. In contrast, the TLR4 mRNA level was not changed by Pneumocystis stimulation. However, this result does not suggest that TLR4 is not involved in the recognition of Pneumocystis, as TLR4 may be activated without a significant increase in mRNA levels (11).
TLR2 recognizes a large variety of microbial molecules due to its ability to collaborate with several other proteins that are either structurally related or unrelated (43). Steele et al. previously reported that Dectin-1 blockage significantly reduced MIP-2 production by alveolar macrophages in response to Pneumocystis (40). This observation is consistent with our finding of the role of TLR2 in response to Pneumocystis infection and the recent report on the collaborative induction of the inflammatory response by Dectin-1 and TLR2 to β-glucans of S. cerevisiae (12). In addition to Dectin-1, the mannose receptor has also previously been reported to be important for the response of alveolar macrophages to Pneumocystis (54). Our data (Fig. 7) showing that TLR2−/− mice challenged with P. murina had reduced cytokine responses further confirm the role of TLR2 in response to the organism. It is likely that the inflammatory response to Pneumocystis depends on the overall function of several different receptors and the interaction of these receptors with TLR2.
Through gain-of-function studies with HEK293 cells transiently expressing TLR2 or TLR4, we found that TLR2, but not TLR4, triggers the NF-κB response to Pneumocystis (Fig. 4C). TLR4 is an essential receptor for LPS (16) and various other ligands (31, 38). TLR4 may signal through a MyD88-dependent pathway to cause NF-κB nuclear translocation and the production of many inflammatory cytokines. TLR4 can also signal through a MyD88-independent pathway, leading to the activation of transcription factor interferon regulatory factor 3, which results in the production of beta interferon (IFN-β). IFN-β in turn induces several IFN-β-inducible genes, such as monocyte chemoattractant protein 5 and IFN-inducible protein 10, which also play important roles in innate immunity (45). In the present study, there was little NF-κB activation after Pneumocystis stimulation in TLR4-tranfected HEK293 cells, suggesting that Pneumocystis does not induce NF-κB activation through TLR4. This is consistent with a previous study by Lebron et al. (23) in which alveolar macrophages from TLR4-deficient and wild-type mice produced equivalent amounts of TNF-α when stimulated with P. carinii β-glucan. However, our result does not necessarily mean that TLR4 is not involved in the immune response to Pneumocystis at all, as HEK293 cells lack many immunoreceptors that are present on the surface of alveolar macrophages. In addition, Evans et al. (10) previously reported that epithelial cells signal Pneumocystis β-glucans differently from alveolar macrophages. Since the response of HEK293 cells to microbial stimulation is more similar to that of epithelial cells, our gain-of-function study in HEK293 cells may not reflect actual responses in alveolar macrophages. A recent study by Ding et al. showed that impaired recognition through TLR4 is responsible for an exacerbated murine Pneumocystis pneumonia (9), indicating that TLR4 does play some role in the response to P. carinii infection. In their study, HEK293 cells expressing TLR4 also displayed no elevated NF-κB activation after stimulation with P. murina antigen. However, they found that there was a reduced level of MIP-2 in the bronchoalveolar lavage fluid of TLR4 mutant mice with PcP, suggesting that TLR4 may mediate the inflammatory response to Pneumocystis through transcription factors other than NF-κB.
In conclusion, our data demonstrate that Pneumocystis stimulates alveolar macrophage NF-κB nuclear translocation and cytokine production through interaction with TLR2. We are currently investigating the hypothesis that TLR2 deficiency leads to an insufficient cytokine response by alveolar macrophages and less efficient protection against long-term Pneumocystis infection in vivo.
Acknowledgments
This study was supported by Public Health Service grant RO1 HL65170 from the National Heart, Lung, and Blood Institute.
Editor: A. Casadevall
REFERENCES
- 1.Akamine, M., F. Higa, N. Arakaki, K. Kawakami, K. Takeda, S. Akira, and A. Saito. 2005. Differential roles of Toll-like receptors 2 and 4 in in vitro responses of macrophages to Legionella pneumophila. Infect. Immun. 73:352-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker-LePain, J. C., M. Sarzotti, and C. V. Nicchitta. 2004. Glucose-regulated protein 94/glycoprotein 96 elicits bystander activation of CD4+ T cell Th1 cytokine production in vivo. J. Immunol. 172:4195-4203. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett, M. S., S. F. Queener, M. M. Durkin, M. A. Shaw, and J. W. Smith. 1992. Inoculated mouse model of Pneumocystis carinii infection. Diagn. Microbiol. Infect. Dis. 15:129-134. [DOI] [PubMed] [Google Scholar]
- 4.Beck, J. M., R. L. Newbury, B. E. Palmer, M. L. Warnock, P. K. Byrd, and H. B. Kaltreider. 1996. Role of CD8+ lymphocytes in host defense against Pneumocystis carinii in mice. J. Lab. Clin. Med. 128:477-487. [DOI] [PubMed] [Google Scholar]
- 5.Benfield, T. L., J. Vestbo, J. Junge, T. L. Nielsen, A. B. Jensen, and J. D. Lundgren. 1995. Prognostic value of interleukin-8 in AIDS-associated Pneumocystis carinii pneumonia. Am. J. Respir. Crit. Care Med. 151:1058-1062. [DOI] [PubMed] [Google Scholar]
- 6.Carter, A. B., M. M. Monick, and G. W. Hunninghake. 1998. Lipopolysaccharide-induced NF-κB activation and cytokine release in human alveolar macrophages is PKC-independent and TK- and PC-PLC-dependent. Am. J. Respir. Cell Mol. Biol. 18:384-391. [DOI] [PubMed] [Google Scholar]
- 7.Chen, W., E. A. Havell, and A. G. Harmsen. 1992. Importance of endogenous tumor necrosis factor alpha and gamma interferon in host resistance against Pneumocystis carinii infection. Infect. Immun. 60:1279-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-10692. [DOI] [PubMed] [Google Scholar]
- 9.Ding, K., A. Shibui, Y. Wang, M. Takamoto, T. Matsuguchi, and K. Sugane. 2005. Impaired recognition by Toll-like receptor 4 is responsible for exacerbated murine Pneumocystis pneumonia. Microbes Infect. 7:195-203. [DOI] [PubMed] [Google Scholar]
- 10.Evans, S. E., P. Y. Hahn, F. McCann, T. J. Kottom, Z. V. Pavlovic, and A. H. Limper. 2005. Pneumocystis cell wall β-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor-κB-dependent mechanisms. Am. J. Respir. Cell Mol. Biol. 32:490-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchini, M., M. Schweizer, P. Matzener, I. Magkouras, K. S. Sauter, J. Mirkovitch, E. Peterhans, and T. W. Jungi. 2005. Evidence for dissociation of TLR mRNA expression and TLR agonist-mediated functions in bovine macrophages. Vet. Immunol. Immunopathol. [Online.] doi: 10.1016/j.vetimm.2005.09.002. [DOI] [PubMed]
- 12.Gantner, B. N., R. M. Simmons, S. J. Canavera, S. Akira, and D. M. Underhill. 2003. Collaborative induction of inflammatory responses by Dectin-1 and Toll-like receptor 2. J. Exp. Med. 197:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 14.Hahn, P. Y., S. E. Evans, T. J. Kottom, J. E. Standing, R. E. Pagano, and A. H. Limper. 2003. Pneumocystis carinii cell wall β-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J. Biol. Chem. 278:2043-2050. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman, O. A., J. E. Standing, and A. H. Limper. 1993. Pneumocystis carinii stimulates tumor necrosis factor-α release from alveolar macrophages through a β-glucan-mediated mechanism. J. Immunol. 150:3932-3940. [PubMed] [Google Scholar]
- 16.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 17.Keely, S. P., J. M. Fischer, M. T. Cushion, and J. R. Stringer. 2004. Phylogenetic identification of Pneumocystis murina sp. nov., a new species in laboratory mice. Microbiology 150:1153-1165. [DOI] [PubMed] [Google Scholar]
- 18.Kirschning, C. J., H. Wesche, T. M. Ayres, and M. Rothe. 1998. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 188:2091-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolls, J. K., J. M. Beck, S. Nelson, W. R. Summer, and J. Shellito. 1993. Alveolar macrophage release of tumor necrosis factor during murine Pneumocystis carinii pneumonia. Am. J. Respir. Cell Mol. Biol. 8:370-376. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan, V. L., A. Meager, D. M. Mitchell, and A. J. Pinching. 1990. Alveolar macrophages in AIDS patients: increased spontaneous tumour necrosis factor-α production in Pneumocystis carinii pneumonia. Clin. Exp. Immunol. 80:156-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen, C. G., A. O. Anderson, E. Appella, J. J. Oppenheim, and K. Matsushima. 1989. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science 243:1464-1466. [DOI] [PubMed] [Google Scholar]
- 22.Lasbury, M. E., P. J. Durant, and C. H. Lee. 2003. Numbers of alveolar macrophages are increased during Pneumocystis pneumonia in mice. J. Eukaryot. Microbiol. 50(Suppl):637-638. [DOI] [PubMed] [Google Scholar]
- 23.Lebron, F., R. Vassallo, V. Puri, and A. H. Limper. 2003. Pneumocystis carinii cell wall β-glucans initiate macrophage inflammatory responses through NF-κB activation. J. Biol. Chem. 278:25001-25008. [DOI] [PubMed] [Google Scholar]
- 24.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 25.Linke, M. J., M. T. Cushion, and P. D. Walzer. 1989. Properties of the major antigens of rat and human Pneumocystis carinii. Infect. Immun. 57:1547-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorenz, E., D. C. Chemotti, A. L. Jiang, and L. D. McDougal. 2005. Differential involvement of Toll-like receptors 2 and 4 in the host response to acute respiratory infections with wild-type and mutant Haemophilus influenzae strains. Infect. Immun. 73:2075-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandell, L., A. P. Moran, A. Cocchiarella, J. Houghton, N. Taylor, J. G. Fox, T. C. Wang, and E. A. Kurt-Jones. 2004. Intact gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via Toll-like receptor 2 but not Toll-like receptor 4. Infect. Immun. 72:6446-6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 29.Meng, G., M. Rutz, M. Schiemann, J. Metzger, A. Grabiec, R. Schwandner, P. B. Luppa, F. Ebel, D. H. Busch, S. Bauer, H. Wagner, and C. J. Kirschning. 2004. Antagonistic antibody prevents Toll-like receptor 2-driven lethal shock-like syndromes. J. Clin. Investig. 113:1473-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris, A., R. M. Wachter, J. Luce, J. Turner, and L. Huang. 2003. Improved survival with highly active antiretroviral therapy in HIV-infected patients with severe Pneumocystis carinii pneumonia. AIDS 17:73-80. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira, A. C., J. R. Peixoto, L. B. de Arruda, M. A. Campos, R. T. Gazzinelli, D. T. Golenbock, S. Akira, J. O. Previato, L. Mendonca-Previato, A. Nobrega, and M. Bellio. 2004. Expression of functional TLR4 confers proinflammatory responsiveness to Trypanosoma cruzi glycoinositolphospholipids and higher resistance to infection with T. cruzi. J. Immunol. 173:5688-5696. [DOI] [PubMed] [Google Scholar]
- 32.O'Riordan, D. M., J. E. Standing, and A. H. Limper. 1995. Pneumocystis carinii glycoprotein A binds macrophage mannose receptors. Infect. Immun. 63:779-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pascale, J. M., M. M. Shaw, P. J. Durant, A. A. Amador, M. S. Bartlett, J. W. Smith, R. L. Gregory, and G. L. McLaughlin. 1999. Intranasal immunization confers protection against murine Pneumocystis carinii lung infection. Infect. Immun. 67:805-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rock, F. L., G. Hardiman, J. C. Timans, R. A. Kastelein, and J. F. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 95:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudmann, D. G., A. M. Preston, M. W. Moore, and J. M. Beck. 1998. Susceptibility to Pneumocystis carinii in mice is dependent on simultaneous deletion of IFN-γ and type 1 and 2 TNF receptor genes. J. Immunol. 161:360-366. [PubMed] [Google Scholar]
- 36.Schmal, H., T. P. Shanley, M. L. Jones, H. P. Friedl, and P. A. Ward. 1996. Role for macrophage inflammatory protein-2 in lipopolysaccharide-induced lung injury in rats. J. Immunol. 156:1963-1972. [PubMed] [Google Scholar]
- 37.Sepkowitz, K. A. 2002. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin. Infect. Dis. 34:1098-1107. [DOI] [PubMed] [Google Scholar]
- 38.Shoham, S., C. Huang, J. M. Chen, D. T. Golenbock, and S. M. Levitz. 2001. Toll-like receptor 4 mediates intracellular signaling without TNF-α release in response to Cryptococcus neoformans polysaccharide capsule. J. Immunol. 166:4620-4626. [DOI] [PubMed] [Google Scholar]
- 39.Silverman, N., and T. Maniatis. 2001. NF-κB signaling pathways in mammalian and insect innate immunity. Genes Dev. 15:2321-2342. [DOI] [PubMed] [Google Scholar]
- 40.Steele, C., L. Marrero, S. Swain, A. G. Harmsen, M. Zheng, G. D. Brown, S. Gordon, J. E. Shellito, and J. K. Kolls. 2003. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the Dectin-1 β-glucan receptor. J. Exp. Med. 198:1677-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stringer, J. R., C. B. Beard, R. F. Miller, and A. E. Wakefield. 2002. A new name (Pneumocystis jiroveci) for Pneumocystis from humans. Emerg. Infect. Dis. 8:891-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swain, S. D., T. W. Wright, P. M. Degel, F. Gigliotti, and A. G. Harmsen. 2004. Neither neutrophils nor reactive oxygen species contribute to tissue damage during Pneumocystis pneumonia in mice. Infect. Immun. 72:5722-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeda, K., and S. Akira. 2005. Toll-like receptors in innate immunity. Int. Immunol. 17:1-14. [DOI] [PubMed] [Google Scholar]
- 44.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 45.Toshchakov, V., B. W. Jones, P. Y. Perera, K. Thomas, M. J. Cody, S. Zhang, B. R. Williams, J. Major, T. A. Hamilton, M. J. Fenton, and S. N. Vogel. 2002. TLR4, but not TLR2, mediates IFN-β-induced STAT1α/β-dependent gene expression in macrophages. Nat. Immunol. 3:392-398. [DOI] [PubMed] [Google Scholar]
- 46.Vassallo, R., J. E. Standing, and A. H. Limper. 2000. Isolated Pneumocystis carinii cell wall glucan provokes lower respiratory tract inflammatory responses. J. Immunol. 164:3755-3763. [DOI] [PubMed] [Google Scholar]
- 47.Vilcek, J., and T. H. Lee. 1991. Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J. Biol. Chem. 266:7313-7316. [PubMed] [Google Scholar]
- 48.Viriyakosol, S., J. Fierer, G. D. Brown, and T. N. Kirkland. 2005. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1. Infect. Immun. 73:1553-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, J., F. Gigliotti, S. Maggirwar, C. Johnston, J. N. Finkelstein, and T. W. Wright. 2005. Pneumocystis carinii activates the NF-κB signaling pathway in alveolar epithelial cells. Infect. Immun. 73:2766-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright, T. W., F. Gigliotti, J. N. Finkelstein, J. T. McBride, C. L. An, and A. G. Harmsen. 1999. Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia. J. Clin. Investig. 104:1307-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright, T. W., C. J. Johnston, A. G. Harmsen, and J. N. Finkelstein. 1997. Analysis of cytokine mRNA profiles in the lungs of Pneumocystis carinii-infected mice. Am. J. Respir. Cell Mol. Biol. 17:491-500. [DOI] [PubMed] [Google Scholar]
- 52.Wright, T. W., C. J. Johnston, A. G. Harmsen, and J. N. Finkelstein. 1999. Chemokine gene expression during Pneumocystis carinii-driven pulmonary inflammation. Infect. Immun. 67:3452-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, G., and S. Ghosh. 2002. Negative regulation of Toll-like receptor-mediated signaling by Tollip. J. Biol. Chem. 277:7059-7065. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, J., J. Zhu, A. Imrich, M. Cushion, T. B. Kinane, and H. Koziel. 2004. Pneumocystis activates human alveolar macrophage NF-κB signaling through mannose receptors. Infect. Immun. 72:3147-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]