Abstract
Yersinia species pathogenic to humans have been extensively characterized with respect to type III secretion and its essential role in virulence. This study concerns the twin arginine translocation (Tat) pathway utilized by gram-negative bacteria to secrete folded proteins across the bacterial inner membrane into the periplasmic compartment. We have shown that the Yersinia Tat system is functional and required for motility and contributes to acid resistance. A Yersinia pseudotuberculosis mutant strain with a disrupted Tat system (tatC) was, however, not affected in in vitro growth or more susceptible to high osmolarity, oxidative stress, or high temperature, nor was it impaired in type III secretion. Interestingly, the tatC mutant was severely attenuated via both the oral and intraperitoneal routes in the systemic mouse infection model and highly impaired in colonization of lymphoid organs like Peyer's patches and the spleen. Our work highlights that Tat secretion plays a key role in the virulence of Y. pseudotuberculosis.
As the integrity of the bacterial cytoplasm is protected by membranes, the necessity of means for transport of molecules between the bacterial compartments, periplasm and cytoplasm, and the surrounding milieu is obvious. Not only do bacteria require the ability to take up nutrients and get rid of waste, transport across the membranes is also highly involved in the ability of bacteria to cause disease. A number of specialized protein secretion systems are essential for delivery of virulence factors across the two membranes of gram-negative pathogens (39). The importance of these systems is reflected in the multitude of mechanisms that have evolved not only for protein translocation across the bacterial membranes but also for targeting of anti-host factors across the host cell membrane. The means of translocation across the bacterial outer membrane have been grouped into five different pathways (I to V) (66). In pathways I, III, and IV, the substrate appears to be targeted directly across the two membranes without any periplasmic intermediates (13, 18, 20, 24, 42, 53), whereas in pathways II (58) and V (32), the process is divided into discrete steps where translocation across the inner membrane occurs as the substrate is targeted to the periplasm either by the general secretory pathway (sec system) (23) or via the more recently discovered twin arginine translocation (Tat) pathway (6).
The most extensively studied secretion and virulence mechanism of the pathogenic Yersinia species Yersinia enterocolitica, Y. pseudotuberculosis, and Y. pestis is the plasmid-encoded type III secretion system (T3SS). Utilizing this mechanism, the pathogens deliver Yop effectors across the bacterial envelope and the host cell membrane in a bacterium-cell contact-dependent process (48, 55). A chromosomal T3SS is also present in all three species and has been shown to be important for virulence in Y. enterocolitica (30). In contrast, a role in virulence for the chromosomal systems encoded by Y. pseudotuberculosis and Y. pestis has not been established (51). The plasmid-encoded T3SS has, on the other hand, been shown to be a powerful tool for subversion of the host immune defense and is essential for virulence in all three human-pathogenic Yersinia species. Hence, T3SS mutants are highly attenuated in mouse infection models. Although Y. pseudotuberculosis, in contrast to Y. pestis, rarely causes systemic infections in humans, it inflicts a systemic plague-like disease in mice (28), making it a relevant and convenient model to study the mechanisms of severe infections.
The rapid progress in genome sequencing of bacterial pathogens has extended the possibilities to identify novel putative virulence determinants, as well as secretion systems (45). The genomes of the closely related species Y. pseudotuberculosis and Y. pestis both contain homologues to genes encoding the components required to assemble a functional Tat system in Escherichia coli (14, 47). The Tat pathway targets folded proteins, identified via an N-terminal signal peptide with an S/T-R-R-x-F-L-K consensus motif, for secretion across the inner membrane of gram-negative bacteria. A majority of the E. coli Tat substrates bind cofactors in the cytoplasm (5, 6), therefore demanding complete folding prior to their export. Tat substrates with no cofactor have, however, also been identified. Halophilic archaea require a way to rapidly stabilize their proteins in protection against the nearly saturated salt conditions in their cytoplasm. Thus, rapid folding of newly synthesized proteins is performed in the cytoplasm, facilitated by the ability of the majority of the exported proteins in these archaea to take the Tat route (34, 54). In E. coli, where Tat has been paradigmatically researched, the minimal components of the Tat protein translocation system are the membrane proteins TatA, TatB, and TatC (8, 59, 70). In many bacteria, a wide range of substrates are secreted by the Tat pathway and as a result, tat mutants often show pleiotropic phenotypes with defects in biogenesis of the cell envelope (36), biofilm formation (35), motility (21, 43), and resistance to various environmental stress conditions (62). However, the Tat system has also been shown to be involved in secretion of virulence determinants like phospholipase toxins in Pseudomonas aeruginosa (43, 69), P. syringae pv. tomato DC3000 (11), and Legionella pneumophila (56), as well as Shiga toxin 1 in enterohemorrhagic E. coli (50).
In this work, we have established that Y. pseudotuberculosis and Y. pestis both express functional Tat translocation systems and further investigated the phenotypes of a Y. pseudotuberculosis tatC mutant strain. Somewhat surprisingly, disruption of the Tat system did not influence in vitro growth under low-nutrient conditions or stress conditions like high osmolarity, high temperature, and oxidative stress. Nor was the tat mutant defective for phenotypes coupled to functional T3S, including secretion of Yop effectors under low-Ca2+ conditions, their translocation into target cells, and resistance against phagocytosis by macrophages. The only in vitro defects we could confirm for the tatC mutant were loss of motility at environmental temperatures and a slight decrease in acid resistance. Interestingly, Tat was demonstrated to have an essential role in Yersinia pathogenicity, as the tatC mutant was severely attenuated for virulence in the systemic mouse infection model. Thus, our work highlights that twin arginine translocation plays a key role in the ability of Y. pseudotuberculosis to cause disease.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Routine culturing of bacteria occurred in Luria-Bertani (LB) broth (17) or on blood agar base (Merck) plates at 26°C (Y. pseudotuberculosis and Y. pestis) or at 37°C (E. coli) with aeration. For analysis of in vitro growth at 26°C or 37°C, defined Yersinia medium (TMH; 63) (nutrient-rich medium) or Yersinia defined minimal medium (YDM; 1× M9 minimal salts, 0.4% glucose, 0.4% Casamino Acids, 10 mM MgCl2, 5 mM K2SO4, 10-μg/ml thiamine) (nutrient-poor medium) was used. When growth was investigated at 37°C, the cultures were pregrown for 1 h at 26°C and then shifted to 37°C. To induce or repress T3S, media were supplemented with 5 mM EGTA and 20 mM MgCl2 (inducing medium) or 2.5 mM CaCl2 (noninducing medium), respectively. Where appropriate, antibiotics were added to the following final concentrations: ampicillin, 100 μg/ml; chloramphenicol, 20 μg/ml. For induction of the tac promoter, IPTG (isopropyl-β-d-thiogalactopyranoside) was added to the growth medium at a final concentration of 0.4 mM.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| Y. pseudotuberculosis | ||
| IP32953 | Wild type, serotype I | E. Carniela |
| IP32953c | IP32953, cured of pYV | S. Garbom,b unpublished |
| IP32953tatC | IP32953, tatC insertion mutation; Cmlr | This study |
| Y. pestis | ||
| KIM5 | Wild type | R. R. Brubakerc,d |
| KIM5tatC | KIM5, tatC insertion mutation; Cmlr | This study |
| E. coli S17-1λpir | RP4-2 Tc::Mu-Km::Tn7 (λpir) | 19 |
| Plasmids | ||
| pNQ705 | Suicide integration vector; Cmlr | 41 |
| pML30 | pNQ705 with bases 19-329 of tatC; Cmlr | This study |
| pMMB66HE | Ptac expression vector; Ampr | 26 |
| pML31 | pMMB66EH with tatC; Ampr | This study |
| pZD51 | pMMB22 with torA::GFP; Ampr | 21 |
Pasteur Institute, Paris, France.
Umeå University, Umeå, Sweden.
Michigan State University, East Lansing.
KIM5 is also called KIM10 or substrain D27 (49).
DNA methods.
Preparation of plasmid DNA, restriction enzyme digests, ligation, and transformation of E. coli strains were performed according to standard methods (57).
Generation of tatC insertion mutants in Yersinia.
An internal fragment of the tatC gene was amplified by PCR with IP32953 as the template and primers 5′-GCACTCGAGCTCCAACCCCTTATCTCTCATCTGA-3′ (SacI site underlined) and 5′-GCTCACTCTAGATAACGGCACCATCAAACGGCG-3′ (XbaI site underlined). Nucleotides in italics correspond to bases 306379 to 306400 and 306690 to 306670, respectively, in the IP32953 sequence (GenBank accession number BX936398). The resulting 311-bp fragment was introduced into XbaI/SacI-digested pNQ705 (41), resulting in the suicide vector pML30. Conjugal mating experiments with S17-1λpir as the donor strain allowed the integration of pML30 within regions of complementary sequences on the chromosomes of Y. pseudotuberculosis IP32953 and Y. pestis KIM5 (41), thereby generating mutants IP32953tatC and KIM5tatC, respectively.
Construction of a vector for trans complementation of tatC mutants.
The expression vector pML31, encoding tatC under the control of the tac promoter, was generated as follows. By PCR with primers 5′-GCACTCGGATCCCGG CTAAATATCAACGCATCGA-3′ (BamHI site underlined) and 5′-GCACTC GAATTCATCTCGTATTCACCAACCTGGC-3′ (EcoRI site underlined), an 814-bp fragment encoding tatC was obtained. Italicized letters correspond to nucleotides 306325 to 306346 and 307160 to 307139, respectively, in the IP32953 sequence (GenBank accession number BX936398). The fragment was introduced into BamHI/EcoRI-digested pMMB66HE (26), resulting in plasmid pML31. Plasmids were transferred into recipient Yersinia by conjugation.
Yop expression and secretion assay.
Induction of type III substrate synthesis and secretion from Y. pseudotuberculosis were performed as previously described (4). Total protein levels were assessed by sampling directly from the bacterial culture suspension containing both proteins secreted into the culture medium and bacteria associated. Sampling of the cleared supernatant allowed assessment of secreted protein levels. All protein fractions were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subjected to immunoblotting. Detection of specific proteins on membrane support was achieved by the use of rabbit polyclonal antiserum raised against all secreted Yops (AgriSera AB), followed by ECL detection (Amersham Pharmacia Biotech).
Use of the torA::GFP reporter system to analyze Tat functionality in Yersinia.
The reporter plasmid pZD51 encoding the Tat signal sequence of trimethylamine N-oxide reductase (TorA) of E. coli fused to green fluorescent protein (GFP), under control of the IPTG-inducible tac promoter (21), was introduced by electroporation into wild-type or tatC mutant Y. pseudotuberculosis and Y. pestis. Due to the leakiness of the tac promoter, no IPTG addition was required to achieve detectable levels of the GFP reporter. The cellular location of GFP was determined by fluorescence microscopy on bacteria grown in liquid culture.
Motility assay.
Motility assays were conducted on 0.3% minimal motility agar containing (wt/vol) 1% peptone and 0.5% NaCl. Where appropriate, antibiotics and IPTG were added.
Cell cultures grown in LB broth overnight were normalized to an optical density at 600 nm of 2.5, and 1 μl of each strain was inoculated onto the surface of the motility plates. Motility was examined at 24, 48, 60, and 96 h of incubation at 22 and 37°C. At least four independent motility assays were carried out for each strain and condition.
Environmental stress experiments.
The effects of high osmolarity, oxidative stress, low pH, and high temperature were determined for strains of Y. pseudotuberculosis grown overnight in LB broth at 26°C and followed established protocols (12, 22, 44, 72, 73). For high-temperature stress experiments, stationary-phase cells were transferred to prewarmed tubes and incubated at 55°C for 5 min. Control bacteria were incubated at ambient temperature (22°C). In the oxidative-stress experiments, cells were incubated for 1 h at 26°C in 40 mM H2O2 in deionized water. For high-osmolarity stress, cells were incubated in 2.4 M NaCl in deionized water for 1 h at 26°C. To evaluate survival at low pH, cells were incubated in LB broth adjusted to pH 3 with HCl for 5 min at ambient temperature. Control bacteria were incubated in deionized water or LB broth. After exposure, the bacteria were pelleted at 12,000 × g for 5 min at 22°C and resuspended in sterile phosphate-buffered saline (PBS) (pH 7.4), and appropriate dilutions were plated on LA agar, LB broth (17) with 1.5% agar, for the wild type and on LA agar containing 20 μg/ml chloramphenicol for tatC mutant cells. Plates were incubated at 26°C for 2 days for CFU counting. All survival experiments were repeated at least three times with duplicate samples of each strain in each individual experiment.
Cultivation and infection of HeLa cells.
Culture maintenance and infections with Yersinia followed established protocols (65). The cytotoxic effect induced in the infected HeLa cells was monitored by light microscopy, and images were collected at successive time points.
Survival in J774 macrophage cell cultures.
Culture maintenance and infections of macrophages with Yersinia were performed essentially as described previously (44), with the exception that the number of viable bacterial cells at different time points after infection was determined after growth on LA agar supplemented with appropriate antibiotics. Duplicate samples were taken at all time points, and the assay was repeated twice.
Phagocytosis inhibition assay.
The phagocytosis inhibition assay followed established protocols (64), with subtle modifications. Y. pseudotuberculosis cultures were adjusted to an optical density at 600 nm of 0.2 in Dulbecco's modified Eagle's medium and subsequently grown at 26°C for 30 min before a shift to 37°C for an additional 2 h. J774 macrophages seeded at a density of 5 × 104 cells onto an eight-chamber slide were infected with bacteria to a final A600 of 0.1 or 0.01. After infection, macrophages were washed and fixed in 2% formalin. Cells were further washed, blocked for 10 min in 0.1 M glycine, washed, and then incubated with anti-Yersinia rabbit antiserum, followed by Alexa Fluor 568 red-conjugated goat anti-rabbit antibodies (Molecular Probes). Macrophages were permeabilized (0.15% saponin), washed, and incubated with anti-Yersinia antiserum (containing 0.15% saponin, 2% bovine serum albumin, and 0.1 M glycine). Diluted in the same solution, Alexa Fluor 488 green-conjugated antibodies (Molecular Probes) were added. This staining procedure allowed us to distinguish between external (red) and internal (green) bacteria by fluorescence microscopy.
Infection of mice.
This study was approved by the Local Ethical Committee on Laboratory Animals in Umeå, Sweden. Mice were housed under conventional conditions, given food and water ad libitum, and allowed to acclimatize for at least 7 days before infection. For oral infection of mice, the parental strain IP32953, the tatC mutant, and as a negative control, an avirulent, plasmid-cured strain (IP32953c) were used. Bacteria grown overnight in LB broth at 26°C were harvested and resuspended in 50 ml of 0.9% NaCl in sterilized tap water to concentrations of 109, 108, and 107 CFU/ml. Each suspension was given to groups of five C57BL/6 (Scanbur BK) female mice deprived of food and water overnight (18 h), after which the mice were allowed to drink the bacterium-containing water ad libitum for 6 h. Each mouse consumed approximately 3 ml of the bacterial suspension. To ensure that the strains tested did not succumb under these conditions during the feeding period, their ability to survive in the drinking water was monitored for 8 h. For intraperitoneal infection, bacteria grown overnight in LB broth at 26°C were harvested and resuspended in PBS at concentrations ranging from approximately 104 to 108 bacteria/ml. For each concentration used, 0.1 ml was injected intraperitoneally into five mice. Aliquots of the diluted cultures were also plated on blood agar base plates with appropriate antibiotics to determine the number of CFU injected. An in vivo segregation assay for the strains in Peyer's patches and the spleen was also performed. The mice were infected orally with a concentration of 109 CFU/ml as described above. Two mice from each group were sacrificed daily, and six Peyer's patches and the spleen were removed by dissection. The organs were homogenized in PBS, and serial dilutions were spread on Yersinia selective agar base (Difco) plates for viable counting. In all of the infection experiments, the mice were monitored at least two times daily for 14 days. Mice that showed severe symptoms like hunched carriage and tousled fur, no longer moved around, and had stopped feeding and drinking were sacrificed according to the instructions of the Local Ethical Committee on Laboratory Animals. Fifty percent infective doses were calculated based on the number of animals that had to be sacrificed as a result of severe symptoms by the Reed-Muench method for estimation of 50% endpoints (52). Based on previous experience, the calculated infective doses are comparable to the 50% lethal doses of earlier studies.
Data mining.
By using the protein and nucleotide BLAST resources at the National Center for Biotechnology Information homepage (http://www.ncbi.nlm.nih.gov/BLAST), genes encoding Tat components (tatA, -B, -C, and -E) and a gene colocalizing with the tat operon (tatD) were identified in the genome sequences of Y. pseudotuberculosis IP32953 and Y. pestis KIM5. The accession numbers retrieved for Y. pseudotuberculosis IP32953 and Y. pestis KIM5 gene products were YP_068804/NP_667790 (TatA), YP_068805/NP_667791 (TatB), YP_068806/NP_667792 (TatC), YP_068807/NP_667793 (TatD), and YP_069628/NP_668496 (TatE). By using BLAST (http://www.ncbi.nlm.nih.gov/BLAST/bl2seq/wblast2.cgi), the amino acid identities between the Yersinia proteins and their E. coli homologues TatA (NP_418280), TatB (YP_026270), TatC (NP_418282), TatD (YP_026271), and TatE (NP_415160) were determined. For identification of potential Tat substrates, pattern searches of the KIM5 and IP32953 genomes were performed with the Pedant genome database (http://pedant.gsf.de). The search pattern used was <X(2,32)-RR-X-[VFLIMA](2)-X(0,10)-{RDEK}(13), i.e., a range of 2 to 32 amino acids after the N terminus prior to the twin arginine, one open residue, two amino acids each being either Val, Phe, Leu, Ile, Met, or Ala, and finally the restraint that the following 23 residues must contain an uncharged stretch of at least 13 amino acids (54). The results from the pattern search were then put through the TatP program (http://www.cbs.dtu.dk/services/TatP) (3). To reduce the number of false positives, we excluded all proteins that had negative results for of all the parameters measured by the program.
RESULTS
The Tat system is functional in Y. pseudotuberculosis strain IP32953 and Y. pestis strain KIM5.
Investigation of the genome sequences of Y. pseudotuberculosis IP32953 and Y. pestis KIM5 revealed the presence of a chromosomal region encoding four genes with high homology to E. coli genes tatA, tatB, tatC, and tatD and with identities ranging from 52 to 77% on the amino acid level. The genetic organization of the tatABCD operon is similar to that of E. coli (7), and the genes are most likely transcribed from a common promoter. In addition, the hemB gene was found to localize downstream of the tatABCD operon and is possibly also part of the same transcriptional unit. In Y. pestis KIM5, tatD is represented by a pseudogene. This is, however, not likely to affect the function of Tat since tatD, given its name due to colocalization with the tatABC operon, encodes a DNase and is not required for Tat translocation in E. coli (71). Further, the Yersinia genomes also contain the monocistronic gene encoding TatE, which has functions overlapping those of TatA and is believed to be the result of gene duplication (59).
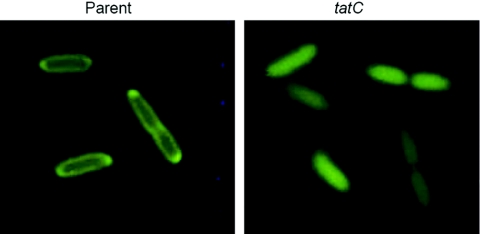
For investigation of Tat functionality, plasmid pZD51 (Table 1) (21), encoding a GFP reporter fused to the E. coli Tat signal peptide from TorA, was introduced into Yersinia. Similar to E. coli, specific targeting of GFP to the periplasm could be observed for wild-type strains of both Y. pseudotuberculosis and Y. pestis (Fig. 1 and data not shown). In contrast, when pZD51 was introduced into tatC mutant strains, the GFP fluorescence was evenly distributed in the bacterial cytoplasm, demonstrating that Tat is disrupted in these strains. From this, we conclude that Yersinia indeed encodes a functional Tat system and that, similar to what has been shown in other gram-negative bacteria, tatC is essential for the function of the system.
FIG. 1.
TatC-dependent export of TorA::GFP in Yersinia. Fluorescence images of the parental IP32953 and mutant IP32953tatC strains carrying plasmid pZD51, encoding the signal peptide from the E. coli Tat substrate TorA fused to GFP. Note the periplasmic location of GFP in the parental strain.
Disruption of the Tat system does not affect Yersinia growth in liquid culture.
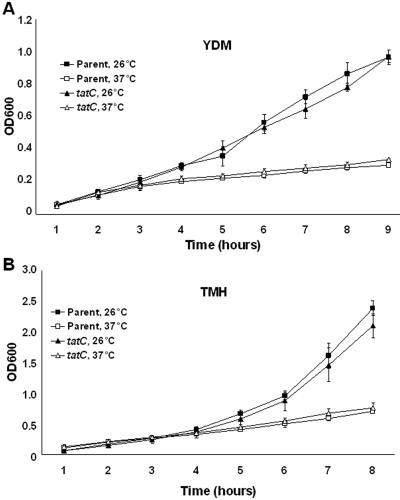
We first wanted to determine the importance of Tat under in vitro conditions. Growth in both rich (TMH) and minimal (YDM) media at 26°C or 37°C was compared for the Y. pseudotuberculosis wild-type strain and the tatC mutant. Irrespective of culture conditions, the mutant did not show any growth deficiency compared to the parental strain (Fig. 2). Therefore, the Tat protein translocation system is unlikely to have a major role in cell growth and division in Yersinia.
FIG. 2.
In vitro growth of Tat mutant Yersinia. Growth of the parental IP32953 and mutant IP32953tatC strains was determined in poor (YDM) (A) and rich (TMH) (B) media at 26°C and 37°C under low-calcium conditions. Growth assays were performed in duplicate, with the error bars representing the standard deviation. For details, see Materials and Methods. OD600, optical density at 600 nm.
A functional Tat system is required for motility under environmental conditions.
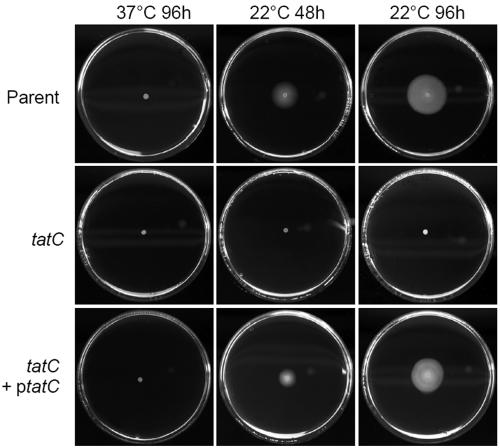
Tat secretion has been shown to be important for the motility of the gram-negative pathogens P. aeruginosa, P. syringae pv. tomato DC3000, E. coli O157:H7, and Agrobacterium tumefaciens (11, 21, 43, 50). In the latter two cases, the Tat-defective strains have been shown not to express flagellin, indicating that components of the flagellar secretion assembly and/or secretion system are Tat substrates. No role for motility per se in connection with the ability of human-pathogenic Yersinia to cause disease has been defined. In fact, both Y. enterocolitica and Y. pseudotuberculosis are motile only at environmental temperatures (2, 37, 38) and the genes encoding flagella are nonfunctional in Y. pestis (47). Cultivation of the tatC mutant and wild-type strains of Y. pseudotuberculosis on semisolid minimal agar revealed that the mutant strain was impaired in motility at 22°C. As expected, neither of the strains was motile at 37°C (Fig. 3). The motility deficiency of the mutant was fully restored by introduction of the tatC gene in trans under the control of the inducible tac promoter from pML31 (Fig. 3). Thus, the phenotype of the mutant could be confirmed to be the result of lack of a functional tatC gene and not due to effects on downstream genes. Our results also indicate that there might be a link between flagellar assembly and/or secretion and Tat secretion also in Yersinia.
FIG. 3.
Analysis of motility of Tat mutant Yersinia. The motility of the parental IP32953, mutant IP32953tatC, and trans-complemented IP32953tatC(pML31) strains was assayed by growth on semisolid minimal medium plates. Results after growth at 22°C or 37°C for 48 and 96 h are shown. For details, see Materials and Methods.
Contribution of Tat to survival under environmental stresses.
Since Tat previously has been implicated in the resistance to a range of environmental stress conditions (62), we wanted to determine whether Tat could play a similar role in Yersinia. Hence, the survival rates of the Y. pseudotuberculosis wild-type and tatC mutant strains after exposure to various stresses were examined. No effect on viability as a result of high temperature, oxidative stress, or high osmolarity could be detected for the mutant, indicating that Tat has no role in protection against these stresses. Under all of these stress conditions, the survival rates of both the wild-type and mutant strains were on the order of 85% or higher. The tatC mutant did, however, display a slightly reduced survival rate (50.4% ± 28.9%) compared to that of the wild type (88.5% ± 8.2%) when exposed to a low pH. The reduced survival of the tatC mutant strain was statistically significant (P < 0.05, Student's two-tailed t test with unequal variance). Introduction of plasmid pML31, carrying tatC under control of the tac promoter, restored the survival rate of the tatC mutant strain to wild-type levels (90.3% ± 9.3%), suggesting that there is indeed a small decrease in acid resistance associated with disruption of the Tat system.
Tat is not coupled to Yersinia T3S.
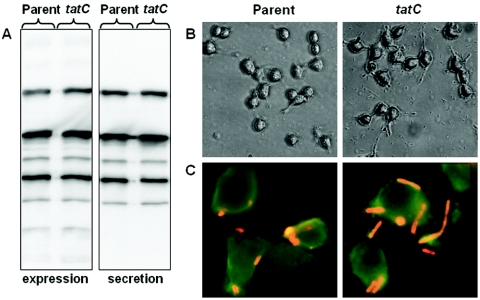
T3S is a virulence mechanism common to human-pathogenic Yersinia species. The T3SS of Yersinia and that of the opportunistic pathogen P. aeruginosa are closely related, and some of the key components are also functionally interchangeable (9, 10, 25). Interestingly, Tat secretion is clearly important for P. aeruginosa virulence (43, 69). In addition to mediating secretion of phospholipases and iron uptake systems, a functional Tat system has been shown to be required for T3S (Michael Vasil, personal communication). PscO, one of the core components of the T3SS, has an N-terminal motif fitting the consensus for Tat substrates, although it remains to be shown that PscO can be secreted via the Tat pathway (M. Vasil, personal communication). To explore whether there is a link between Tat and T3S also in Yersinia, we assayed the tatC mutant for phenotypes coupled to T3S. Initially, we investigated the ability of the tatC mutant to produce and secrete Yop proteins under T3S-inducing conditions in vitro (growth in calcium-depleted medium at 37°C). The mutant strain was found to express and secrete wild-type levels of Yops into the culture medium (Fig. 4A). Accordingly, the tatC mutant was fully capable of delivering Yop effectors into host cells, as visualized by induction of a YopE-mediated cytotoxic effect on HeLa cells (Fig. 4B), as well as of resisting phagocytosis by macrophages (Fig. 4C). Thus, in contrast to the highly related T3SS of P. aeruginosa, the T3SS of Yersinia is not dependent on a functional Tat translocon for function, and importantly, no putative Tat secretion signal can be identified in the N terminus of YscO, the PscO homologue of Yersinia (data not shown).
FIG. 4.
Analysis of T3S-related phenotypes. Parental IP32953 and mutant IP32953tatC were compared for phenotypes related to T3S. (A) Yops in the total protein fraction (left) or secreted into the extracellular medium (right) under T3S-inducing conditions were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and identified by immunoblot analysis with anti-Yop antiserum. (B) Yersinia strains were allowed to infect a monolayer of growing HeLa cells. After 3 h of infection, the effect on the HeLa cells was recorded by phase-contrast microscopy. Note the extensive rounding of the HeLa cells induced by translocated YopE. (C) Fluorescence microscopy of Yersinia-infected J774 macrophages, where extracellular bacteria, resistant to phagocytosis, are labeled in red. For details, see Materials and Methods.
Tat is essential for Yersinia virulence.
Finally, we wanted to determine if the Tat secretion system plays a role in Yersinia virulence. To test this, we used the mouse infection model to study the virulence of Y. pseudotuberculosis strain IP32953. This strain is very closely related to Y. pestis and causes a systemic plague-like disease in mice. When mice were infected via the oral route or intraperitoneally, the tatC mutant strain was revealed to be highly attenuated. The kinetics after oral infection is shown in Fig. 5. For the intraperitoneal route, the 50% infective doses (see Materials and Methods) were calculated to be 2.2 × 103 bacteria for wild-type strain IP32953 and 3.6 × 107 bacteria for the tatC mutant. This is comparable to the 50% infective dose of the virulence plasmid-cured strain, which was 2.2 × 107 bacteria. Thus, the level of attenuation of the tatC mutant for both the oral and intraperitoneal routes was similar to that of a virulence plasmid-cured strain lacking the T3SS. We have previously shown that in mice infected via the oral route, the Peyer's patches are rapidly colonized where virulent strains overcome the primary host defense and further disseminate to colonize the spleen and liver (33). In contrast, mutants affected in the T3SS are highly attenuated in the mouse model, and while fully capable of initially colonizing the Peyer's patches, these mutants are unable to disseminate from this locus to cause a systemic infection (27, 33). To get a better understanding of the virulence defect of Tat secretion-negative strain IP32953tatC, we compared the infection kinetics in lymphoid organs of orally infected mice with that of isogenic wild-type strain IP32953, as well as a virulence plasmid-cured derivative, IP32953c, lacking the T3SS. We found that the tatC mutant strain was severely impaired in the colonization of both Peyer's patches and the spleen, with bacterial numbers several log units lower than for wild-type Yersinia and comparable to those of the avirulent, plasmid-cured strain (Table 2). This indicates either that the tatC mutant does not enter these organs or that it is rapidly cleared by the primary immune system. In conclusion, our work has established that the Tat system has an important and unique role in the virulence of human-pathogenic Yersinia.
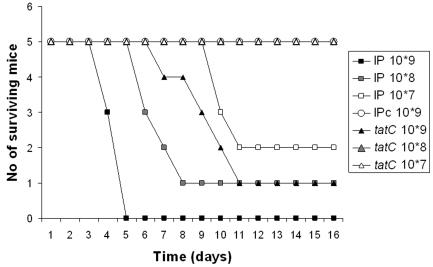
FIG. 5.
Oral infection of mice. Survival curves were determined for mice infected with parental IP32953, mutant IP32953tatC, and virulence plasmid-cured strain IP32953c over a 14-day period. Mice were infected orally with successive 10-fold dilutions (107 to 109) of a bacterial suspension and monitored at least twice daily for 14 days. For details, see Materials and Methods.
TABLE 2.
Number of colony-forming bacteria in Peyer's patches and spleens of mice infected orally with strains of Y. pseudotuberculosis
| Day and site | No. of CFU after infection with:
|
|||||
|---|---|---|---|---|---|---|
| Parent strain IP32953
|
IP32953tatC
|
IP32953c
|
||||
| Mouse 1 | Mouse 2 | Mouse 1 | Mouse 2 | Mouse 1 | Mouse 2 | |
| 1 | ||||||
| Peyer's patches | 9.4 × 105 | 5.2 × 105 | 30 | 10 | <10 | <10 |
| Spleen | 1.2 × 102 | 4.2 × 103 | <10 | 10 | <10 | <10 |
| 2 | ||||||
| Peyer's patches | 2.0 × 104 | 2.7 × 104 | 60 | 20 | 1.4 × 102 | 20 |
| Spleen | 2.7 × 103 | 9.8 × 102 | 80 | <10 | <10 | <10 |
| 3 | ||||||
| Peyer's patches | 1.6 × 105 | 8.5 × 105 | 30 | 5.0 × 102 | 80 | 3.8 × 102 |
| Spleen | 2.4 × 104 | 1.6 × 104 | 30 | 50 | <10 | <10 |
| 4 | ||||||
| Peyer's patches | 2.7 × 106 | 1.5 × 104 | 1.3 × 102 | 80 | 50 | 40 |
| Spleen | 1.9 × 105 | 1.9 × 104 | 70 | <10 | 10 | 4.9 × 102 |
| 5 | ||||||
| Peyer's patches | NAa | NA | 3.0 × 102 | 70 | 40 | 10 |
| Spleen | NA | NA | <10 | <10 | 2.3 × 102 | 20 |
NA, not applicable. On day 5, all mice infected with parent strain IP32953 had been sacrificed due to severe symptoms (see Materials and Methods).
DISCUSSION
Specialized protein secretion systems comprise essential virulence mechanisms in many bacterial pathogens. For human-pathogenic Yersinia species, the T3SS, which targets the primary host defense by delivery of effectors into host cells, is the most extensively studied virulence strategy. The focus of this work was the Tat mechanism, which we here verify to be functional in both Y. pseudotuberculosis and Y. pestis. Importantly, we also confirm that a functional Tat system is essential for the ability of Y. pseudotuberculosis to cause a systemic infection in mice via both the oral and intraperitoneal routes. Our findings indicate that Yersinia encodes specific virulence factors that are secreted by the Tat system.
In pathogens where Tat-mediated protein translocation has been studied so far, the system has been found to be involved in several important processes like biogenesis of the cell envelope, biofilm formation, assembly of the electron transport chain, survival under various environmental stress conditions, and motility (11, 21, 35, 36, 43, 62). The facts that Tat-negative bacteria display pleiotropic defects and are often impaired for in vitro growth make it difficult to establish a more direct contribution of Tat substrates to virulence. The situation seems to be different in Yersinia, where the tatC mutant strain displayed no growth defects during in vitro growth at either environmental or host temperature in rich and poor media alike. We also investigated the importance of the Tat system for survival under different environmental stress conditions and found that the tatC mutant was not impaired in the ability to survive after exposure to high temperature, oxidative stress, or high osmolarity. In line with this is also or finding that only 11 of the 27 predicted or verified Tat substrates in E. coli (46) can be identified as potential Tat substrates in Yersinia (Table 3 and data not shown).
TABLE 3.
Putative Tat substrates of Y. pseudotuberculosis IP32953 and Y. pestis KIM5a
| Physiological role | Cellular localization |
Y. pseudotuberculosis
|
Y. pestis
|
E. coli Tat substrate | ||
|---|---|---|---|---|---|---|
| Accession no. | Protein | Accession no. | Protein | |||
| Respiration | ||||||
| Nitrate reduction | Periplasmic | YP_071269 | NapF | NP_668763 | NapF | NapG |
| Nitrate reduction | Periplasmic | YP_071267 | NapA | NapA | ||
| DMSOb reduction | Inner membrane | YP_069346 | DmsA | NP_668198 | DmsA | DmsA |
| DMSO reduction | Inner membrane | YP_071198 | DmsA | NP_668839 | DmsA | |
| Formate oxidation | Periplasmic? | YP_072403 | FdoG | FdoG | ||
| Cell cycle | ||||||
| Cell division | Periplasmic | YP_071879 | SufI | NP_670801 | SufI | SufI |
| Cell wall amidase | Periplasmic | YP_071528 | AmiC | NP_670460 | AmiC | AmiC |
| Iron acquisition | ||||||
| Ferrichrome binding | Periplasmic | NP_668133 | FhuD | FhuD | ||
| Transport protein | Inner membrane | YP_070126 | YbtP | NP_669704 | YbtP | |
| Transport protein | Inner membrane | YP_070127 | YbtQ | NP_669703 | YbtQ | |
| Transport lipoprotein | Periplasm | YP_070252 | NP_669757 | YcdO | ||
| Transport | ||||||
| HlyD family | Inner membrane/extracellular? | YP_068640 | ||||
| HlyD family | Inner membrane/extracellular? | YP_069921 | NP_670114 | |||
| Transport | Periplasmic | YP_069763 | NP_670302 | |||
| Sugar transport | Periplasmic | YP_069857 | NP_670191 | |||
| Sugar transport | Periplasmic | NP_668205 | ||||
| Phosphate transport | Inner membrane | NP_668720 | ||||
| Aliphatic sulfonate transport | Inner membrane | NP_667585 | ||||
| Sulfate transport | Membrane | YP_071223 | CysZ | NP_668810 | ||
| Others | ||||||
| CA | ? | YP_071573 | NP_670506 | |||
| Proline-specific aminopeptidase | ? | YP_071686 | PepP | NP_670569 | PepP | |
| Copper homcostasis | Periplasmic | YP_069263 | NP_668113 | CueO | ||
| Sulfatase | ? | YP_071579 | NP_670514 | |||
| Unknown | Membrane | YP_069840 | ||||
| Membrane | NP_668871 | |||||
| ? | YP_070253 | NP_669756 | YcdB | |||
| Exported/outer membrane | YP_069963 | NP_670063 | YcbK | |||
| ? | NP_669146 | |||||
| Extracellular | NP_670676 | |||||
| Extracellular | YP_071753 | NP_670675 | ||||
Putative substrates were retrieved with the TatP software (see Materials and Methods).
DMSO, dimethyl sulfoxide.
As in many other pathogens, we did observe a motility defect when the Yersinia Tat system was disrupted (21, 43, 50). We do not believe that this defect per se is of high significance for Yersinia virulence, as Y. pseudotuberculosis is only motile at environmental temperatures (2) and the most virulent species, Y. pestis, is nonmotile due to several mutations in operons encoding gene products involved in flagellar secretion and assembly (47). However, if the deficiency in motility is the result of impaired secretion of flagellar components, we cannot exclude the possibility that other proteins secreted by the flagellar T3SS contribute to virulence, similar to what has been found in Y. enterocolitica (74).
Upon disruption of the Tat system, some bacterial species display impaired cell division, leading to chain formation. In the case of a Yersinia tatC mutant, elongated cell morphology was occasionally observed (Fig. 4C). This was, however, most likely not due to defects in cell division but rather an effect of chloramphenicol selection, a phenomenon previously observed for Yersinia (Fabienne Deleuil, unpublished observation).
The T3SS of P. aeruginosa is closely related to the plasmid-encoded system of Yersinia. Interestingly, in P. aeruginosa there appears to be a link between the Tat system and T3S. Introduction of mutations that disrupt the Tat system causes defects in T3S, possibly due to PscO, one of the core T3SS components, which has a putative Tat signal peptide and thus may be secreted via the Tat system (M. Vasil, personal communication). Recently, a P. syringae tatC mutant strain was also shown to display decreased T3S-dependent translocation of anti-host factors into plant cells, indicating that functional Tat is required for efficient type III translocation in this pathogen (11). When we examined the Yersinia tatC mutant, we could, however, not see any defects in phenotypes coupled to functional T3S. The mutant was indistinguishable from the isogenic wild-type strain with respect to Yop expression and secretion, the ability to induce a YopE-mediated cytotoxic effect on infected HeLa cells, and the ability to block phagocytosis by J774 macrophages. Thus, the virulence defect of the tatC mutant is not an indirect effect caused by impaired function of the T3SS.
When investigating the susceptibility of the tatC mutant to various environmental stresses, we detected a small decrease in acid resistance. One critical phase of infection where the pathogen faces a low pH is inside macrophages, where Yersinia is believed to reside at early stages of infection. We found that the tatC mutant was not impaired for survival in the macrophage-like cell line J774.1. Even if J774 cells are less efficient in bacterial killing compared to activated macrophages, we still believe that these results are relevant, as J774 cells have been shown to cause impaired intracellular survival of phoP mutants in Y. pestis, as well as in Y. pseudotuberculosis (29, 44). In both cases, the impaired intracellular survival was coupled to a moderate decrease in virulence. Impaired acid resistance could impact the ability to cause disease via the oral route, as the pathogen is exposed to a low pH during its entry into the gastrointestinal tract. Importantly, however, the tatC mutant strain was highly attenuated not only via the oral route but also in intraperitoneal infection of mice, when the gastrointestinal tract is circumvented.
When in silico prediction methods were used to identify putative Tat substrates in the genomes of Y. pseudotuberculosis IP32953 and Y. pestis KIM5, most of the potential substrates were shared by the two strains (Table 3). Among the putative Tat substrates, we identified a few which could explain the phenotypes in which the tatC mutant differs from the wild-type strain. One of the substrates encodes a potential carbonic anhydrase (CA) (accession number YP_071573; Table 3). This is interesting since CAs have been shown to play a role in pH regulation and have been proposed to be important for survival of intracellular pathogens within their hosts (60). Prokaryotic CAs have, in Helicobacter pylori and cyanobacteria, been shown to localize to all bacterial compartments, the cytoplasm, the inner and outer membranes, and the periplasm, and also extracellularly (15, 61). A recent publication shows a periplasmically located CA of H. pylori to be required for in vitro acid resistance (40). Other interesting potential substrates, with respect to virulence, include proteins involved in iron acquisition, which could have a significant impact on the ability of Yersinia to establish an infection. In addition, several putative substrate genes encode homologues of systems potentially involved in the transport of sugar, amino acids, phosphate, and sulfur sources (Table 3), and there are also a number of potential Tat substrates of unknown function.
Our findings that the Tat pathway is critical for the ability of Y. pseudotuberculosis to cause a systemic infection in mice has implications also for Y. pestis as the two species are very closely related (1) and cause similar systemic infections in mice. We have verified that the Tat pathway is functional also in Y. pestis KIM5 and that most of the putative secretion targets are shared between the two genomes (Table 3), suggesting that there is a Tat substrate(s) that is involved in virulence also in Y. pestis.
The identification of Tat as a key virulence mechanism could be important for future development of novel component vaccines against Y. pestis. A subunit vaccine is currently under development that is based on two virulence proteins, LcrV of the T3SS and capsule-like antigen fraction 1 (F1). The new vaccine is very promising and induces high levels of protection even against an aerosol challenge with Y. pestis (31, 67, 68). This shows that the principle of using secreted virulence factors as vaccine works well for a pathogen like Y. pestis, which is believed to reside mainly extracellularly during infection. One potential pitfall of the new vaccine is the occurrence of natural isolates of Y. pestis that lack F1 with fully retained virulence (16). Therefore, there is an urgent need to identify additional surface-located or secreted proteins that are required for Yersinia virulence to evaluate for protection. The essential role played by the Tat pathway in virulence suggests that candidate proteins that are promising as vaccine components could be found among the Tat substrates of Yersinia. Work to identify the Tat substrates of Yersinia and to verify the role of the Tat system in Y. pestis is under way and is likely to uncover a novel major virulence mechanism of pathogenic Yersinia.
Acknowledgments
Michael Vasil at the Department of Microbiology, University of Colorado Health Sciences Center, Denver, contributed with valuable discussions concerning Tat and is also thanked for sharing data prior to publication. We further thank Zhiyong Ding and Peter J. Christie at the Department of Microbiology and Molecular Genetics at the University of Texas—Houston Medical School for kindly lending us plasmid pZD51 and Sara Garbom at the Department of Molecular Biology, Umeå University, for providing strain IP32953c. Marléne Lundström is acknowledged for excellent technical assistance.
This work has received funding from the J. C. Kempe Memorial Fund and the Swedish Research Council.
Editor: J. B. Bliska
REFERENCES
- 1.Achtman, M., K. Zurth, G. Morelli, G. Torrea, A. Guiyoule, and E. Carniel. 1999. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 96:14043-14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson, S., J. P. Throup, G. S. Stewart, and P. Williams. 1999. A hierarchical quorum-sensing system in Yersinia pseudotuberculosis is involved in the regulation of motility and clumping. Mol. Microbiol. 33:1267-1277. [DOI] [PubMed] [Google Scholar]
- 3.Bendtsen, J. D., H. Nielsen, D. Widdick, T. Palmer, and S. Brunak. 2005. Prediction of twin-arginine signal peptides. BMC Bioinformatics 6:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergman, T., S. Hakansson, A. Forsberg, L. Norlander, A. Macellaro, A. Backman, I. Bolin, and H. Wolf-Watz. 1991. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J. Bacteriol. 173:1607-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berks, B. C. 1996. A common export pathway for proteins binding complex redox cofactors? Mol. Microbiol. 22:393-404. [DOI] [PubMed] [Google Scholar]
- 6.Berks, B. C., T. Palmer, and F. Sargent. 2005. Protein targeting by the bacterial twin-arginine translocation (Tat) pathway. Curr. Opin. Microbiol. 8:174-181. [DOI] [PubMed] [Google Scholar]
- 7.Berks, B. C., F. Sargent, and T. Palmer. 2000. The Tat protein export pathway. Mol. Microbiol. 35:260-274. [DOI] [PubMed] [Google Scholar]
- 8.Bogsch, E. G., F. Sargent, N. R. Stanley, B. C. Berks, C. Robinson, and T. Palmer. 1998. An essential component of a novel bacterial protein export system with homologues in plastids and mitochondria. J. Biol. Chem. 273:18003-18006. [DOI] [PubMed] [Google Scholar]
- 9.Bröms, J. E., A. L. Forslund, A. Forsberg, and M. S. Francis. 2003. Dissection of homologous translocon operons reveals a distinct role for YopD in type III secretion by Yersinia pseudotuberculosis. Microbiology 149:2615-2626. [DOI] [PubMed] [Google Scholar]
- 10.Bröms, J. E., C. Sundin, M. S. Francis, and A. Forsberg. 2003. Comparative analysis of type III effector translocation by Yersinia pseudotuberculosis expressing native LcrV or PcrV from Pseudomonas aeruginosa. J. Infect. Dis. 188:239-249. [DOI] [PubMed] [Google Scholar]
- 11.Bronstein, P. A., M. Marrichi, S. Cartinhour, D. J. Schneider, and M. P. Delisa. 2005. Identification of a twin-arginine translocation system in Pseudomonas syringae pv. tomato DC3000 and its contribution to pathogenicity and fitness. J. Bacteriol. 187:8450-8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brzostek, K., A. Raczkowska, and A. Zasada. 2003. The osmotic regulator OmpR is involved in the response of Yersinia enterocolitica O:9 to environmental stresses and survival within macrophages. FEMS Microbiol. Lett. 228:265-271. [DOI] [PubMed] [Google Scholar]
- 13.Buchanan, S. K. 2001. Type I secretion and multidrug efflux: transport through the TolC channel-tunnel. Trends Biochem. Sci. 26:3-6. [DOI] [PubMed] [Google Scholar]
- 14.Chain, P. S., E. Carniel, F. W. Larimer, J. Lamerdin, P. O. Stoutland, W. M. Regala, A. M. Georgescu, L. M. Vergez, M. L. Land, V. L. Motin, R. R. Brubaker, J. Fowler, J. Hinnebusch, M. Marceau, C. Medigue, M. Simonet, V. Chenal-Francisque, B. Souza, D. Dacheux, J. M. Elliott, A. Derbise, L. J. Hauser, and E. Garcia. 2004. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 101:13826-13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chirica, L. C., C. Petersson, M. Hurtig, B. H. Jonsson, T. Boren, and S. Lindskog. 2002. Expression and localization of alpha- and beta-carbonic anhydrase in Helicobacter pylori. Biochim. Biophys. Acta 1601:192-199. [DOI] [PubMed] [Google Scholar]
- 16.Davis, K. J., D. L. Fritz, M. L. Pitt, S. L. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Pathology of experimental pneumonic plague produced by fraction 1-positive and fraction 1-negative Yersinia pestis in African green monkeys (Cercopithecus aethiops). Arch. Pathol. Lab. Med. 120:156-163. [PubMed] [Google Scholar]
- 17.Davis, R. W., D. Botstein, and J. R. Roth. 1980. Advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 18.Delepelaire, P. 2004. Type I secretion in gram-negative bacteria. Biochim. Biophys. Acta 1694:149-161. [DOI] [PubMed] [Google Scholar]
- 19.de Lorenzo, V., and K. N. Timmis. 1994. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 235:386-405. [DOI] [PubMed] [Google Scholar]
- 20.Ding, Z., K. Atmakuri, and P. J. Christie. 2003. The outs and ins of bacterial type IV secretion substrates. Trends Microbiol. 11:527-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding, Z., and P. J. Christie. 2003. Agrobacterium tumefaciens twin-arginine-dependent translocation is important for virulence, flagellation, and chemotaxis but not type IV secretion. J. Bacteriol. 185:760-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorrell, N., S. R. Li, P. H. Everest, G. Dougan, and B. W. Wren. 1998. Construction and characterisation of a Yersinia enterocolitica O:8 ompR mutant. FEMS Microbiol. Lett. 165:145-151. [DOI] [PubMed] [Google Scholar]
- 23.Economou, A. 2000. Bacterial protein translocase: a unique molecular machine with an army of substrates. FEBS Lett. 476:18-21. [DOI] [PubMed] [Google Scholar]
- 24.Francis, M. S., K. Schesser, A. Forsberg, and H. Wolf-Watz. 2004. Type III secretion systems in animal- and plant-interacting bacteria, p. 362-392. In P. Cossart, S. Normark, and R. Rappuoli (ed.), Cellular microbiology, 2nd edition. ASM Press, Washington, D.C.
- 25.Frithz-Lindsten, E., A. Holmstrom, L. Jacobsson, M. Soltani, J. Olsson, R. Rosqvist, and A. Forsberg. 1998. Functional conservation of the effector protein translocators PopB/YopB and PopD/YopD of Pseudomonas aeruginosa and Yersinia pseudotuberculosis. Mol. Microbiol. 29:1155-1165. [DOI] [PubMed] [Google Scholar]
- 26.Furste, J. P., W. Pansegrau, R. Frank, H. Blocker, P. Scholz, M. Bagdasarian, and E. Lanka. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119-131. [DOI] [PubMed] [Google Scholar]
- 27.Galyov, E. E., S. Hakansson, and H. Wolf-Watz. 1994. Characterization of the operon encoding the YpkA Ser/Thr protein kinase and the YopJ protein of Yersinia pseudotuberculosis. J. Bacteriol. 176:4543-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gemski, P., J. R. Lazere, T. Casey, and J. A. Wohlhieter. 1980. Presence of a virulence-associated plasmid in Yersinia pseudotuberculosis. Infect. Immun. 28:1044-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grabenstein, J. P., M. Marceau, C. Pujol, M. Simonet, and J. B. Bliska. 2004. The response regulator PhoP of Yersinia pseudotuberculosis is important for replication in macrophages and for virulence. Infect. Immun. 72:4973-4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haller, J. C., S. Carlson, K. J. Pederson, and D. E. Pierson. 2000. A chromosomally encoded type III secretion pathway in Yersinia enterocolitica is important in virulence. Mol. Microbiol. 36:1436-1446. [DOI] [PubMed] [Google Scholar]
- 31.Heath, D. G., G. W. Anderson, Jr., J. M. Mauro, S. L. Welkos, G. P. Andrews, J. Adamovicz, and A. M. Friedlander. 1998. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine 16:1131-1137. [DOI] [PubMed] [Google Scholar]
- 32.Henderson, I. R., F. Navarro-Garcia, M. Desvaux, R. C. Fernandez, and D. Ala'Aldeen. 2004. Type V protein secretion pathway: the autotransporter story. Microbiol. Mol. Biol. Rev. 68:692-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holmstrom, A., R. Rosqvist, H. Wolf-Watz, and A. Forsberg. 1995. Virulence plasmid-encoded YopK is essential for Yersinia pseudotuberculosis to cause systemic infection in mice. Infect. Immun. 63:2269-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutcheon, G. W., and A. Bolhuis. 2003. The archaeal twin-arginine translocation pathway. Biochem. Soc. Trans. 31:686-689. [DOI] [PubMed] [Google Scholar]
- 35.Ize, B., I. Porcelli, S. Lucchini, J. C. Hinton, B. C. Berks, and T. Palmer. 2004. Novel phenotypes of Escherichia coli tat mutants revealed by global gene expression and phenotypic analysis. J. Biol. Chem. 279:47543-47554. [DOI] [PubMed] [Google Scholar]
- 36.Ize, B., N. R. Stanley, G. Buchanan, and T. Palmer. 2003. Role of the Escherichia coli Tat pathway in outer membrane integrity. Mol. Microbiol. 48:1183-1193. [DOI] [PubMed] [Google Scholar]
- 37.Kapatral, V., and S. A. Minnich. 1995. Co-ordinate, temperature-sensitive regulation of the three Yersinia enterocolitica flagellin genes. Mol. Microbiol. 17:49-56. [DOI] [PubMed] [Google Scholar]
- 38.Kapatral, V., J. W. Olson, J. C. Pepe, V. L. Miller, and S. A. Minnich. 1996. Temperature-dependent regulation of Yersinia enterocolitica class III flagellar genes. Mol. Microbiol. 19:1061-1071. [DOI] [PubMed] [Google Scholar]
- 39.Lee, V. T., and O. Schneewind. 2001. Protein secretion and the pathogenesis of bacterial infections. Genes Dev. 15:1725-1752. [DOI] [PubMed] [Google Scholar]
- 40.Marcus, E. A., A. P. Moshfegh, G. Sachs, and D. R. Scott. 2005. The periplasmic alpha-carbonic anhydrase activity of Helicobacter pylori is essential for acid acclimation. J. Bacteriol. 187:729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milton, D. L., A. Norqvist, and H. Wolf-Watz. 1992. Cloning of a metalloprotease gene involved in the virulence mechanism of Vibrio anguillarum. J. Bacteriol. 174:7235-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagai, H., and C. R. Roy. 2003. Show me the substrates: modulation of host cell function by type IV secretion systems. Cell. Microbiol. 5:373-383. [DOI] [PubMed] [Google Scholar]
- 43.Ochsner, U. A., A. Snyder, A. I. Vasil, and M. L. Vasil. 2002. Effects of the twin-arginine translocase on secretion of virulence factors, stress response, and pathogenesis. Proc. Natl. Acad. Sci. USA 99:8312-8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oyston, P. C., N. Dorrell, K. Williams, S. R. Li, M. Green, R. W. Titball, and B. W. Wren. 2000. The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis. Infect. Immun. 68:3419-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pallen, M. J., R. R. Chaudhuri, and I. R. Henderson. 2003. Genomic analysis of secretion systems. Curr. Opin. Microbiol. 6:519-527. [DOI] [PubMed] [Google Scholar]
- 46.Palmer, T., F. Sargent, and B. C. Berks. 2005. Export of complex cofactor-containing proteins by the bacterial Tat pathway. Trends Microbiol. 13:175-180. [DOI] [PubMed] [Google Scholar]
- 47.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Churcher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeno-Tarraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413:523-527. [DOI] [PubMed] [Google Scholar]
- 48.Pettersson, J., R. Nordfelth, E. Dubinina, T. Bergman, M. Gustafsson, K. E. Magnusson, and H. Wolf-Watz. 1996. Modulation of virulence factor expression by pathogen target cell contact. Science 273:1231-1233. [DOI] [PubMed] [Google Scholar]
- 49.Philipovskiy, A. V., C. Cowan, C. R. Wulff-Strobel, S. H. Burnett, E. J. Kerschen, D. A. Cohen, A. M. Kaplan, and S. C. Straley. 2005. Antibody against V antigen prevents Yop-dependent growth of Yersinia pestis. Infect. Immun. 73:1532-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pradel, N., C. Ye, V. Livrelli, J. Xu, B. Joly, and L. F. Wu. 2003. Contribution of the twin arginine translocation system to the virulence of enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 71:4908-4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pujol, C., and J. B. Bliska. 2003. The ability to replicate in macrophages is conserved between Yersinia pestis and Yersinia pseudotuberculosis. Infect. Immun. 71:5892-5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 53.Remaut, H., and G. Waksman. 2004. Structural biology of bacterial pathogenesis. Curr. Opin. Struct. Biol. 14:161-170. [DOI] [PubMed] [Google Scholar]
- 54.Rose, R. W., T. Bruser, J. C. Kissinger, and M. Pohlschroder. 2002. Adaptation of protein secretion to extremely high-salt conditions by extensive use of the twin-arginine translocation pathway. Mol. Microbiol. 45:943-950. [DOI] [PubMed] [Google Scholar]
- 55.Rosqvist, R., K. E. Magnusson, and H. Wolf-Watz. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13:964-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rossier, O., and N. P. Cianciotto. 2005. The Legionella pneumophila tatB gene facilitates secretion of phospholipase C, growth under iron-limiting conditions, and intracellular infection. Infect. Immun. 73:2020-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 58.Sandkvist, M. 2001. Biology of type II secretion. Mol. Microbiol. 40:271-283. [DOI] [PubMed] [Google Scholar]
- 59.Sargent, F., E. G. Bogsch, N. R. Stanley, M. Wexler, C. Robinson, B. C. Berks, and T. Palmer. 1998. Overlapping functions of components of a bacterial Sec-independent protein export pathway. EMBO J. 17:3640-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith, K. S., and J. G. Ferry. 2000. Prokaryotic carbonic anhydrases. FEMS Microbiol. Rev. 24:335-366. [DOI] [PubMed] [Google Scholar]
- 61.Soltes-Rak, E., M. E. Mulligan, and J. R. Coleman. 1997. Identification and characterization of a gene encoding a vertebrate-type carbonic anhydrase in cyanobacteria. J. Bacteriol. 179:769-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stanley, N. R., K. Findlay, B. C. Berks, and T. Palmer. 2001. Escherichia coli strains blocked in Tat-dependent protein export exhibit pleiotropic defects in the cell envelope. J. Bacteriol. 183:139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Straley, S. C., and W. S. Bowmer. 1986. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect. Immun. 51:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sundberg, L., and A. Forsberg. 2003. TyeA of Yersinia pseudotuberculosis is involved in regulation of Yop expression and is required for polarized translocation of Yop effectors. Cell. Microbiol. 5:187-202. [DOI] [PubMed] [Google Scholar]
- 65.Sundin, C., M. C. Wolfgang, S. Lory, A. Forsberg, and E. Frithz-Lindsten. 2002. Type IV pili are dispensable for type III delivery of exoenzymes by Pseudomonas aeruginosa. Microb. Pathog. 33:265-277. [DOI] [PubMed] [Google Scholar]
- 66.Thanassi, D. G., and S. J. Hultgren. 2000. Multiple pathways allow protein secretion across the bacterial outer membrane. Curr. Opin. Cell Biol. 12:420-430. [DOI] [PubMed] [Google Scholar]
- 67.Titball, R. W., and E. D. Williamson. 2001. Vaccination against bubonic and pneumonic plague. Vaccine 19:4175-4184. [DOI] [PubMed] [Google Scholar]
- 68.Titball, R. W., and E. D. Williamson. 2004. Yersinia pestis (plague) vaccines. Expert Opin. Biol. Ther. 4:965-973. [DOI] [PubMed] [Google Scholar]
- 69.Voulhoux, R., G. Ball, B. Ize, M. L. Vasil, A. Lazdunski, L. F. Wu, and A. Filloux. 2001. Involvement of the twin-arginine translocation system in protein secretion via the type II pathway. EMBO J. 20:6735-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weiner, J. H., P. T. Bilous, G. M. Shaw, S. P. Lubitz, L. Frost, G. H. Thomas, J. A. Cole, and R. J. Turner. 1998. A novel and ubiquitous system for membrane targeting and secretion of cofactor-containing proteins. Cell 93:93-101. [DOI] [PubMed] [Google Scholar]
- 71.Wexler, M., F. Sargent, R. L. Jack, N. R. Stanley, E. G. Bogsch, C. Robinson, B. C. Berks, and T. Palmer. 2000. TatD is a cytoplasmic protein with DNase activity. No requirement for TatD family proteins in sec-independent protein export. J. Biol. Chem. 275:16717-16722. [DOI] [PubMed] [Google Scholar]
- 72.Williams, K., P. C. Oyston, N. Dorrell, S. Li, R. W. Titball, and B. W. Wren. 2000. Investigation into the role of the serine protease HtrA in Yersinia pestis pathogenesis. FEMS Microbiol. Lett. 186:281-286. [DOI] [PubMed] [Google Scholar]
- 73.Yamamoto, T., T. Hanawa, S. Ogata, and S. Kamiya. 1996. Identification and characterization of the Yersinia enterocolitica gsrA gene, which protectively responds to intracellular stress induced by macrophage phagocytosis and to extracellular environmental stress. Infect. Immun. 64:2980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Young, G. M., D. H. Schmiel, and V. L. Miller. 1999. A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc. Natl. Acad. Sci. USA 96:6456-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]