Abstract
Toll-like receptors (TLRs) play an important role in the control of infection with Borrelia burgdorferi. Deficiencies in TLR-2 or the shared TLR adapter molecule MyD88 have been shown to result in greatly increased bacterial burdens in mice. However, although in vitro studies have shown that the activation of TLR pathways by B. burgdorferi results in the release of inflammatory cytokines, studies in deficient mice have shown either no change or increased rather than decreased inflammation in infected animals. In this study, we looked at mechanisms to explain the increase in inflammation in the absence of MyD88. We found that MyD88-deficient mice infected with B. burgdorferi did not show increased inflammation at sites typically associated with Lyme disease (joints and heart). However, there was markedly increased inflammation in the muscles, kidneys, pancreas, and lungs of deficient animals. Muscle inflammation was typically seen perivascularly and perineuronally similar to that seen in infected humans. Chemotactic chemokines and cytokines were greatly increased in the muscle and kidneys of MyD88-deficient animals but not in the joints or heart tissue, suggesting that MyD88-independent pathways for recognizing B. burgdorferi and inducing these chemokines are present in the muscle and kidneys. Interleukin-18 signaling through MyD88 does not appear to play a role in either control of infection or inflammation.
Infection with Borrelia burgdorferi, the causative agent of Lyme disease, results in a broad array of clinical manifestations, including, but not limited to, erythema migrans skin lesions, myocarditis, meningitis, neuritis, and arthritis (4, 25, 36). Pathology in these organs is thought to be due to local stimulation of the host immune system resulting in the infiltration of inflammatory cells. Host innate immunity is thought to play a primary role in the initial inflammatory response to B. burgdorferi.
Toll-like receptors (TLRs) play an integral role in mammalian innate immune defenses. TLR-2 has been shown to recognize multiple components of bacterial cell walls including peptidoglycans and lipoproteins (2). Outer surface lipoproteins of B. burgdorferi containing the Pam3Cys modification are recognized by TLR-2; the activation of TLR-2 by B. burgdorferi lipoproteins has been shown in vitro to result in the activation of peripheral blood mononuclear cells and the release of proinflammatory cytokines and chemokines (18).
Mouse studies of TLR-2 have shown a different role for TLRs in B. burgdorferi infection. As would be predicted, the lack of TLR-2 greatly diminishes the ability of the mouse to control infection, resulting in higher numbers of spirochetes in tissue and blood despite preserved development of an appropriate adaptive immune response. However, the mechanism by which TLR-2 is responsible for controlling infection does not appear to be mediated by the ability to recruit inflammatory cells, since TLR-2-deficient mice develop more severe, rather than less severe, arthritis compared with their wild-type counterparts (42).
Myeloid differentiation factor 88 (MyD88) is a common adaptor molecule for TLR, interleukin-1 (IL-1), and IL-18 signaling (3, 38). MyD88 is utilized by all of the TLRs except TLR-3 in the signaling pathway that results in the activation of NFκB, leading to the production of inflammatory mediators. MyD88 knockout mice have been shown to have increased susceptibility to infection with several pathogens including Staphylococcus aureus (37, 40), Listeria monocytogenes (33), and Mycobacterium avium (12); fungal pathogens such as Candida albicans (7); and parasites such as Toxoplasma gondii (32), Leishmania major (10), and the intestinal nematode Trichuris muris (17). Similar to TLR-2-deficient mice, MyD88-deficient mice infected with B. burgdorferi show higher tissue burdens of spirochetes (8, 24). Joint inflammation in B. burgdorferi-infected MyD88 knockout mice has been variously reported by two different groups as either unchanged from the wild type or increased, similar to what is seen with TLR-2 knockout mice (8, 24). Carditis, which is the other major manifestation of B. burgdorferi infection in mice, was unchanged from wild-type levels in MyD88-deficient mice (24). In distinction to the effects of TLR/MyD88 deficiency in B. burgdorferi-infected mice, exposure to streptococcal cell wall products in MyD88−/− mice resulted in decreased joint inflammation in mice compared with that in wild-type mice (19).
Given the conceptually surprising results of increased inflammation in B. burgdorferi infection of TLR/MyD88-deficient mice, the discrepancies in results from MyD88 mice in previous studies, and the dissimilarity between the effects of MyD88 deficiency on B. burgdorferi-induced arthritis and the effects on inflammation in other diseases (13, 14, 27, 35), we sought to more fully characterize inflammation that occurs in MyD88 mice in response to B. burgdorferi infection. Cytokine and chemokine expression patterns in B. burgdorferi-infected MyD88 mice have not previously been reported. Here, we present our data showing inflammatory patterns in B. burgdorferi-infected MyD88-deficient mice.
MATERIALS AND METHODS
Mice and B. burgdorferi infection.
MyD88-deficient mice were maintained as heterozygous breeding pairs at the fifth-generation backcross on the C57BL/6 background. MyD88−/−, MyD88+/+, and MyD88+/− littermates were genotyped as described previously (22). Homozygous IL-18-deficient mice were backcrossed nine times to a C57BL/6 background. The procedures used were reviewed and approved by the Tufts University Institutional Animal Care and Use Committee. Four- to 6-week-old mice were infected intradermally by needle inoculation with low-passage B. burgdorferi (strain N40; 1 × 104 cells) grown in Barbour-Stoenner-Kelly H (Sigma Co., St. Louis, MO) medium. Mice were sacrificed 2 weeks postinfection. Cartilage was microdissected from the ankle joints by using a stereomicroscope, and total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Successful infection of individual mice was confirmed by culturing ear samples in Barbour-Stoenner-Kelly H medium and monitoring the growth of B. burgdorferi by dark-field microscopy as we have previously reported (6).
Histological analysis.
Tissues were first fixed either in Bowen's fixative (hind legs) for 24 h and then placed in 70% ethanol or in 10% neutral buffered formalin (all other soft tissue). The hind legs were then skinned, split in half longitudinally in the center, processed for decalcification, and finally embedded in paraffin. Other tissues were processed whole. The slides were processed and stained with hematoxylin and eosin (HE) by the Histology Core Facility at New England Medical Center as previously described (6). The slides were scored for histopathology in a blinded manner on a scale of 1 to 5, with 1 being the least inflammation and 5 being the most inflammation.
Quantitative real-time RT-PCR.
Total RNA was purified by using Trizol (Invitrogen) according to the manufacturer's instructions. First-strand synthesis of cDNA from total RNA was performed by using Improm II reverse transcriptase (RT) (Promega, Madison, WI) according to the manufacturer's instructions. Control reactions performed in the absence of reverse transcriptase were used to control for contamination by genomic DNA. cDNA samples contaminated by genomic DNA were discarded, and the original RNA was treated with DNase before the reverse transcriptase reaction was repeated. Quantitation of cDNA from specific mRNA transcripts was accomplished by real-time quantitative RT-PCR (iCycler; Bio-Rad, Hercules, CA) by using SYBR green technology (Quantitect SYBR green PCR kit; QIAGEN, Valencia, CA) as previously described (6). The primers used are listed in Table 1.
TABLE 1.
Primer sequences for real-time RT-PCR
| Primer | Sequencea | Annealing temp (°C) |
|---|---|---|
| RecA | F, 5′-GTGGATCTATTGTATTAGATGAGG CTCTCG-3′ | 60 |
| R, 5′-GCCAAAGTTCTGCAACATTAACAC CTAAAG-3′ | ||
| IFN-γ | F, 5′-TACTGCCACGGCACAGTCATTGAA-3′ | 60 |
| R, 5′-GCAGCGACTCCTTTTCCGCTTCCT-3′ | ||
| IL-10 | F, 5′-AGAGCTGCGGACTGCCTTCA-3′ | 60 |
| R, 5′-AATGCTCCTTGATTTCTGGG-3′ | ||
| TNF-α | F, 5′-ATGAGCACAGAAAGCATGATC-3′ | 60 |
| R, 5′-TACAGGCTTGTCACTCGAATT-3′ | ||
| IL-12p40 | F, 5′-CGTTTATGTTGTAGAGGTGG-3′ | 56.8 |
| R, 5′-TGTGGAGCAGCAGATGTGAG-3′ | ||
| IL-12p35 | F, 5′-CCCTTGCCCTCCTAAACCAC-3′ | 60 |
| R, 5′-TAGTAGCCAGGCAACTCTCG-3′ | ||
| GM-CSF | F, 5′-CCCGCTCACCCATCACTGTC-3′ | 60 |
| R, 5′-CGGAGTTGGGGGGCAGTATG-3′ | ||
| CXCL-1 | F, 5′-GGCGCCTATCGCCAATGAGCTG-3′ | 60 |
| R, 5′-CTTGGGGACACCTTTTAGC-3′ | ||
| CXCL-2 | F, 5′-GGGTTGACTTCAAGAACATCCAGA-3′ | 60 |
| R, 5′-GGGCTTCAGGGTCAAGGCAAAC-3′ | ||
| MCP-1 | F, 5′-GCTGTTCACAGTTGCCGGCT-3′ | 56.8 |
| R, 5′-CATTAGCTTCAGATTTACGG-3′ | ||
| RANTES | F, 5′-CCCTCTGCACCCCCGTACCT-3′ | 60 |
| R, 5′-CCATTTTCCCAGGACCGAGT-3′ | ||
| MIP-1α | F, 5′-CTCAACATCATGAAGGTCTC-3′ | 60 |
| R, 5′-GGCATTCAGTTCCAGGTCAG-3′ | ||
| MIP-1β | F, 5′-CTCTCTCTCCTCTTGCTCGT-3′ | 65.1 |
| R, 5′-CTCCATGGGAGACACGCGTC-3′ |
F, forward; R, reverse.
Quantification of B. burgdorferi DNA in mouse tissue.
DNA from different mouse tissues was isolated using the QIAGEN genomic DNA isolation kit, and real-time PCR was performed to assess the number of B. burgdorferi isolates present in different tissues as described previously (8). The copy numbers of the recA gene were normalized to the tissue weight and compared with standards.
Statistical analysis.
Experiments were repeated three to five times as indicated below. The statistical significance between groups was analyzed by using the nonparametric Mann-Whitney U test. Differences were considered statistically significant when the P value was equal to or less than 0.05.
RESULTS
MyD88 deficiency does not cause increased arthritis or carditis in B. burgdorferi-infected mice.
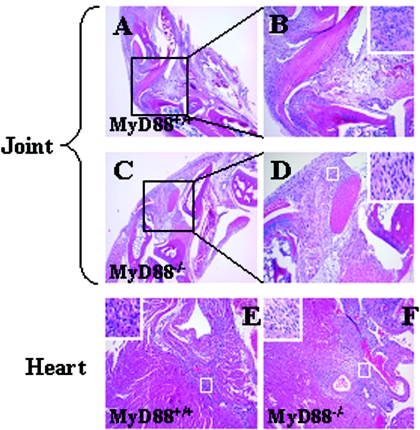
Arthritis and carditis are the two most prominent manifestations of disease in the murine model of Lyme disease. Previous studies of B. burgdorferi infection of MyD88−/− mice have differed on the impact of the loss of MyD88 on the development of arthritis. We injected MyD88−/− mice or their wild-type littermates with B. burgdorferi N40 or sham. Mice were sacrificed at various time points after infection. Joints and cardiac tissue from uninfected MyD88−/− mice appeared normal and were indistinguishable from those from wild-type mice. All mice infected with B. burgdorferi showed greater inflammation in joints and the heart than uninfected mice. We found no significant differences in arthritis or carditis between MyD88−/− and wild-type mice (Fig. 1 and Table 2). Inflammatory infiltrates in both MyD88−/− and wild-type animals appeared to be a mixture of mononuclear cells and neutrophils with a slight predominance of neutrophils in the joints, as has previously been reported (8). No differences in the cellular composition between the infiltrates of MyD88−/− mice and those of wild-type mice were noted.
FIG. 1.
MyD88 deficiency does not cause increased arthritis or carditis in B. burgdorferi-infected mice. MyD88−/− and MyD88+/+ mice were infected with B. burgdorferi as described in Materials and Methods and were sacrificed 2 weeks after infection. Representative HE-stained sections of joints (A to D) and heart (E and F) of both wild-type (A, B, and E) and MyD88−/− (knockout) (C, D, and F) mice are shown. B and D are the magnified pictures of the boxed areas of A and B, respectively. The insets are the magnified views of the areas in the boxes.
TABLE 2.
Pathology of MyD88-deficient mice infected with B. burgdorferia
| Genotype | Avg score ± SD (no. of mice)
|
||||
|---|---|---|---|---|---|
| Joint | Muscle | Kidney | Lung | Pancreas | |
| MyD88+/+ | 1.9 ± 0.576 (10) | 0.1 ± 0.316 (10) | 0.5 ± 0.577 (4) | 0.8 ± 0.447 (5) | 0.0 ± 0.0 (5) |
| MyD88−/− | 1.8 ± 0.523 (10) | 2.1 ± 0.568 (10) | 1.4 ± 0.726 (9) | 2.2 ± 0.447 (5) | 1 ± 0.0 (5) |
| P | 0.716 | <0.00001 | 0.048 | 0.005 | 0.003 |
The overall histopathology was scored in a blinded manner by two different individuals on a scale of 0 (no lesion) to 5 (severe lesion), and the average of all scores is shown.
MyD88 deficiency results in the development of myositis, alveolitis, glomerulonephritis, and pancreatitis.
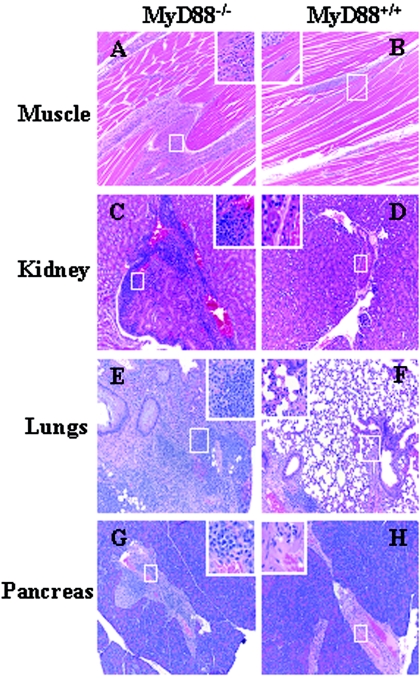
In addition to joint and cardiac tissue, we also examined organs that are not typically affected by infection with B. burgdorferi. Brain, spinal cord, eye, lung, spleen, pancreas, kidney, and muscle tissue from B. burgdorferi-infected MyD88−/− and wild-type mice were fixed, stained, and scored for inflammation in a blinded manner. Again, no differences were seen in inflammation between uninfected wild-type and knockout mice. Significantly higher levels of inflammation were seen in the lungs (P = 0.005), pancreas (P = 0.003), kidney (P = 0.048), and muscle (P < 0.00001) tissues of MyD88−/− mice (Table 2). No differences in inflammation were seen in brain, spinal cord, eye, or spleen from infected wild-type and knockout mice (data not shown).
In the muscle, inflammation was greatest in interfasicular areas and localized around vascular and neuronal sheaths (Fig. 2). Vasculitis and neuritis is seen in human Lyme disease but has been uncommonly reported in infection of mice. Again, similar to joint tissues, infiltrates were mixed, with a slight predominance of neutrophils compared with mononuclear cells. Inflammation in the kidneys was seen as glomerulonephritis. Cellular thickening of interstitial spaces and perivascular inflammatory infiltrates were also seen (Fig. 2). Inflammation of the pancreas was primarily periductal (Fig. 2). Inflammation in the lungs showed significantly high perivascular cellular infiltration with thickening of the interstitial space (Fig. 2). The inflammation in all tissue started to appear at 2 weeks but peaked at 3 weeks postinfection (data not shown).
FIG. 2.
MyD88 deficiency results in the B. burgdorferi infection-induced development of myositis, glomerulonephritis, alveolitis, and pancreatitis. MyD88−/− and MyD88+/+ mice were infected with B. burgdorferi as described in Materials and Methods and were sacrificed 3 weeks after infection. Representative HE-stained sections of muscle (A and B), kidney (C and D), lungs (E and F), and pancreas (G and H) of both MyD88−/− mice (A, C, E, and G) and wild-type littermate MyD88 control mice (B, D, F, and H) are shown. The insets are the magnified views of the areas in the boxes.
B. burgdorferi-infected MyD88−/− mice have a higher bacterial load in all inflamed organs.
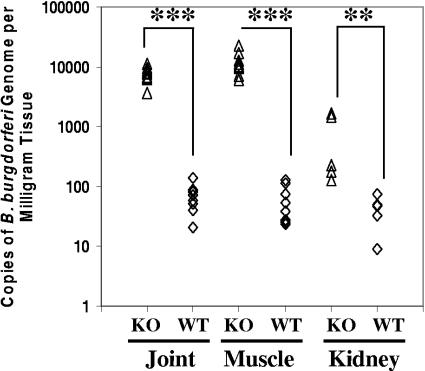
Spirochete numbers in MyD88−/− mice have been shown to be elevated in the joints and bladders of infected mice. We wished to determine whether spirochete burdens in specific tissues could account for some the differences in inflammation seen in the various tissues. Bolz et al. have previously shown that spirochete levels in blood and spleen were not affected and remained low in MyD88−/− mice in spite of the high levels of B. burgdorferi in joint, heart, and ear (8). Notably, we did not see any evidence of increased inflammation in the spleen histologically, which fits well with this finding. We chose to compare spirochete burdens in two tissues, kidney and muscle, that were found to exhibit increased inflammation in MyD88−/− mice but not typically in immunocompetent mice to the spirochete numbers in an organ, the joint, where pathology is commonly seen in inbred strains. Spirochete burdens were measured by quantitative PCR from mice infected for 3 weeks with B. burgdorferi. Significantly higher numbers of B. burgdorferi were seen in all three tissues: joint (P < 0.00001; 2.5 logs higher), muscle (P < 0.00001; 2.5 logs higher), and kidney (P = 0.009; 1 log higher) (Fig. 3A). Joint and muscle tissue of MyD88−/− mice showed similar levels of increases between wild-type and knockout mice and comparable numbers of spirochetes overall; the kidney had a lower level of increase as well as a lower overall burden.
FIG. 3.
B. burgdorferi-infected MyD88−/− mice have higher bacterial loads in all inflamed organs. MyD88−/− and MyD88+/+ mice were infected with B. burgdorferi as described in Materials and Methods and sacrificed 3 weeks after infection. DNA was isolated from joint, muscle, and kidney tissues and processed to quantitate B. burgdorferi DNA levels using real-time RT-PCR for a single-copy B. burgdorferi gene, recA. Numbers represent copies of B. burgdorferi recA normalized to the weight of tissue used to isolate DNA. Ten mice each were used for studies of wild-type (WT) and MyD88−/− (knockout [KO]) joints and muscle tissue; five mice each were used for studies of kidney tissue. ***P < 0.001; **P < 0.01.
MyD88 deficiency alters the kinetics of proinflammatory chemokine, cytokine, and adhesion molecule gene transcription.
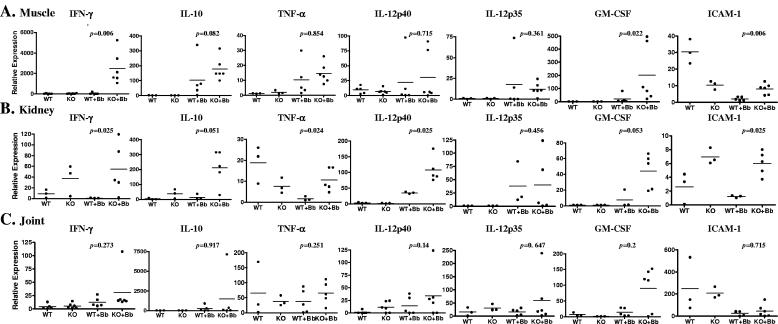
In order to understand the mechanism of differences in inflammation observed between affected and unaffected tissues, we compared the expression pattern of key immunomodulators in the muscles, joints, and kidneys of wild-type and MyD88 knockout mice. We examined transcription of cytokines and chemokines that may play a role in attracting inflammatory cells to affected tissues. Among the proinflammatory cytokines, we examined the transcription of gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and IL-12p40; we also examined expression of the anti-inflammatory cytokine IL-10. IFN-γ was significantly elevated in both muscle (P = 0.006) and kidney (P = 0.025) but not in joint (P = 0.273) in B. burgdorferi-infected MyD88−/− mice compared to MyD88+/+ controls (Fig. 4). The expression of IL-10 and TNF-α in B. burgdorferi-infected MyD88−/− muscle showed a trend towards elevation compared to MyD88+/+ littermates but did not reach significance. This appeared to be due to a single outlier wild-type mouse, which showed elevations in all of these cytokines. When this mouse is removed from the analysis, both IL-10 and TNF-α were significantly higher in the B. burgdorferi-infected MyD88−/− muscle than in MyD88+/+ muscle. TNF-α (P = 0.024) and IL-12p40 (P = 0.025) but not IL-12p35 (P = 0.456) levels were significantly elevated in B. burgdorferi-infected MyD88−/− mouse kidneys compared to those of wild-type controls (Fig. 4B). Again, expression of IL-10 showed a trend towards an increase in MyD88−/− mouse kidneys but did not reach significance (P = 0.051). No differences in IL-10 (P = 0.917), TNF-α (P = 0.251), IL-12p40 (P = 0.14), and IL-12p35 (P = 0.647) were seen in joint tissues (Fig. 4C). Granulocyte macrophage colony-stimulating factor (GM-CSF) expression was also induced significantly in B. burgdorferi-infected muscle (P = 0.022) but not in kidneys (p = 0.053) or joint tissue (P = 0.2) of MyD88−/− mice.
FIG. 4.
MyD88 deficiency alters B. burgdorferi-induced proinflammatory cytokine expression patterns. MyD88−/− (knockout [KO]) and MyD88+/+ (wild-type [WT]) mice were injected with B. burgdorferi (Bb) or sham as described in Materials and Methods and sacrificed 2 weeks after infection. Total RNA was extracted from the muscle (A), kidney (B), and joint (cartilage) (C), and expression of IFN-γ, IL-10, TNF-α, IL-12p40, IL-12p35, and GM-CSF was examined by real-time RT-PCR. P values at the top of individual graphs are comparisons between infected wild-type and knockout mice. The scales on the y axis are different for each individual graph. ICAM-1, intercellular adhesion molecule 1.
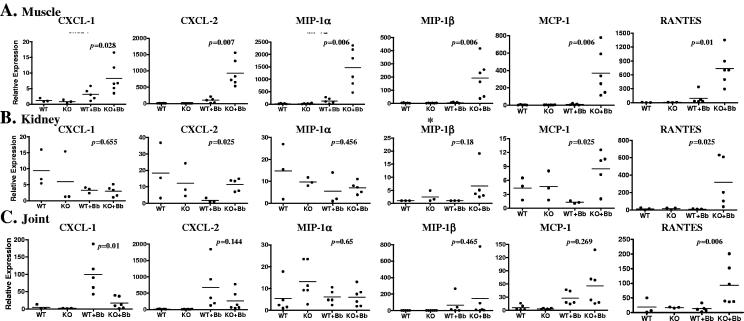
The chemokines we examined included the neutrophil chemoattractants CXC chemokine ligand 1 (CXCL-1) and CXCL-2, macrophage inflammatory protein 1α (MIP-1α), MIP-1β, monocyte chemoattractant protein 1 (MCP-1), and RANTES (Fig. 5). All of the neutrophil and monocyte/lymphocyte chemoattractants tested in muscle tissue were elevated in B. burgdorferi-infected MyD88−/− mice compared with wild-type littermates (CXCL-1, P = 0.028; CXCL-2, P = 0.007; MIP-1α, P = 0.006, MIP-1β, P = 0.006; MCP-1, P = 0.006; RANTES, P = 0.01) (Fig. 5). In kidney, CXCL-2 (P = 0.025), MCP-1 (P = 0.025), and RANTES (P = 0.025) were significantly elevated in B. burgdorferi-infected MyD88−/− mice compared to kidneys from MyD88+/+ mice (Fig. 6). There was no significant change in expression of CXCL-1 (P = 0.655) and MIP-1α (P = 456) in kidney tissue of MyD88−/− and wild-type mice. Expression of MIP-1β was undetectable in kidneys from both MyD88−/− and wild-type mice. In joint tissue, where inflammation was seen in response to B. burgdorferi infection but only small differences were seen between MyD88−/− and wild-type mice, only RANTES (P = 0.006) was significantly elevated in B. burgdorferi-infected MyD88−/− mice compared to wild-type mice, whereas CXCL-1 (P = 0.01) was significantly elevated in B. burgdorferi-infected wild-type mice compared to MyD88−/− mice (Fig. 5). CXCL-2, MIP-1α, MIP-1β, and MCP-1 were all similarly induced in MyD88−/− and wild-type animals (Fig. 5). These data confirm that differences in inflammation in muscle and kidney tissues of MyD88−/− mice compared with the lack of difference in inflammation in joint tissues are the result of changes in the induction of chemotactic chemokines and inflammatory cytokines.
FIG. 5.
MyD88 deficiency alters B. burgdorferi-induced proinflammatory chemokine expression patterns. MyD88−/− (knockout [KO]) and MyD88+/+ (wild-type [WT]) mice were injected with B. burgdorferi (Bb) or sham as described in Materials and Methods and sacrificed 3 weeks after infection. Total RNA was extracted from the muscle (A), kidney (B), and joint (cartilage) (C), and expression of CXCL-1, CXCL-2, MIP-1α, MIP-1β, MCP-1, and RANTES was examined by real-time RT-PCR. P values at the top of individual graphs represent comparisons between infected wild-type and knockout mice. The scales on the y axis are different for each individual graph.
FIG. 6.
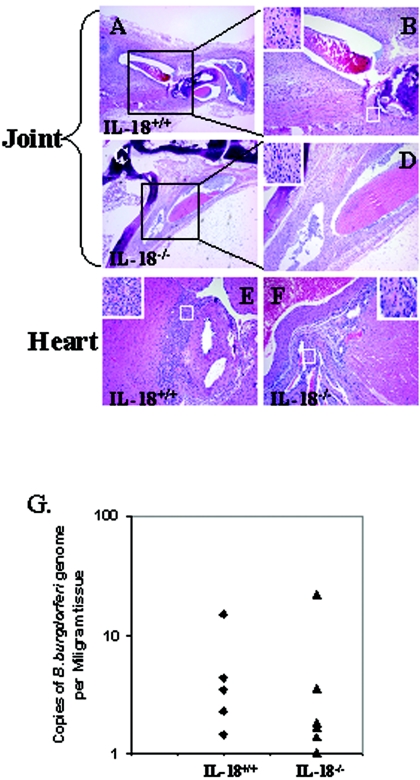
B. burgdorferi-infected mice develop arthritis and carditis in the absence of IL-18. IL-18−/− mice and their wild-type littermates (IL-18+/+) were infected with B. burgdorferi as described in Materials and Methods and sacrificed 3 weeks after infection, and their joints and hearts were processed for either histology or DNA isolation. Representative HE-stained sections of joints (A to D) and heart (E and F) of both wild-type (A, B, and E) and IL-18−/− (knockout) (C, D, and F) mice are shown. B and D are the magnified pictures of the boxed areas of A and B, respectively. The insets are the magnified views of the areas in the boxes. (G) DNA was isolated from joints of IL-18−/− (n = 6) and wild-type (n = 5) mice and processed to quantitate B. burgdorferi DNA levels using real-time RT-PCR for a single-copy B. burgdorferi gene, recA. Numbers represent copies of B. burgdorferi recA normalized to the weight of tissue used to isolate DNA.
B. burgdorferi-infected mice develop arthritis and carditis in the absence of IL-18.
IL-18, which has been shown to be important for the development of Lyme arthritis (16, 29), is a member of the IL-1 superfamily, which mediates signaling through the IL-1R superfamily signaling cascade that includes MyD88 (21). Thus, in order to understand the role of IL-18 in the differences in inflammation observed between different tissues in MyD88−/− and MyD88+/+ mice, we compared the kinetics of disease development in B. burgdorferi-infected IL-18−/− and IL-18+/+ littermates. Histopathology revealed cellular infiltrates in the joints and hearts of B. burgdorferi-infected IL-18−/− mice that were indistinguishable from those observed in infected wild-type littermates (Fig. 6 and Table 3). There was no significant difference in the borrelial load (P = 0.361) in the joint of IL-18−/− mice and their wild-type littermates (Fig. 6G). There was no change in joint swelling between the IL-18−/− and wild-type mice (data not shown). There was no inflammation observed in muscle, kidney, liver, and pancreas of both IL-18−/− and IL-18+/+ mice. These results clearly demonstrate that IL-18-dependent signaling does not account for the differences in inflammation observed between different tissues in MyD88−/− and MyD88+/+ mice.
TABLE 3.
Pathology of IL-18-deficient mice infected with B. burgdorferia
| Genotype | Avg score ± SD (no. of mice)
|
|
|---|---|---|
| Joint | Heart | |
| IL-18+/+ | 1.6 ± 0.55 (5) | 1.7 ± 0.488 (7) |
| IL-18−/− | 1.6 ± 0.5 (10) | 1.4 ± 0.224 (5) |
| P | 0.81 | 0.115 |
The overall histopathology was scored in a blinded manner on a scale of 0 (no lesion) to 5 (severe lesion), and the average of all scores for each group is shown.
DISCUSSION
The immune response to B. burgdorferi is a double-edged sword for the infected host. Host immune responses, both innate and adaptive, are important for the control of infection and reduction of spirochete burden. However, inflammation that is generated by the host immune response is also responsible for much of the pathology that is associated with the organism. In our study and in previous studies by Liu et al. and Bolz et al., it has clearly been shown that the development of inflammation can be mediated through pathways independent of TLR innate immune control of infection (8, 24). Additionally, we have found that inflammation in MyD88−/− mice occurs in tissues that are not typically affected by B. burgdorferi infection, including the lungs, pancreas, kidney, and muscle tissues. Inflammation in these tissues appears to be directly dependent upon the presence of the spirochete, as tissues that do not harbor significant numbers of spirochetes, such as the spleen, did not show evidence of inflammation.
Although prior publications have differed on changes in joint inflammation with the MyD88 deficiency, taken together, the three studies suggest only a mild difference, if any, in joint inflammation between wild-type and knockout mice. In the study by Bolz et al., there was an increase in joint inflammation reported, but this was a small effect of less than 1.5 histologic grades (on a scale of 0 to 5) (8); in the study by Liu et al. (24), there was no difference in histologic inflammation in the joint, and we did not see any difference in joint inflammation between MyD88−/− and wild-type mice. These small differences between the studies may be due to the timing of the examination, ages of the mice, or differences in the origin of the mice used (the mice used in our study were of a background identical to that used by Bolz et al. [8]), but overall, our assessment is that there is not a large difference in histologic joint inflammation.
The similarities in joint inflammation in MyD88−/− and wild-type mice are supported by our examination of inflammatory cytokines and chemokines in the different tissues. Of the 13 cytokines, chemokines, and adhesion molecules we examined, only CXCL-1 and RANTES were increased in the joints of MyD88−/− mice compared with wild-type mice, whereas in tissues showing greatly increased inflammation, such as muscle, the majority of inflammatory chemokines and cytokines were significantly elevated.
It is curious that increases in cytokine/chemokine expression and inflammation in MyD88−/− mice compared with littermate controls are not seen in tissues that are most commonly affected in B. burgdorferi infection of mice (the heart and the joints) but instead are seen in typically uninvolved tissues such as muscle. One possible explanation for this is that spirochete numbers were greatly increased in the MyD88−/− mice and that the presence of such unusually high numbers of organisms resulted in the activation of inflammatory pathways that are typically not turned on when fewer organisms are present. However, this alone cannot explain the differences between the tissues since the spirochete numbers in joints (where there was no increase in inflammation) and muscle (where there is a significant increase in inflammation) were similar. It is possible that inflammation in cardiac and joint tissue is already maximized in wild-type mice and that the addition of more spirochetes does not result in increases in inflammation compared with muscle and other tissues, where inflammation is typically low. Another possibility is that despite their in vitro role in activating NFκB and inducing inflammatory cytokines, in vivo TLR signaling may, on balance, activate pathways that suppress inflammation. Certain pathogens use TLR-based strategies to evade host defenses. Yersinia enterocolitica and Candida albicans induce immune suppression through TLR-2-mediated IL-10 release, and TLR-2-deficient mice are more resistant to lethal Yersinia and Candida infections (7, 28, 34). In Lyme arthritis, IL-10 has been shown to mediate reductions in joint inflammation, but this reduction is accompanied by an increase in spirochete burden (9). IL-10 levels in response to B. burgdorferi infection do not appear to be decreased in MyD88 knockout mice, so it is less likely that increases in inflammation are due to a loss of IL-10-mediated suppression. Oral administration of CpG oligodeoxynucleotide, the ligand for TLR-9, ameliorates inflammation in several animal models of colitis (31). B. burgdorferi DNA does contain CpG, and MyD88−/− mice would be expected to lose signaling through TLR-9. Whether the increase in inflammation in MyD88−/− mice is due to the loss of signaling through TLR-9 has not been tested.
IL-18 is an important mediator of both innate and adaptive immune responses and has been shown to play a role in Lyme neuroborreliosis and rheumatoid arthritis (11, 15, 16, 23, 26, 29). MyD88 is an adaptor molecule for the IL-18 receptor signaling pathway (21). IL-18 is expressed in the rheumatoid arthritis synovial membrane in macrophages, which correlates with TNF-α and IL-1β expression and disease severity (20). IL-18-deficient mice exhibit reduced incidence and severity of collagen-induced arthritis (5, 30), and IL-18 neutralization ameliorates streptococcus-induced arthritis (41). Thus, IL-18 is an important molecule for the development of arthritis. However, our results showed that the spirochetal load and inflammation pattern observed in the various tissues of IL-18−/− and IL-18+/+ mice were indistinguishable, indicating that IL-18-dependent signaling does not account for the inflammation observed in different tissues in MyD88-deficient mice.
In summary, we have shown that a deficiency of MyD88−/− results in increased inflammation in tissues not typically severely affected by B. burgdorferi such as muscle and kidney, but deficiency does not appear to affect inflammation in more typically affected tissues such as the joints and heart. The mechanism for the increased inflammation appears to be through increases in the expression of inflammatory cytokines and chemokines in the muscle and kidney tissues that are not seen in joint tissue. The MyD88-independent signaling pathways that are responsible for the induction of these inflammatory mediators remain unknown and are the focus of intense investigation.
Acknowledgments
We thank Kathleen Rogers for her help with genotyping and breeding of the mice. We also thank Douglas Golenbock, Honorine Ward, and Eduoard Vannier for their many helpful discussions.
This work was supported by grants from the National Institutes of Health, R01AI44240 (L.T.H.), R01 AI50043 (L.T.H), and U01AI058266 (L.T.H.); the American Lung Association (A.K.B.); the Earle P. Charlton Research fund (A.K.B.); and the Natalie V. Zucker Research Grant (A.K.B.).
Editor: D. L. Burns
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S. 2000. Toll-like receptors: lessons from knockout mice. Biochem. Soc. Trans. 28:551-556. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 4.Asbrink, E., and A. Hovmark. 1988. Early and late cutaneous manifestations in Ixodes-borne borreliosis (erythema migrans borreliosis, Lyme borreliosis). Ann. N. Y. Acad. Sci. 539:4-15. [DOI] [PubMed] [Google Scholar]
- 5.Banda, N. K., A. Vondracek, D. Kraus, C. A. Dinarello, S. H. Kim, A. Bendele, G. Senaldi, and W. P. Arend. 2003. Mechanisms of inhibition of collagen-induced arthritis by murine IL-18 binding protein. J. Immunol. 170:2100-2105. [DOI] [PubMed] [Google Scholar]
- 6.Behera, A. K., E. Hildebrand, J. Scagliotti, A. C. Steere, and L. T. Hu. 2005. Induction of host matrix metalloproteinases by Borrelia burgdorferi differs in human and murine Lyme arthritis. Infect. Immun. 73:126-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellocchio, S., C. Montagnoli, S. Bozza, R. Gaziano, G. Rossi, S. S. Mambula, A. Vecchi, A. Mantovani, S. M. Levitz, and L. Romani. 2004. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J. Immunol. 172:3059-3069. [DOI] [PubMed] [Google Scholar]
- 8.Bolz, D. D., R. S. Sundsbak, Y. Ma, S. Akira, C. J. Kirschning, J. F. Zachary, J. H. Weis, and J. J. Weis. 2004. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J. Immunol. 173:2003-2010. [DOI] [PubMed] [Google Scholar]
- 9.Brown, J. P., J. F. Zachary, C. Teuscher, J. J. Weis, and R. M. Wooten. 1999. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect. Immun. 67:5142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Veer, M. J., J. M. Curtis, T. M. Baldwin, J. A. DiDonato, A. Sexton, M. J. McConville, E. Handman, and L. Schofield. 2003. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur. J. Immunol. 33:2822-2831. [DOI] [PubMed] [Google Scholar]
- 11.Dinarello, C. A. 2004. Interleukin-18 and the treatment of rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 30:417-434. [DOI] [PubMed] [Google Scholar]
- 12.Feng, C. G., C. A. Scanga, C. M. Collazo-Custodio, A. W. Cheever, S. Hieny, P. Caspar, and A. Sher. 2003. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J. Immunol. 171:4758-4764. [DOI] [PubMed] [Google Scholar]
- 13.Fremond, C. M., V. Yeremeev, D. M. Nicolle, M. Jacobs, V. F. Quesniaux, and B. Ryffel. 2004. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J. Clin. Investig. 114:1790-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuse, K., G. Chan, Y. Liu, P. Gudgeon, M. Husain, M. Chen, W. C. Yeh, S. Akira, P. P. Liu, Y. Naiki, K. S. Michelsen, N. W. Schroder, R. Alsabeh, A. Slepenkin, W. Zhang, S. Chen, B. Wei, Y. Bulut, M. H. Wong, E. M. Peterson, and M. Arditi. 2005. Myeloid differentiation factor-88 plays a crucial role in the pathogenesis of coxsackievirus B3-induced myocarditis and influences type I interferon production. Circulation 112:2276-2285. [DOI] [PubMed] [Google Scholar]
- 15.Gracie, J. A. 2004. Interleukin-18 as a potential target in inflammatory arthritis. Clin. Exp. Immunol. 136:402-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grusell, M., M. Widhe, and C. Ekerfelt. 2002. Increased expression of the Th1-inducing cytokines interleukin-12 and interleukin-18 in cerebrospinal fluid but not in sera from patients with Lyme neuroborreliosis. J. Neuroimmunol. 131:173-178. [DOI] [PubMed] [Google Scholar]
- 17.Helmby, H., and R. K. Grencis. 2003. Essential role for TLR4 and MyD88 in the development of chronic intestinal nematode infection. Eur. J. Immunol. 33:2974-2979. [DOI] [PubMed] [Google Scholar]
- 18.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by Toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 19.Joosten, L. A., M. I. Koenders, R. L. Smeets, M. Heuvelmans-Jacobs, M. M. Helsen, K. Takeda, S. Akira, E. Lubberts, F. A. van de Loo, and W. B. van den Berg. 2003. Toll-like receptor 2 pathway drives streptococcal cell wall-induced joint inflammation: critical role of myeloid differentiation factor 88. J. Immunol. 171:6145-6153. [DOI] [PubMed] [Google Scholar]
- 20.Joosten, L. A., T. R. Radstake, E. Lubberts, L. A. van den Bersselaar, P. L. van Riel, P. L. van Lent, P. Barrera, and W. B. van den Berg. 2003. Association of interleukin-18 expression with enhanced levels of both interleukin-1beta and tumor necrosis factor alpha in knee synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 48:339-347. [DOI] [PubMed] [Google Scholar]
- 21.Kashiwamura, S., H. Ueda, and H. Okamura. 2002. Roles of interleukin-18 in tissue destruction and compensatory reactions. J. Immunother. 25:S4-S11. [DOI] [PubMed] [Google Scholar]
- 22.Leadbetter, E. A., I. R. Rifkin, A. M. Hohlbaum, B. C. Beaudette, M. J. Shlomchik, and A. Marshak-Rothstein. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416:603-607. [DOI] [PubMed] [Google Scholar]
- 23.Liew, F. Y., X. Q. Wei, and I. B. McInnes. 2003. Role of interleukin 18 in rheumatoid arthritis. Ann. Rheum. Dis. 62:ii48-ii50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, N., R. R. Montgomery, S. W. Barthold, and L. K. Bockenstedt. 2004. Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect. Immun. 72:3195-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Logigian, E. L., R. F. Kaplan, and A. C. Steere. 1990. Chronic neurologic manifestations of Lyme disease. N. Engl. J. Med. 323:1438-1444. [DOI] [PubMed] [Google Scholar]
- 26.Matsui, K., H. Tsutsui, and K. Nakanishi. 2003. Pathophysiological roles for IL-18 in inflammatory arthritis. Exp. Opin. Ther. Targets 7:701-724. [DOI] [PubMed] [Google Scholar]
- 27.Naiki, Y., K. S. Michelsen, N. W. Schroder, R. Alsabeh, A. Slepenkin, W. Zhang, S. Chen, B. Wei, Y. Bulut, M. H. Wong, E. M. Peterson, and M. Arditi. 2005. MyD88 is pivotal for the early inflammatory response and subsequent bacterial clearance and survival in a mouse model of Chlamydia pneumoniae pneumonia. J. Biol. Chem. 280:29242-29249. [Online.] [DOI] [PubMed] [Google Scholar]
- 28.Netea, M. G., R. Sutmuller, C. Hermann, C. A. Van der Graaf, J. W. Van der Meer, J. H. van Krieken, T. Hartung, G. Adema, and B. J. Kullberg. 2004. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J. Immunol. 172:3712-3718. [DOI] [PubMed] [Google Scholar]
- 29.Pietruczuk, A., R. Swierzbinska, M. Pietruczuk, and T. Hermanowska-Szpakowicz. 2004. Role of interleukin-18, interleukin-1beta and its soluble receptor (sIL-1RII) in early and late Lyme borreliosis. Pol. Merkuriusz Lek. 17:446-450. [In Polish.] [PubMed] [Google Scholar]
- 30.Plater-Zyberk, C., L. A. Joosten, M. M. Helsen, P. Sattonnet-Roche, C. Siegfried, S. Alouani, F. A. van De Loo, P. Graber, S. Aloni, R. Cirillo, E. Lubberts, C. A. Dinarello, W. B. van Den Berg, and Y. Chvatchko. 2001. Therapeutic effect of neutralizing endogenous IL-18 activity in the collagen-induced model of arthritis. J. Clin. Investig. 108:1825-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rachmilewitz, D., F. Karmeli, K. Takabayashi, T. Hayashi, L. Leider-Trejo, J. Lee, L. M. Leoni, and E. Raz. 2002. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology 122:1428-1441. [DOI] [PubMed] [Google Scholar]
- 32.Scanga, C. A., J. Aliberti, D. Jankovic, F. Tilloy, S. Bennouna, E. Y. Denkers, R. Medzhitov, and A. Sher. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 168:5997-6001. [DOI] [PubMed] [Google Scholar]
- 33.Seki, E., H. Tsutsui, N. M. Tsuji, N. Hayashi, K. Adachi, H. Nakano, S. Futatsugi-Yumikura, O. Takeuchi, K. Hoshino, S. Akira, J. Fujimoto, and K. Nakanishi. 2002. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J. Immunol. 169:3863-3868. [DOI] [PubMed] [Google Scholar]
- 34.Sing, A., D. Rost, N. Tvardovskaia, A. Roggenkamp, A. Wiedemann, C. J. Kirschning, M. Aepfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits Toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 196:1017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skerrett, S. J., H. D. Liggitt, A. M. Hajjar, and C. B. Wilson. 2004. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J. Immunol. 172:3377-3381. [DOI] [PubMed] [Google Scholar]
- 36.Steere, A. 1989. Lyme disease. N. Engl. J. Med. 321:586-596. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki, N., S. Suzuki, G. S. Duncan, D. G. Millar, T. Wada, C. Mirtsos, H. Takada, A. Wakeham, A. Itie, S. Li, J. M. Penninger, H. Wesche, P. S. Ohashi, T. W. Mak, and W. C. Yeh. 2002. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature 416:750-756. [Online.] [DOI] [PubMed] [Google Scholar]
- 38.Takeda, K., and S. Akira. 2005. Toll-like receptors in innate immunity. Int. Immunol. 17:1-14. [DOI] [PubMed] [Google Scholar]
- 39.Takeda, K., H. Tsutsui, T. Yoshimoto, O. Adachi, N. Yoshida, T. Kishimoto, H. Okamura, K. Nakanishi, and S. Akira. 1998. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 8:383-390. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392-5396. [DOI] [PubMed] [Google Scholar]
- 41.Tissi, L., B. McRae, T. Ghayur, C. von Hunolstein, G. Orefici, F. Bistoni, and M. Puliti. 2004. Role of interleukin-18 in experimental group B streptococcal arthritis. Arthritis Rheum. 50:2005-2013. [DOI] [PubMed] [Google Scholar]
- 42.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168:348-355. [DOI] [PubMed] [Google Scholar]