Abstract
We previously reported the isolation of a novel protein gene family, termed SAP (serine-, alanine-, and proline-rich protein), from Trypanosoma cruzi. Aided by the availability of the completed genome sequence of T. cruzi, we have now identified 39 full-length sequences of SAP, six pseudogenes and four partial genes. SAPs share a central domain of about 55 amino acids and can be divided into four groups based on their amino (N)- and carboxy (C)-terminal sequences. Some SAPs have conserved N- and C-terminal domains encoding a signal peptide and a glycosylphosphatidylinositol anchor addition site, respectively. Analysis of the expression of SAPs in metacyclic trypomastigotes by two-dimensional electrophoresis and immunoblotting revealed that they are likely to be posttranslationally modified in vivo. We have also demonstrated that some SAPs are shed into the extracellular medium. The recombinant SAP exhibited an adhesive capacity toward mammalian cells, where binding was dose dependent and saturable, indicating a possible ligand-receptor interaction. SAP triggered the host cell Ca2+ response required for parasite internalization. A cell invasion assay performed in the presence of SAP showed inhibition of internalization of the metacyclic forms of the CL strain. Taken together, these results show that SAP is involved in the invasion of mammalian cells by metacyclic trypomastigotes, and they confirm the hypothesis that infective trypomastigotes exploit an arsenal of surface glycoproteins and shed proteins to induce signaling events required for their internalization.
Infection by the protozoan parasite Trypanosoma cruzi, which is the etiological agent of Chagas' disease, a debilitating and incurable disease, affects 16 to 18 million people in the American continent (39). To infect mammalian hosts, T. cruzi relies on the ability to invade host cells, replicate intracellularly, and spread from cell to cell. Metacyclic trypomastigotes, the developmental forms found in the insect vector, establish the initial parasite-host cell interaction by adhering to cells as a prelude to invasion. Upon invasion, metacyclic forms differentiate into amastigotes that transform into trypomastigotes after several multiplication cycles. Once the host cells rupture, trypomastigotes are released into circulation and disseminate to various organs and tissues, where they enter cells and go through new multiplication cycles. To invade target cells, metacyclic forms and blood trypomastigotes engage a plethora of surface and secreted molecules, some of which are involved in triggering the signaling pathways both in the parasite and the host cell, leading to intracellular Ca2+ mobilization, a process essential for parasite internalization (22, 31, 36, 40).
Studies with in vitro-generated metacyclic trypomastigotes and tissue culture-derived trypomastigotes (TCT) have shown that these infective forms engage distinct sets of surface molecules that interact differentially with host components. The metacyclic stage-specific surface glycoprotein GP82, which binds to target cells and induces a bidirectional Ca2+ response (31), adheres to gastric mucin and also promotes invasion of the gastric mucosal epithelium upon oral infection (12, 25). Members of the TCT GP85 family, which have been implicated in target cell entry, bind to components of the extracellular matrix, such as fibronectin and laminin (19, 26). Notwithstanding their differential adhesive properties, metacyclic trypomastigote GP82 and TCT GP85 are related molecules belonging to the transsialidase (TS) superfamily (4, 11). Another group of surface glycoproteins implicated in cell invasion that are differentially expressed in metacyclic forms and TCT are constituted by mucin-like molecules. Mucins from metacyclic forms are protease-resistant GP35/50 molecules (23, 33), which induce Ca2+ signaling bidirectionally (31), whereas TCT mucins are larger molecules, which migrate in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as diffuse bands between 70 and 200 kDa (2).
T. cruzi secreted molecules also play a role in host cell invasion. Cruzipain, the major cysteine proteinase, participates in the process of TCT internalization (21) by generating bradykinin from cell-bound kininogen, thus activating bradykinin receptor and inducing Ca2+ mobilization (32). A soluble factor of unknown structure secreted by TCT but not by noninfective epimastigotes has been reported to trigger Ca2+ response in host cells (29). There are possibly other soluble T. cruzi molecules with signal-inducing activity contributing to parasite internalization. Here we address the question whether members of a novel serine-, alanine-, and proline-rich protein (SAP) family (9) could be such molecules. In addition to the identification and characterization of members of the SAP family in the T. cruzi genome, we report the cell adhesion and Ca2+ signal-inducing properties of SAP.
MATERIALS AND METHODS
Parasites, mammalian cells, and cell invasion assays.
T. cruzi strains G (41) and CL (6) and clone CL Brener (43) were used in this study. Parasites were maintained by cyclic passage in mice and in axenic cultures in liver infusion tryptose medium. Metacyclic trypomastigotes were purified by passage through DEAE-cellulose columns, as described previously (38). HeLa cells, the human carcinoma-derived epithelial cells, were grown at 37°C in Dulbecco's minimal essential medium supplemented with 10% fetal calf serum (FCS), streptomycin (100 μg/ml), and penicillin (100 U/ml) in a humidified 5% CO2 atmosphere. Cell invasion assays were carried out as previously described (42). Briefly, 1.5 × 106 to 3.0 × 106 metacyclic forms were seeded onto each well of 24-well plates containing 13-mm-diameter round glass coverslips coated with 1.5 × 105 HeLa cells. After 1 h of incubation at 37°C, the duplicate coverslips were washed in phosphate-buffered saline (PBS), pulsed for 1 min with water to lyse externally associated parasites, and washed in PBS. The cells were fixed with methanol, and after staining with Giemsa stain, the number of intracellular parasites was counted in a total of at least 500 cells.
Determination of intracellular Ca2+ concentration.
To measure mammalian cell cytosolic free Ca2+ ([Ca2+]i), we proceeded as follows. Cells were washed in Tyrode solution (137 mM NaCl-2.7 mM KCl-12 mM NaHCO3-0.36 mM NaH2PO4-0.53 mM MgCl2-1.36 mM CaCl2-5.5 mM glucose), pH 7.6. After the concentration of cells was adjusted to 2 × 106/ml, they were incubated with 5 μM fura-2 acetoxymethyl ester for 2 h at room temperature and nonincorporated fura-2 was washed out. Fluorescence was read in a fluorophotometer, the SPEX AR-CM system, with dual-wavelength excitation (340 and 380 nm) and emission at 510 nm. The increase in mammalian cell [Ca2+]i resulting from the addition of SAP recombinant protein was calculated as described previously (31). For each preparation we determined Rmax and Rmin, which correspond to the fluorescence ratio at 340 and 380 nm in the presence of saturating Ca2+ after treatment with 50 mM digitonin and in the absence of Ca2+ upon addition of 10 mM EGTA, respectively. The trypan blue dye exclusion method was used to determine the HeLa cells' viability after incubation with SAP recombinant protein (4 μg/ml) in the presence of Tyrode or RPMI. More than 98% of the cells are viable after this treatment.
T. cruzi supernatants.
Metacyclic trypomastigotes purified as described above were washed in PBS and incubated in this solution at a concentration of 1 × 108 parasites/ml for 16 h at 28°C. The parasites were centrifuged at 3,000 × g for 15 min, and parasite-free supernatants were filtered with 0.22-μm-pore-size filters (Millipore). The supernatants were concentrated under vacuum (Speed Vac) and kept at −20°C.
The integrity of parasites was assessed by checking their mobility under a phase-contrast light microscope and counting them in a Neubauer chamber. After incubation in PBS, very few parasites showed alteration in their morphology or mobility under phase-contrast light microscope. The estimated percentage of lysis was less than 2.0% (0.76% and 1.4% for the CL and G strains, respectively). Furthermore, metacyclic trypomastigotes maintained overnight in PBS preserved full infectivity towards HeLa cells, the rate of invasion being similar to that of freshly prepared parasites.
Purification of recombinant SAP.
A fragment of the SAP 1 gene (GenBank AF324829.1) encoding the central domain (CD) of the molecule, which is highly conserved in all SAPs (amino acids 32 to 193), was cloned in fusion with Schistosoma japonicum glutathione S-transferase (GST) in plasmid pGEX-A (34). The recombinant protein (SAP-CD) was produced in Escherichia coli DH5α by transforming the bacterium with the construct. After growth in LB medium and induction with 1 mM isopropyl-β-d-thiogalactopyranoside for 4 h, the transformed bacteria were washed in PBS, sonicated, and centrifuged at 12,000 × g for 10 min at 4°C. The bacterial lysates were passed through prepacked glutathione Sepharose 4B (Amersham Pharmacia Biotech), resuspended in SDS-PAGE sample buffer, boiled, and subjected to electrophoresis. The gel was treated with iced 250 mM KCl to visualize the band of high intensity of the expected size for the recombinant protein on a black background by using molecular size markers ranging from 94 kDa to 14 kDa (Amersham Pharmacia Biotech) as a reference. The excised band was put into a 50-ml tube containing bidistilled water. After 48 h under constant shaking, the eluate was dialyzed for 48 h at 4°C against 10 mM ammonium bicarbonate at 250 mA and then against bidistilled water for an additional 48 h. The final preparation was vacuum dried. The amount of purified protein was determined in microtiter plates by using the Coomassie Plus assay reagent (Pierce), followed by reading the optical density at 620 nm. To ascertain that the correct protein was obtained, the purified preparations were analyzed by silver staining of SDS-polyacrylamide gel and immunoblotting. The same procedure as that used to purify recombinant SAP was used for GST purification.
Production of anti-SAP antibodies.
Polyclonal monospecific antibodies to recombinant SAP were generated by immunizing BALB/c mice with four doses of the purified molecule (5 μg/mouse) plus Al(OH)3 as adjuvant (0.5 mg/mouse) by the intraperitoneal route at 10-day intervals. Ten days after the last immunizing dose, the animals were bled and the sera were stored at −20°C until used.
Cell binding assay of recombinant SAP.
HeLa cells (5 × 104) placed in 96-well microtiter plates were grown overnight at 37°C. After fixation with 4% paraformaldehyde in PBS, the cells were washed in PBS and blocked with PBS containing 10% FCS (PBS-FCS) for 1 h at room temperature. Various amounts of purified recombinant SAP or GST were added, and the incubation proceeded for 1 h at 37°C. Cells were washed in PBS and sequentially incubated for 1 h at 37°C with anti-SAP antibodies diluted in PBS-FCS and anti-mouse immunoglobulin (Ig) conjugated to peroxidase. Following washes in PBS, the bound enzyme was revealed by using o-phenylenediamine, as described previously (28).
Southern blot analysis and pulsed-field gel electrophoresis.
DNA samples isolated from epimastigotes as previously described (10) were digested with restriction enzymes, separated by electrophoresis on agarose gels (0.8%), stained with ethidium bromide (0.5 μg/ml), and transferred to nylon membranes in 20× SSC (0.15 M NaCl and 0.015 M sodium citrate). The membranes were prehybridized in a solution containing 50% formamide-5× SSC-5× Denhardt's solution-0.5% SDS-5 mM EDTA-0.1 mg tRNA per ml at 42°C for 2 h and then hybridized overnight at the same temperature with a 32P-labeled probe, which consisted of random primed labeled DNA fragments corresponding to different regions of the SAP gene. Following hybridization, the membranes were subjected to three washes (30 min each at 56°C) in 2× SSC containing 0.1% SDS and two additional washes at 56°C in 0.1× SSC containing 0.1% SDS. They were then exposed to X-ray film. Separation of T. cruzi chromosomal DNA was carried out by pulsed-field gel electrophoresis in a Gene Navigator apparatus (Pharmacia) using a hexagonal electrode array (8). Gels were stained with ethidium bromide, photographed, transferred to nylon filters, and hybridized as described above. Slot blots containing genomic DNA from different species of the genus Trypanosoma (T. rangeli, T. blanchardi, T. cruzi, T. rabinowitschae, T. dionisii, T. conorhini, Trypanosoma sp., T. cyclops, and T. evansi) were kindly provided by Marta M. Teixeira (Department of Parasitology, ICB, Universidade de São Paulo). Solutions containing 10 μg of genomic DNA were heated for 5 min at 100°C and chilled on ice. Samples were applied to Zeta Probe membranes (Bio-Rad) using a slot blot apparatus (Bio-Rad). The membranes were treated with 0.5 M NaOH for 5 min; neutralized with 1 M Tris-HCl, pH 8.0, 1.5 M NaCl for 5 min; and washed with 3× SSC for 1 min. DNA was fixed by exposure to UV light for 5 min and baking at 80°C for 30 min.
Cloning of SAP genes by reverse transcriptase PCR.
Total RNA was extracted from metacyclic trypomastigotes with Trizol. First-strand cDNA was prepared using the SuperScript preamplification system according to the manufacturer's instructions (Life Technologies). Specific forward and reverse primers (forward, 5′ CCATTGTGGTATAACTGCACGGAT 3′; reverse, 5′ CTTTGGATCTGGTAGAGATTCGGA 3′) based on the nucleotide sequences of the SAP gene (GenBank accession number AF324829) were used to amplify sequences from different members of the family by PCR. The T. cruzi tubulin gene (forward, 5′ ACCGATGTTGCGGCGATGCTTGAC 3′; reverse, 3′ CCGCGCGCTGCACCTTGGCAA 3′) was used as an internal control. Sequences were amplified with appropriate primers using Taq DNA polymerase in a final volume of 50 μl at a denaturing temperature of 95°C for 30 s, an annealing temperature of 55°C for 30 s, and an elongation temperature of 72°C for 30 s. The amplified PCR products were then cloned into plasmid pUC18 vector and transformed into E. coli strain DH5α.
Gene prediction and homology searches.
Nucleotide sequences of cDNA clones were determined by the dideoxynucleotide chain termination method using Taq dye terminator cycle sequencing chemistry in an ABI PRISM 377 DNA sequencer. BLASTX, TBLASTX, and BLASTP (3) algorithms were used to search for homologous nucleic acid or protein SAP sequences in the T. cruzi GeneDB and GenBank databases, respectively, at htpp://www.genedb.org/ and http://www.ncbi.nlm.nih.gov/. This analysis resulted in 33 contigs carrying complete copies of SAP (GenBank AAHK01000015, AAHK01000139, AAHK01000985, AAHK01001646, AAHK01000410, AAHK01000051, AAHK01000068, AAHK01000371, AAHK01001855, AAHK01000695, AAHK01000036, AAHK01000809, AAHK01000177, AAHK01000334, AAHK01000241, AAHK01002159, AAHK01000303, AAHK01000002, AAHK01000557, AAHK01001647, AAHK01000008, AAHK01000221, AAHK01000381, AAHK01000014, AAHK01000469, AAHK01000537, AAHK01000137, AAHK01000155, AAHK01000310, AAHK01000048, AAHK01000191, AAHK01000026, and AAHK01000041). All open reading frames identified within T. cruzi contigs were annotated manually and analyzed using BLASTX, TBLASTX, and BLASTP to search for homologous nucleic acid or protein sequences in the GeneDB and GenBank databases. Motif scanning in predicted protein sequences was performed in the ExPASy proteomics server at htpp://www.expasy.org/, which screens for signal peptide cleavage sites (SignalP) (5); O-GalNAc (mucin-type) glycosylation sites (NetOGlyc); N-glycosylation sites (NetNGlyc); glycosylphosphatidylinositol (GPI) anchor and cleavage sites (DGPI); and Ser, Thr, and Tyr phosphorylation sites (NetPhos).
SDS-PAGE and Western blotting.
T. cruzi samples were boiled in 0.2% SDS for 5 min, chilled on ice for 1 min, solubilized in buffer (7 M urea, 2 M thiourea, 1% dithiothreitol, 2% Triton X-100, with protease inhibitors; 100 μM phenylmethylsulfonyl fluoride, 100 μM leupeptin, 5 mM EDTA) for 30 min with occasional agitation, and then centrifuged at 15,000 rpm for 10 min in a bench top microcentrifuge (27). The supernatant was subjected to two-dimensional (2D) gel electrophoresis by isoelectric focusing, using 13-cm immobilized pH gradient gel strips with 2.0% (pH 4.0 to 7.0) ampholines (Amersham Biosciences) for 17,000 V · h. After equilibration for 5 min in 62.5 mM Tris-HCl buffer (pH 6.8) containing 50 mM dithiothreitol, 2.3% SDS, and 10% glycerol, each gel strip was placed on the top of a 12% SDS-polyacrylamide gel and electrophoresed for about 14 h at 8 mA/gel, along with standard molecular mass proteins, which were added to a well at the gel edge. The gel was either stained by silver staining and dried or stained with Ponceau S and blotted onto nitrocellulose membranes. The Western blots were probed with the appropriate antisera and, after reaction with anti-mouse Ig conjugated to horseradish peroxidase, were revealed by chemiluminescence using the ECL Western blotting detection reagent and hyperfilm-MP (Amersham Biosciences).
RESULTS
Characterization of the SAP gene family.
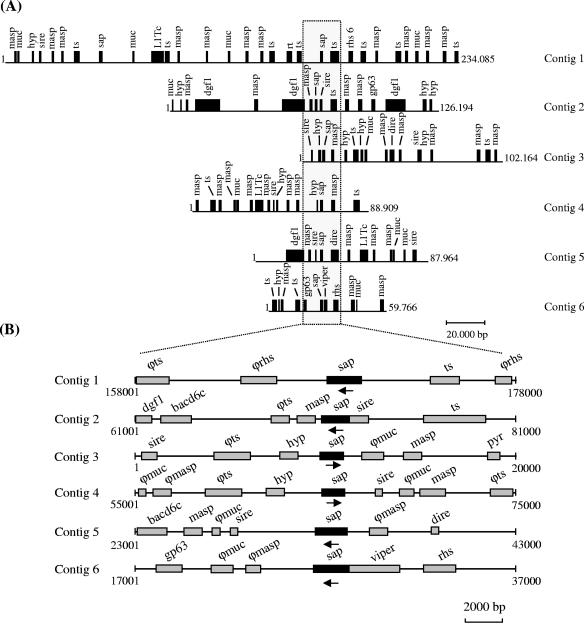
We further characterized the SAP gene family, which was discovered in the course of screening an expression genomic library of T. cruzi with antisera to metacyclic trypomastigotes (9). Through BLASPN and BLASTP searches against the T. cruzi GeneDB database, we identified 39 full-length copies of SAP that are distributed in 33 contigs (length ranging from 7,117 to 234,085 nucleotides): 28 contigs showed a single copy, four contigs carried two copies, and one contig had three copies of SAP (Fig. 1A). In a given contig, the distance between two SAP genes varied from 8 kb (contig 6972) to 120 kb (contig 7644). The contigs appeared to be enriched in surface antigen (pseudo)-genes(MASP, TS, GP63, mucin, and DGF-1), RHS (retrotransposon hot spot protein), and retrotransposon-like elements (VIPER, L1Tc, SIRE, and DIRE) (Fig. 1A and B). We also identified six pseudogenes and four partial genes. Most SAP genes were located near genes from group II of the TS superfamily (16) and repetitive sequences previously described in the intergenic spacers of the pyrimidine biosynthetic (PYR) gene cluster (17) (Fig. 1A and B).
FIG. 1.
Characterization of SAP gene family. (A) Representation of T. cruzi contigs harboring SAP genes, each line representing the entire contig. The length (in base pairs) is indicated to the right of each contig. (B) The dotted rectangle delineates the zoom-in area from panel A. The areas shown are 20 kb in length, and the exact position in the contigs is indicated below each line. Locus names appearing above genes correspond to their systematic names as submitted to GenBank and assigned in GeneDB. The symbol ϕ indicates a pseudogene. Loci are as follows: sap, serine-, alanine-, and proline-rich protein; masp, mucin-associated surface protein; ts, surface glycoprotein from group II of the transsialidase superfamily; muc, mucin; L1Tc, non-long terminal repeat retrotransposon encoding ape (AP-endonuclease); rt (reverse transcriptase) and gag (RNase H); rhs, retrotransposon hot spot protein; gp63, surface metalloproteinase; dgf-1, dispersed gene family 1 (putative surface protein); hyp (hypothetical protein), putative coding sequence with no assigned function; sire, short interspersed repetitive element; dire, degenerated ingi/L1Tc-related element; viper, vestigial interposed retroelement; bacd6c, T. cruzi telomeric clone BAC 6D6; pyr, intergenic spacer of pyrimidine biosynthetic gene cluster. The arrows indicate the orientation of the SAP gene. The contigs indicated have the following GenBank accession numbers: 1, AAHK01000002; 2, AAHK01000014; 3, AAHK01000026; 4, AAHK01000048; 5, AAHK01000051; 6, AAHK01000139.
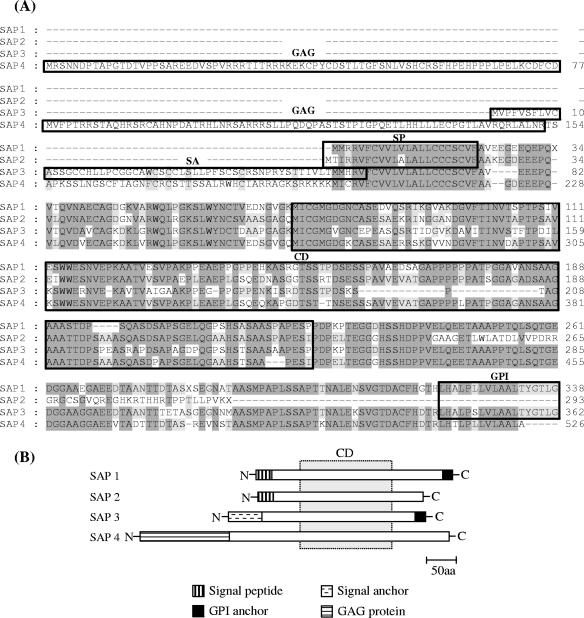
SAPs share a CD of about 55 amino acids, and they could be divided into four groups based on their amino (N)- and carboxy (C)-terminal sequences (Fig. 2). SAP 1 is made up of 31 polypeptides of about 339 amino acids (∼38.2 kDa) characterized by the presence of conserved N- and C-terminal domains encoding a signal peptide and a GPI anchor addition site, respectively. After the first methionine, there is a typical N-terminal signal sequence composed of basic residues followed by hydrophobic amino acids with a putative cleavage site, VFA-VE (5). The SAP 2 group is composed of two proteins (∼284 residues, ∼32 kDa) that display the predicted N-terminal signal sequence but differ from SAP 1 in the absence of a GPI anchor addition site. The SAP 3 group has five members that encode peptides of about 399 amino acids (∼44.8 kDa) with an N-terminal sequence predicted to be a signal anchor sequence with a high score but no identifiable cleavage site. SAP 4 (596 residues, ∼67.1 kDa) is a chimera containing the N-terminal domain of T. cruzi gag protein (encoded by the retrotransposon L1Tc) combined with the central and C-terminal domains of SAP.
FIG. 2.
Multiple alignment of amino acid sequences from SAP variants. (A) Alignments were done by Clustal W with the MegAlign program (DNAstar Inc.). Dashes were introduced to maximize the alignment. Sequences corresponding to the conserved residues are shaded in dark gray (>80% conservation) and light gray (60 to 80% conservation), and the absence of shading denotes residues with <60% conservation. The N-terminal signal peptide (SP), the N-terminal signal anchor sequence (SA), the CD of approximately 155 amino acids common to all SAPs, and the C-terminal GPI anchor addition site are boxed. The N-terminal domain of the T. cruzi gag protein encoded by SAP 4 is also boxed. (B) Schematic drawing of SAPs indicating the regions of identity between different members of the SAP family. The dotted rectangle denotes the CD common to all SAPs.
To analyze the transcripts of members of the SAP family in metacyclic trypomastigotes, we sequenced cDNA clones obtained by reverse transcriptase PCR from phylogenetically distant strains G and CL (7) using specific primers based on the SAP 1 nucleotide sequence. The cDNA clones from the two strains (GenBank accession numbers DQ130018 and DQ130019, respectively) were very similar (87% identity), displaying 89% identity with SAP 1 at nucleotide and amino acid levels. A search of the GenBank and GeneDB databases revealed identity between SAP 1 and 19 expressed tag sequences, confirming that SAP-related sequences are also transcribed in epimastigotes and amastigotes. Transcripts of SAP 3 and SAP 4 were also identified in metacyclic forms.
From the predicted SAP amino acid sequences, there are 17 to 41 potential sites for phosphorylation, 2 to 5 sites for N glycosylation, and 27 to 36 sites for O glycosylation. The deduced SAP peptides were characterized by a high content of alanine (12.2 to 17.3%), proline (7.06 to 13.5%), serine (7.2 to 11.7%), glycine (6.7 to 11.2%), glutamic acid (6.0 to 11.2%), and threonine (4.2 to 8.9%), with serine, alanine, and proline residues distributed in diverse repeats (AAS, AAP, AAS, APS, SAA, SSA, SPP, PAP, AAPP, SAPA, SAAP, SSPA, SSAP, SAAA, APPPP, SAAAS, PPSPP, SSPPA, AAAPP, PSAAAS, PPPPPPA, SASAASPA, SASAASAA, APPPPPPA, and SASAASSPA). SAPs are likely to contain acid-labile O-linked glycans because their reactivity with specific antibodies was abolished when metacyclic trypomastigote extracts were treated with sodium periodate (data not shown). SAPs deposited in the T. cruzi Genome Project database displayed some degree of similarity at amino acid level with a few members of the MASP (mucin-associated surface protein) family (15) and mucins (TcMUC and TcMUC II) (16), sharing 45 to 65% identity with a MASP sequence of ∼180 amino acids and 51 to 81% identity with a region of 80 amino acids located at the C-terminal domain of mucins from the TcMUC subfamily (Fig. 3).
FIG. 3.
Comparison of amino acid sequences of SAP with mucins and MASP of T. cruzi. Alignments were done by Clustal W with the MegAlign program (DNAstar Inc.). Conserved residues are shaded in dark gray (>80% conservation) and light gray (40 to 80% conservation). Sequences are as follows: SAP 1 and SAP 4; MASP, mucin-associated surface protein (GenBank EAN80883); TcMUCa (GenBank EAN81022); TcMUCII (GenBank EAN89849); and TcMUCb (GenBank EAN83206). The C-terminal GPI anchor addition site is overlined.
Genomic organization of SAP family.
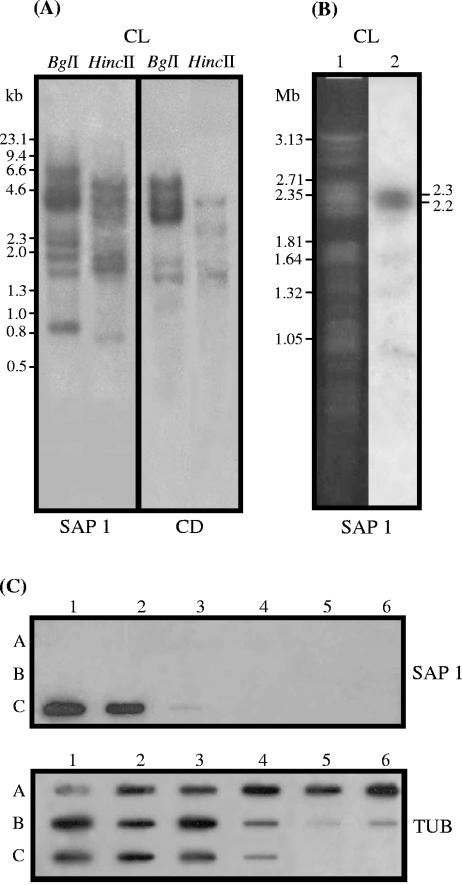
When T. cruzi (clone CL Brener) genomic DNA digested with BglI or HincII was hybridized with SAP probes, multiple bands suggestive of a family of polymorphic genes were revealed. More bands were detected by the probe containing the complete open reading frame of SAP 1 (1,038 bp) than by the fragment (511 bp) encoding the CD of 155 amino acids common to all SAPs (Fig. 4A). The latter probe hybridized to a 2.75-Mb chromosomal band of high intensity and hybridized weakly with two bands of 1.25 and 0.85 Mb (Fig. 4B). This suggests that the copies of SAP may be concentrated in a few loci, rather than distributed throughout the genome, as is the case in several families of T. cruzi surface molecules such as TS-like proteins, mucins, MASP, and GP63 proteases (4, 8, 10, 13, 16, 20, 35). Taken together, the data from Southern blot hybridization, chromosome mapping, and computational analysis of DNA sequences from T. cruzi DB indicate that SAP genes belong to a relatively small family.
FIG. 4.
Genomic organization of SAP-related sequences. (A) Southern blot of T. cruzi (clone CL Brener) genomic DNA digested with BglI or HincII and hybridized with the 32P-labeled insert of SAP 1 or the fragment encoding the central domain of approximately 155 amino acids common to all SAPs. Numbers correspond to the molecular sizes in kilobases. (B) Chromosomal mapping of SAP-related sequences. Chromosomal bands of epimastigotes (clone CL Brener) were separated by pulsed-field gel electrophoresis, stained with ethidium bromide (lane 1), transferred onto nylon membranes, and hybridized with the 32P-labeled insert of SAP 1 (lane 2). Numbers correspond to the molecular sizes in megabases. (C) Demonstration of species specificity of SAP genes by slot blot hybridization using genomic DNA from different species from the genus Trypanosoma. (Rows A) Lanes: 1, T. rangeli (Saimiri); 2, T. rangeli (Preguiça); 3, T. rangeli (P.G.); 4, T. rangeli (Palma-2); 5, T. rangeli (Choachi); 6, T. rangeli (San Augustin). (Rows B) Lanes: 1, T. lewisi; 2, T. blanchardi; 3, T. rabinowitschae; 4, T. conorhini; 5, T. cyclops; 6, T. evansi. (Rows C) Lanes: 1, T. cruzi (G); 2, T. cruzi (Y); 3, T. dionisii; 4, Trypanosoma sp. strain 60. Samples were hybridized with random primed 32P-labeled SAP probe. After exposition, the membranes were incubated at 100°C for 30 min in 0.1% SDS and rehybridized with the tubulin gene probe.
To investigate whether SAP-related sequences are present in other trypanosomatids, genomic DNA of 10 species of the genus Trypanosoma was hybridized with a SAP probe. Only T. cruzi reacted positively (Fig. 4C). This result was further confirmed by PCR amplification using primers derived from the SAP gene, which exclusively amplified T. cruzi DNA (data not shown), indicating that the SAP sequences are species specific.
Expression of the SAP family.
Identification of T. cruzi native proteins that share antigenic determinants with SAPs was carried out by subjecting metacyclic trypomastigote extracts to 2D electrophoresis and immunoblotting, using anti-SAP antibodies (Fig. 5A). In the CL strain, anti-SAP antibodies reacted with 24 proteins ranging from 52 to 81.5 kDa, focusing at pH 5 to 7. Those of highest intensity were four 81.5-kDa spots at pH 5.1 to 5.4 and four ∼53-kDa spots at pH 5.5 to 6.0. The profile of the G strain differed considerably from that of the CL strain, with fewer spots. Of about 10 proteins detected in the G strain, the most intense were four ∼53-kDa spots at pH 5.1 to 5.4 and two 59-kDa spots at pH 4.4 to 5.7 (Fig. 5A). The difference in size between the native SAPs and those deduced from DNA sequences could be due to the posttranslational modifications of the proteins which have a large number of potential sites for O glycosylation and phosphorylation, in addition to sites for N glycosylation.
FIG. 5.
Identification of SAP-related proteins from metacyclic trypomastigotes of T. cruzi. (A) 2D-PAGE immunoblot. Total protein (300 μg) from the indicated parasite strains was solubilized and separated by isoelectric focusing, followed by second-dimensional separation in a 12% SDS-polyacrylamide gel. Proteins were transferred onto a nitrocellulose membrane and stained with Ponceau S. After blocking and incubation with anti-SAP mouse antisera in PBS-milk, the membranes were washed in PBS containing 0.05% Tween 20 and incubated with anti-mouse IgG conjugated to horseradish peroxidase. The bands were revealed by chemiluminescence using the ECL Western blotting detection reagent and hyperfilm-MP (Amersham). (B) Shedding of SAP into the extracellular medium. Metacyclic forms were incubated overnight in PBS at 28°C (1 × 108 parasites/ml). After centrifugation, the supernatant was filtrated, concentrated, subjected to SDS-PAGE, and blotted onto a nitrocellulose membrane, which was processed as in panel A. Note the difference in reactivity between CL and G strain proteins towards anti-SAP antibodies.
To determine the cellular localization of SAP-related proteins, metacyclic trypomastigotes were analyzed by immunofluorescence followed by visualization with a confocal microscope. Anti-SAP antibodies reacted with surface and cytoplasmic components of parasites permeabilized with saponin and fixed with formaldehyde and also reacted at low intensity with intact live parasites (data not shown). The possibility that SAP-related molecules were shed into the extracellular medium was examined by maintaining metacyclic forms overnight in PBS and collecting the supernatants for analysis by immunoblotting. A broad band of ∼55 kDa was detected in both strains, with the difference that the intensity of SAP from the CL strain was much greater than that of SAP from the G strain (Fig. 5B).
Binding of SAP to host cells and Ca2+ mobilization.
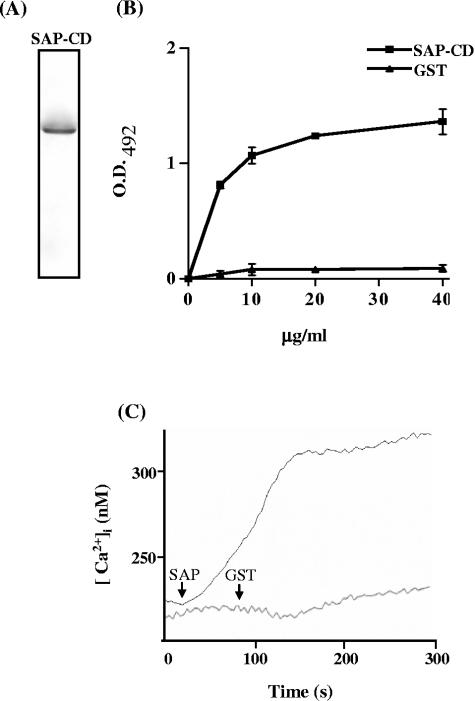
To determine whether SAP had cell adhesion and signal-inducing properties, we used the purified recombinant protein containing the conserved central domain (SAP-CD), which was analyzed by silver staining of an SDS-polyacrylamide gel to ascertain that it was free of contaminant (Fig. 6A). HeLa cells immobilized on the bottom of microtiter plates were incubated with increasing concentrations of SAP-CD, and the bound peptide was detected by anti-SAP antibodies. SAP-CD, but not GST, bound to HeLa cells in a dose-dependent and saturable manner (Fig. 6B). We investigated the possibility that SAP-CD triggered a Ca2+ response in target cells by measuring the increase in intracellular Ca2+ concentration in HeLa cells preloaded with fura-2 upon exposure to SAP-CD, following the procedure detailed in reference 31. As shown in Fig. 6C, HeLa cells responded to SAP-CD by mobilizing Ca2+ in a sustained manner but were unresponsive to GST.
FIG. 6.
Cell binding and Ca2+ signal-inducing activity of SAP-CD. (A) The purity of the recombinant SAP-CD was ascertained by silver staining of an SDS-polyacrylamide gel containing 5 μg of protein. (B) Increasing concentrations of SAP-CD were added to wells in enzyme-linked immunosorbent assay plates containing adherent HeLa cells. After the washes, cells were sequentially incubated with anti-SAP antibodies and anti-mouse IgGs conjugated to peroxidase. The bound enzyme was revealed by o-phenylenediamine as substrate. The GST recombinant protein was used as a control. Representative results of one of four comparable experiments are shown. The values are the means ± standard deviations of triplicates. (C) A total of 2.5 μg of SAP-CD or GST was added (arrow) to fura-2-loaded HeLa cells in a cuvette containing 2.5 ml of Tyrode solution at the time indicated. The results of one out of three representative experiments are presented.
Effect of SAP on target cell entry of metacyclic trypomastigotes.
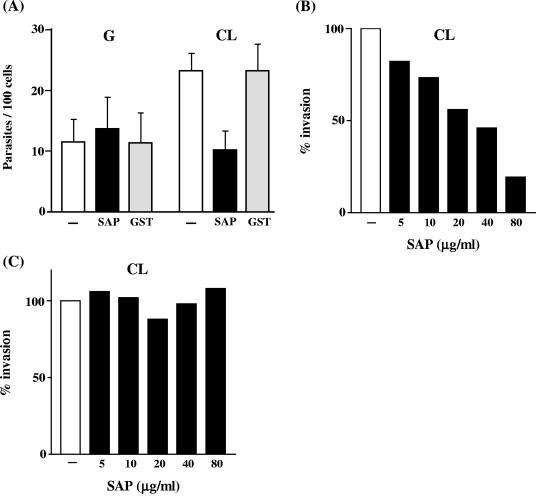
As SAP-CD induces a sustained Ca2+ response, we reasoned that treatment of target cells with SAP-CD could impair the internalization of parasites. HeLa cells were treated with SAP-CD or GST for 15 min and then incubated with metacyclic forms in the presence of the recombinant protein. After 1 h, the cells were washed and stained. HeLa cell entry of CL strain metacyclic forms was significantly inhibited by SAP-CD but not by GST, compared with untreated controls (P < 0.0005). Infectivity of the G strain was not affected by SAP-CD (Fig.7A). When the inhibitory effects of different SAP-CD concentrations were tested, HeLa cell invasion by CL strain metacyclic forms was found to be inhibited by SAP-CD even at 5 μg/ml, albeit to a lower degree (Fig. 7B). We also examined whether the addition of SAP-CD to HeLa cells at the same time as the parasites could have a different effect. As shown in Fig. 7C, there was no inhibitory effect when SAP and parasites were added simultaneously to HeLa cells.
FIG. 7.
Effect of SAP-CD on host cell entry of T. cruzi metacyclic trypomastigotes. (A) HeLa cells were treated for 30 min with SAP-CD at 80 μg/ml, before incubation with the parasites. After incubation for 1 h in the presence of SAP-CD, the cells were washed and stained with Giemsa stain. The number of internalized parasites was counted in a total of at least 500 Giemsa-stained cells. The values are the means ± standard deviations of five independent experiments performed in duplicate. The difference between the control and SAP-treated cells in CL strain invasion is significant (P < 0.0005) by Student's t test. (B) HeLa cells were preincubated with various concentrations of SAP-CD, and its effect on invasiveness of the T. cruzi CL strain was assessed as in panel A. A value of 100% invasion was ascribed to the untreated control. Representative results of two experiments carried out in duplicate are shown. These showed differences of less than 10%. (C) SAP-CD and parasites (CL strain) were added at the same time to HeLa cells. After incubation for 1 h, the cells were washed and stained with Giemsa stain. A value of 100% was ascribed to the untreated control. Representative results of two experiments carried out in duplicate are shown. They showed differences of less than 10%.
DISCUSSION
Multigene families encoding surface antigens are common in trypanosomes (10, 13, 15, 16, 37) and protozoa in general. Our results show that SAPs are also products of a multigene family. SAP genes were found near retrotransposon-like elements and genes encoding diverse surface molecules (Fig. 1). In T. cruzi, regions of synteny with other trypanosomatids (Trypanosoma brucei and Leishmania major) are interrupted by genes belonging to these multigene families (18), suggesting that these regions could be genetically unstable. Some of the genes (SAP, MASP, TS-like, mucin, and DGF-1) located in the regions of synteny break appear to be species specific, and the dramatic expansion of these gene families could be associated with this preferential location in the T. cruzi genome. A search against the African trypanosome sequences did not reveal any orthologs. That the SAP gene family was unique to T. cruzi was confirmed by Southern blot hybridization and PCR amplification of genomic DNA from different species of the genus Trypanosoma, using specific probes (Fig. 4). This suggests that the acquisition of genetic information for SAP may have occurred after the separation of the T. cruzi ancestor from other trypanosomatid lineages. Of interest is the existence of a chimera of the N-terminal conserved domain of a gag-related protein combined with the N- and C-terminal domains of SAP. Chimeras containing the N- or C-terminal conserved domain of MASP combined with the N- or C-terminal domain of mucin or the C-terminal domain from the TS superfamily or a combination of mucin and TS-like genes have been reported previously (1, 15). Mosaic genes seem to be a common feature of the T. cruzi genome, and they could have evolved by segmental gene conversion, which could even involve the exchange of gene segments between members of the family with limited identity or with the pseudogene pool. Like other surface protein gene families in T. cruzi, the SAP family contains pseudogenes, which could contribute to the SAP repertoire through recombination, a hypothesis that could explain the existence of numerous pseudogenes of the TS-like family and adenylate cyclase family in the genome of T. cruzi (35, 37). Trypanosoma brucei apparently uses defective genes in building bona fide variant surface glycoprotein (30).
Metacyclic trypomastigotes expressed SAPs with molecular masses ranging from 45 to 82.7 kDa (Fig. 5A). However, the theoretical molecular masses of mature SAP (without signal peptide and GPI anchor sequences) were within the 26.5- to 53.9-kDa range, indicating that SAPs are likely to be posttranslationally modified in vivo. Modifications could include N and O glycosylation, as well as phosphorylation. Although the deduced amino acid sequence of SAP contained two elements typical of several GPI-anchored surface proteins in trypanosomes, namely, the presence of an N-terminal leader sequence and a C-terminal hydrophobic extension predicted to act as a signal sequence for addition of a GPI anchor, SAPs were barely detected on the cell surface. On the other hand, metacyclic forms were found to secrete a SAP of ∼55 kDa (Fig. 5B).
Sequences found at the central domain are conserved and seem to be unique to the SAP family. They could provide a signature for this family (Fig. 2). We found that the recombinant protein SAP-CD, containing the central domain conserved in the SAP family, binds to and triggers Ca2+ mobilization in HeLa cells (Fig. 6). SAP is the first shed T. cruzi molecule of known identity that directly triggers a Ca2+ response in host cells. Although a Ca2+ signal-inducing soluble factor from TCT has been reported (29), its identity has never been determined. As regards cruzipain, the major T. cruzi cysteine proteinase, it indirectly promotes target cell Ca2+ mobilization through kinin receptor by acting on cell-bound kininogen and generating bradykinin (32). SAP may be an additional factor contributing to T. cruzi infectivity of metacyclic trypomastigotes, in a strain-dependent manner. Preincubation of HeLa cells with SAP-CD was found to significantly inhibit invasion by metacyclic forms of the CL strain but not the G strain (Fig. 6C). As SAP-CD induces Ca2+ signaling (Fig. 6B), preincubation with SAP-CD could desensitize the cells and therefore impair not only the SAP-induced response but, more importantly, the signal-inducing activity of GP82. This surface molecule attaches to and triggers Ca2+ mobilization in host cells, promoting internalization of CL strain metacyclic forms (31). Another possibility is that Ca2+-mediated events, rather than desensitization of cells, are interfering with parasite invasion. A picture that we envisage is that, soon after recognition of GP82 by its as-yet-undefined receptor, SAP released within the microdomain formed by juxtaposition of parasite and host cell plasma membranes triggers Ca2+ signaling which, added to that induced by GP82, further enhances the response, leading to productive infection. The fact that SAP-CD added at the same time as the parasites did not enhance the invasion rate (Fig. 6D) supports this assumption. Invasion activity of G strain metacyclic forms is not associated with SAP, and this may be due to reduced expression of SAPs. In contrast to the CL strain, which displayed 24 SAPs, of which eight appeared as major spots, in immunoblots of 2D gels revealed with anti-SAP antibodies, the G strain expressed 10 SAPs of low intensity (Fig. 5A). We speculate that, due to the low level of SAP secretion in the G strain, the critical concentration of SAP necessary for signaling activity in the referred microdomain is not reached.
This difference in expression of SAP between CL and G isolates adds to a number of other differences observed in previous studies. Metacyclic trypomastigotes of the CL isolate are highly invasive in vitro (28) and efficiently invade mouse gastric mucosal epithelium upon oral infection (12, 25). During invasion, they engage the bidirectional Ca2+ signal-inducing surface molecule GP82 (31), which establishes the cross talk with the target cell. It has been proposed that, in the parasite, the interaction of GP82 with its receptor triggers the activation of a protein kinase and phospholipase C that generates inositol-1,4,5-triphosphate (InsP3), which releases Ca2+ from internal stores (24). The poorly invasive G strain metacyclic forms rely on mucin-like surface molecules GP35/50 to enter host cells (31, 42). The proposed signaling route activated in G strain involves adenylate cyclase and generation of cyclic AMP, with Ca2+ mobilization from acidocalcisomes (24), the acidic vacuoles containing a Ca2+/H+ exchange system (14). In addition, we found that GP82 and GP35/50 induce activation of distinct signal transduction pathways in host cells (unpublished observations). These differences are consistent with the finding that CL and G strains belong to highly divergent genetic T. cruzi subgroups (7).
Overall, our results have shown that SAP is involved in the invasion of mammalian cells by metacyclic trypomastigotes. SAP binding to the host cell induces activation of signal transduction pathways, leading to intracellular Ca2+ mobilization, which is a requirement for T. cruzi internalization. These results confirm that T. cruzi exploits multiple ligand-receptor interactions to invade mammalian host cells.
Acknowledgments
This work was supported by grants from FAPESP and CNPq (Brazil) to J.F.D. and N.Y. and from the International Atomic Energy Agency (IAEA) to J.F.D. R.C.P.B. and D.F. were awarded doctoral fellowships by FAPESP and CAPES, respectively.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Allen, C. L., and J. M. Kelly. 2001. Trypanosoma cruzi: mucin pseudogenes organized in a tandem array. Exp. Parasitol. 97:173-177. [DOI] [PubMed] [Google Scholar]
- 2.Almeida, I. C., M. A. Ferguson, S. Schenkman, and L. R. Travassos. 1994. Lytic anti-α-galactosyl antibodies from patients with chronic Chagas' disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol glycoproteins of Trypanosoma cruzi. Biochem. J. 304:793-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 15:403-410. [DOI] [PubMed] [Google Scholar]
- 4.Araya, J. E., M. I. Cano, N. Yoshida, and J. F. da Silveira. 1994. Cloning and characterization of a gene for the stage-specific 82 kDa surface antigen of metacyclic trypomastigotes of Trypanosoma cruzi. Mol. Biochem. Parasitol. 65:161-169. [DOI] [PubMed] [Google Scholar]
- 5.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 6.Brener, Z., and E. Chiari. 1963. Variações morfológicas observadas em diferentes amostras de Trypanosoma cruzi. Rev. Inst. Med. Trop. São Paulo 5:220-224. [PubMed] [Google Scholar]
- 7.Briones, M. R. S., R. P. Souto, B. S. Stolf, and B. Zingalez. 1999. The evolution of two Trypanosoma cruzi subgroups inferred from rRNA genes can be correlated with the interchange of American mammalian faunas in the Cenozoic and has implications to pathogenicity and host specificity. Mol. Biochem. Parasitol. 104:219-232. [DOI] [PubMed] [Google Scholar]
- 8.Cano, M. I., A. Gruber, M. Vazquez, A. Cortés, M. J. Levin, A. Gonzalez, W. Degrave, E. Rondinelli, J. L. Ramirez, C. Alonso, J. M. Requena, and J. Franco da Silveira. 1995. Molecular karyotype of clone CL Brener chosen for the Trypanosoma cruzi genome project. Mol. Biochem. Parasitol. 71:273-278. [DOI] [PubMed] [Google Scholar]
- 9.Carmo, M. S., M. R. M. Santos, L. M. Cummings, J. E. Araya, L. M. Yamauchi, N. Yoshida, R. A. Mortara, and J. Franco da Silveira. 2001. Isolation and characterisation of genomic and cDNA clones coding for a serine-, alanine-, and proline-rich protein of Trypanosoma cruzi. Int. J. Parasitol. 31:259-264. [DOI] [PubMed] [Google Scholar]
- 10.Carmo, M. S., M. R. M. Santos, M. I. Cano, J. E. Araya, N. Yoshida, and J. Franco da Silveira. 2002. Expression and organization of the gene family encoding a 90 kDa surface glycoprotein of metacyclic trypomastigotes of Trypanosoma cruzi. Mol. Biochem. Parasitol. 125:201-206. [DOI] [PubMed] [Google Scholar]
- 11.Colli, W., and M. J. M. Alves. 1999. Relevant glycoconjugates on the surface of Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz 94(Suppl. 1):37-49. [DOI] [PubMed] [Google Scholar]
- 12.Cortez, M., M. R. Silva, I. Neira, D. Ferreira, G. R. Sasso, A. O. Luquetti, A. Rassi, and N. Yoshida. 7 September 2005, posting date. Trypanosoma cruzi surface molecule gp90 downregulates invasion of gastric mucosal epithelium in orally infected mice. Microbes Infect. [Online.] doi: 10.1016/j.micinf.2005.05.016. [DOI] [PubMed]
- 13.Cuevas, I. C., J. J. Cazzulo, and D. O. Sanchez. 2003. gp63 homologues in Trypanosoma cruzi: surface antigens with metalloprotease activity and a possible role in host cell infection. Infect. Immun. 71:5739-5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Docampo, R., D. A. Scott, A. E. Vercesi, and S. N. J. Moreno. 1995. Intracellular Ca2+ storage in acidocalcisomes of Trypanosoma cruzi. Biochem. J. 310:1005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Sayed, N. M. A., P. J. Myler, D. C. Bartholomeu, D. Nilsson, G. Aggarwal, et al. 2005. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas' disease. Science 309:409-415. [DOI] [PubMed] [Google Scholar]
- 16.Frasch, A. C. C. 2000. Functional diversity in members of the trans-sialidase and mucin families in Trypanosoma cruzi. Parasitol. Today 16:282-286. [DOI] [PubMed] [Google Scholar]
- 17.Gao, G., T. Nara, J. Nakajima-Shimada, and T. Aoki. 1999. Novel organization and sequences of five genes encoding all six enzymes for de novo pyrimidine biosynthesis in Trypanosoma cruzi. J. Mol. Biol. 285:149-161. [DOI] [PubMed] [Google Scholar]
- 18.Ghedin, E., F. Bringaud, J. Peterson, P. Myler, M. Berriman, A. Ivens, B. Andersson, E. Bontempi, J. Eisen, S. Angiuoli, D. Wanless, A. Von Arx, L. Murphy, N. Lennard, S. Salzberg, M. D. Adams, O. White, N. Hall, K. Stuart, C. M. Fraser, and N. M. El-Sayed. 2004. Gene synteny and evolution of genome architecture in trypanosomatids. Mol. Biochem. Parasitol. 134:183-191. [DOI] [PubMed] [Google Scholar]
- 19.Giordano, R., R. Chammas, S. S. Veiga, W. Colli, and M. J. M. Alves. 1994. An acidic component of the heterogeneous Tc-85 protein family from the surface of Trypanosoma cruzi is a laminin binding glycoprotein. Mol. Biochem. Parasitol. 65:85-94. [DOI] [PubMed] [Google Scholar]
- 20.Giordano, R., D. L. Fouts, D. Tewari, W. Colli, J. Manning, and M. J. M. Alves. 1999. Cloning of a surface membrane glycoprotein specific for the infective forms of Trypanosoma cruzi having adhesive properties of laminin. J. Biol. Chem. 274:3461-3468. [DOI] [PubMed] [Google Scholar]
- 21.Meirelles, M. N., L. Juliano, E. Carmona, S. G. Silva, E. M. Costa, A. C. Murta, and J. Scharfstein. 1992. Inhibitors of the major cysteinyl proteinase (gp57/51) impair host cell invasion and arrest the intracellular development of Trypanosoma cruzi in vivo. Mol. Biochem. Parasitol. 52:175-184. [DOI] [PubMed] [Google Scholar]
- 22.Moreno, S. N. J., J. Silva, A. E. Vercesi, and R. Docampo. 1994. Cytosolic-free calcium elevation in Trypanosoma cruzi is required for cell invasion. J. Exp. Med. 180:1535-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortara, R. A., S. Silva, M. F. Araguth, S. A. Blanco, and N. Yoshida. 1992. Polymorphism of the 35- and 50-kilodalton surface glycoconjugates of Trypanosoma cruzi metacyclic trypomastigotes. Infect. Immun. 60:4673-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neira, I., A. T. Ferreira, and N. Yoshida. 2002. Activation of distinct signal transduction pathways in Trypanosoma cruzi isolates with differential capacity to invade host cells. Int. J. Parasitol. 32:405-414. [DOI] [PubMed] [Google Scholar]
- 25.Neira, I., F. A. Silva, M. Cortez, and N. Yoshida. 2003. Involvement of Trypanosoma cruzi metacyclic trypomastigote surface molecule gp82 in adhesion to gastric mucin and invasion of epithelial cells. Infect. Immun. 71:557-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouaissi, M. A., J. Cornette, and A. Capron. 1986. Identification and isolation of Trypanosoma cruzi trypomastigote cell surface protein with properties expected of a fibronectin receptor. Mol. Biochem. Parasitol. 19:201-211. [DOI] [PubMed] [Google Scholar]
- 27.Paba, J., J. M. Santana, A. R. Teixeira, W. Fontes, M. V. Sousa, and C. A. Ricart. 2004. Proteomic analysis of the human pathogen Trypanosoma cruzi. Proteomics 4:1052-1059. [DOI] [PubMed] [Google Scholar]
- 28.Ramirez, M. I., R. C. Ruiz, J. E. Araya, J. F. da Silveira, and N. Yoshida. 1993. Involvement of the stage-specific 82-kilodalton adhesion molecule of Trypanosoma cruzi metacyclic trypomastigotes in host cell invasion. Infect. Immun. 61:3636-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez, A., M. G. Rioult, A. Ora, and N. Andrews. 1995. Trypanosome-soluble factor induces IP3 formation, intracellular Ca2+ mobilization and microfilament rearrangement in host cells. J. Cell Biol. 129:1263-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roth, C. W., S. Longacre, A. Raibaud, T. Baltz, and H. Eisen. 1986. The use of incomplete genes for the construction of a Trypanosoma equiperdum variant surface glycoprotein gene. EMBO J. 5:1065-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiz, R. C., S. Favoreto, Jr., M. L. Dorta, M. E. M. Oshiro, A. T. Ferreira, P. M. Manque, and N. Yoshida. 1998. Infectivity of Trypanosoma cruzi isolates is associated with differential expression of surface glycoproteins with differential Ca2+ signalling activity. Biochem. J. 330:505-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scharfstein, J., V. Schmitz, V. Morandi, M. M. A. Capella, A. P. C. A. Lima, A. Morrot, L. Juliano, and W. Muller-Ester. 2000. Host cell invasion by Trypanosoma cruzi is potentiated by activation of bradykinin B2 receptors. J. Exp. Med. 192:1289-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schenkman, S., M. A. J. Ferguson, N. Heise, M. L. Cardoso de Almeida, R. A. Mortara, and N. Yoshida. 1993. Mucin-like glycoproteins linked to the membrane by glycosylphosphatidylinositol anchor are the major acceptors of sialic acid in a reaction catalysed by trans-sialidase in metacyclic forms of Trypanosoma cruzi. Mol. Biochem. Parasitol. 59:293-304. [DOI] [PubMed] [Google Scholar]
- 34.Smith, D. B., and K. S. Johnson. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31-40. [DOI] [PubMed] [Google Scholar]
- 35.Takle, G. B., J. O'Connor, A. J. Young, and G. A. Cross. 1992. Sequence homology and absence of mRNA defines a possible pseudogene member of the Trypanosoma cruzi gp85/sialidase multigene family. Mol. Biochem. Parasitol. 56:117-127. [DOI] [PubMed] [Google Scholar]
- 36.Tardieux, I., M. H. Nathanson, and N. W. Andrews. 1994. Role in host cell invasion of Trypanosoma cruzi-induced cytosolic-free Ca2+ transients. J. Exp. Med. 179:1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor, M. C., D. K. Muhia, D. A. Baker, A. Mondragon, P. B. Schaap, and J. M. Kelly. 1999. Trypanosoma cruzi adenylyl cyclase is encoded by a complex multigene family. Mol. Biochem. Parasitol. 104:205-217. [DOI] [PubMed] [Google Scholar]
- 38.Teixeira, M. M. G., and N. Yoshida. 1986. Stage-specific surface antigens of metacyclic trypomastigotes of Trypanosoma cruzi identified by monoclonal antibody. Mol. Biochem. Parasitol. 18:271-282. [DOI] [PubMed] [Google Scholar]
- 39.WHO Expert Committee. 2002. Control of Chagas disease. WHO Tech. Rep. Ser. 905:i-vi, 1-109, back cover. [PubMed] [Google Scholar]
- 40.Yakubu, M. A., S. Majunder, and F. Kierszenbaum. 1994. Changes in Trypanosoma cruzi infectivity by treatment that affects calcium ion levels. Mol. Biochem. Parasitol. 66:119-125. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida, N. 1983. Surface antigens of metacyclic trypomastigotes of Trypanosoma cruzi. Infect. Immun. 40:836-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida, N., R. A. Mortara, M. F. Araguth, J. C. Gonzalez, and M. Russo. 1989. Metacyclic neutralizing effect of monoclonal antibody 10D8 directed to the 35- and 50-kilodalton surface glycoconjugates of Trypanosoma cruzi. Infect. Immun. 57:1663-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zingales, B., M. E. S. Pereira, R. P. Oliveira, K. A. Almeida, E. S. Umezawa, R. P. Souto, N. Vargas, M. I. Cano, J. Franco Da Silveira, N. S. Nehme, C. M. Morel, Z. Brener, and A. Macedo. 1997. Trypanosoma cruzi genome project: biological characteristics and molecular typing of clone CL Brener. Acta Trop. 68:159-173. [DOI] [PubMed] [Google Scholar]