Abstract
Illness due to respiratory virus infection is often induced by excessive infiltration of cells into pulmonary tissues, leading to airway occlusion. We show here that infection with Trichinella spiralis results in lower levels of tumor necrosis factor in bronchoalveolar lavage fluid and inhibits cellular recruitment into the airways of mice coinfected with influenza A virus. Infiltration of neutrophils and CD4+ and CD8+ lymphocytes was reduced, resulting in animals gaining weight more rapidly following the initial phase of infection. Influenza resulted in a generalized increase in vascular permeability in pulmonary tissues, and this was suppressed by parasite infection, although the effects were restricted to the early phase of trichinosis. Moreover, the number of cells producing interleukin-10 (IL-10), and the local levels of this cytokine, were reduced, suggesting that amelioration of pulmonary pathology by parasite infection occurs independently of IL-10 production.
Coinfection with different pathogens is a common occurrence which can alter the progression of disease (33, 36, 38). These relationships are complex, as the immune response can regulate survival and proliferation of the pathogen and be responsible for tissue damage. The effect of helminths on concurrent infections is of particular interest, given their prevalence in human populations, their ability to drive strong type 2 responses, and a wide range of immunomodulatory effects (27). As type 1 and type 2 responses are generally antagonistic, it has been suggested that helminth parasites may result in increased progression of disease when associated with bacteria and viruses, for which type 1 responses are generally regarded as beneficial in the control of infection (7, 22). There has been considerable debate on the impact of helminth infection on management of other diseases and whether this would be improved by anthelminthic treatment of the target populations (10, 11, 29).
Studies in animal models have in some cases correlated helminth coinfection with a shift in lymphocyte polarization, resulting, for example, in defective induction of type 1 cytokines and cytotoxic T-cell activity and a delay in viral clearance (1). However, the impact of one infectious agent upon another in terms of resultant immune responses, clearance of pathogens, and development of pathology varies greatly dependent upon the organisms involved, the timing of infection, the anatomical distribution of the pathogens, and their mode of replication within the host (13, 16, 26, 32). Indeed, recent studies have shown that treatment of Ugandan individuals with an antischistosomal drug resulted in transient increases in human immunodeficiency virus type 1 loads and sustained decreases in CD4+ T-cell counts (8).
We investigated the effects of Trichinella spiralis on influenza A infection. The rationale is, first, that these organisms colonize distinct anatomical sites, allowing an analysis of systemic rather than local bystander effects. T. spiralis organisms initially invade intestinal epithelia, later disseminating to skeletal muscle, whereas influenza virus is localized to the respiratory tract. Approximately 2 million children are estimated to die of acute respiratory infection per annum, 70% of these in Africa and Southeast Asia, where helminth infections are also highly prevalent (40). Influenza occurs in seasonal epidemics which affect 5 to 15% of the world's population, and there is considerable concern that the recent explosion of the H5N1 avian influenza strain in Asia may result in a global pandemic in the human population (39).
The enteric phase of T. spiralis infection generally induces a type 2-biased response, particularly in fast-responder (resistant) strains of mice, which becomes mixed during the systemic phase (18, 23). In contrast, replication of influenza virus in epithelial cells lining the airways is initially controlled by innate immune mechanisms, prior to specific adaptive responses which ultimately result in subsequent protection against reinfection. A prior set of experiments indicated that coinfection of mice with Nematospiroides dubius (Heligomosomoides polygyrus) and influenza A resulted in lower antihemagglutinin titers, reduced lung consolidation, and an indication of lower viral titers than in mice infected with influenza alone, although the mechanisms underlying these effects were not investigated (9). We show here that infection with T. spiralis inhibits cellular recruitment into the airways of mice infected with influenza, resulting in a more rapid gain of weight during the adaptive phase of the antiviral immune response. Interestingly, this effect was observed only during the early phase of parasite infection, not during long-term residence in skeletal muscle, and was not accompanied by local expansion of interleukin-10 (IL-10)-producing cells.
MATERIALS AND METHODS
Infection procedures.
Male NIH mice (6 to 8 weeks old) were infected by oral gavage with 500 infective larvae. At different times after parasite infection, or independently, mice were inoculated intranasally with 50 hemagglutinin (HA) units of influenza A virus X31 (HA titer, 4,096) diluted in phosphate-buffered saline. Animals were monitored and their weights determined on a daily basis.
Influenza-specific plaque assay.
The plaque assay was performed as previously described (19). Cells were removed from the upper left lobe of the mouse lung, and viral plaques were determined on Madin-Darby canine kidney (MDCK) cells using anti-influenza nucleocapsid A antibody (clone AA5H; Serotec).
Recovery of cells, flow cytometry, and histochemical staining.
Single-cell suspensions from mediastinal lymph nodes (MdLN), lungs, and bronchoalveolar lavage (BAL) fluid were prepared as previously described (21) and then stained with allophycocyanin-conjugated rat anti-mouse CD8a, 1 μg ml−1 (clone 53-6.7; BD Biosciences); phycoerythrin (PE)-Cy5-conjugated rat anti-mouse CD4, 1 μg ml−1 (clone RM4-5; Caltag Laboratories); rat anti-mouse CD49b/pan NK cell marker, 2.5 μg ml−1 (clone DX5; BD Biosciences); PE-conjugated rat anti-mouse IL-10, 2 μg ml−1 (clone JES5-2A5; Caltag); or PE-conjugated rat anti-mouse gamma interferon (IFN-γ), 2 μg ml−1 (clone XMG1.1; Caltag). Suspensions were then analyzed on a FACSCalibur flow cytometer using CELL-Quest software.
Cell suspensions from BAL fluid were enumerated, centrifuged onto glass slides, dried, fixed, and stained in hematoxylin and eosin (VWR). At least 400 cells per slide were counted to determine the relative percentages of lymphocytes, macrophages, eosinophils, and neutrophils. For histochemistry, lungs were removed from mice at different time points postinfection and fixed in 10% normal buffered formalin; 4-μm paraffin-embedded sections were cut and stained with hematoxylin and eosin.
Measurement of albumin in bronchoalveolar lavage fluid.
Samples and murine-albumin standards were mixed with an equal volume of the fluorescent probe 8-anilino-1-napthalenesulphonic acid (Sigma) at 0.2 mM, 0.25 M sucrose, and 0.05 M Tris-HCl (pH 7.4) at room temperature. Fluorescence was measured at 380 nm excitation and 460 nm emission wavelengths, and albumin concentrations were determined from a standard curve (37).
Cytokine assay.
Enzyme-linked immunosorbent assay plates were coated overnight at 4°C with antimouse IL-10 (BD Biosciences) at 4 μg ml−1 or antimouse IFN-γ (R&D Systems) at 6 μg ml−1. Plates were washed and blocked, 30 μl of BAL fluid was added, and plates were incubated at room temperature for 2 h. Samples were washed prior to addition of either biotinylated antimouse IL-10 at 1 μg ml−1 (BD Biosciences) or biotinylated antimouse IFN-γ at 400 ng ml−1 and developed with avidin-horseradish peroxidase conjugate (BD Biosciences)-3,3′,5,5′-tetramethylbenzidine (TMB) substrate and then read at 450 nm.
Apoptosis and in vivo cellular proliferation.
Cells from MdLN were stained with PE-conjugated anti-annexin-V (BD Biosciences), used neat, and either PE-Cy5-conjugated rat anti-mouse CD4 (clone RM4-5; Caltag) or peridinin-chlorophyl-protein complex-conjugated rat anti-mouse CD8a (clone 53-6.7; BD Biosciences), both used at 1:200. Cells were analyzed by flow cytometry after addition of 1 μM To-pro-3 (Molecular Probes) to the final suspension. Cellular proliferation in vivo was determined as previously described (19). Mice were given 0.8 mg ml−1 bromodeoxyuridine (BrdU) in their drinking water from the initial day of influenza A infection until sacrifice; single-cell suspensions were prepared from MdLN, stained with fluorescein isothiocyanate (FITC)-conjugated anti-BrdU (BD Biosciences), and analyzed by flow cytometry.
Statistical analysis.
Groups of five mice were used, and experiments were repeated at least twice. Data are shown as means ± 1 standard deviation (SD). Data were analyzed using two-way analysis of variance and Bonferroni's posttest. P values of ≤0.05 were considered to be statistically significant (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001).
RESULTS
Coinfection of mice during the enteric phase of trichinosis results in reduced lung pathology and accelerated recovery of weight.
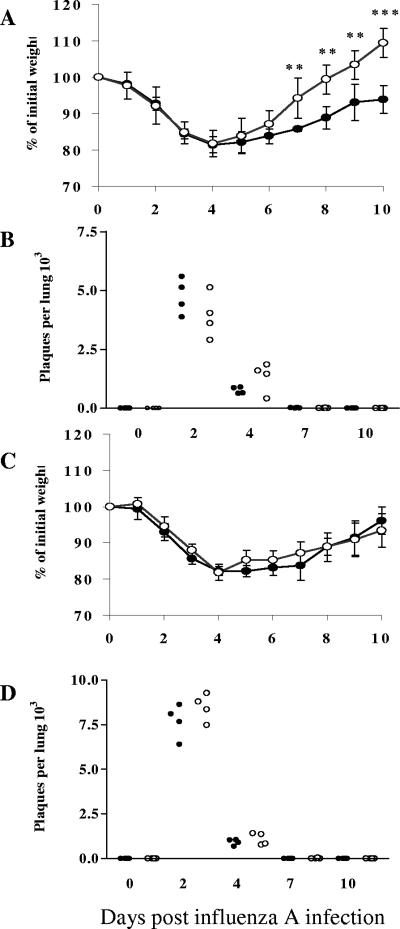
In order to assess the effects of T. spiralis at different stages of development on the course of influenza infection, male NIH mice were inoculated with virus during the peak of the enteric phase coincident with release of newborn larvae, i.e., day 7, and also during the systemic phase when parasites were encysted in skeletal muscle, i.e., day 60 postinfection (p.i.). A dose of 50 HA units administered intranasally to control mice resulted in animals dropping to approximately 80% of their initial weight at day 4, followed by progressive recovery (Fig. 1A). Infectious viral particles were recovered from the lungs at days 2 and 4 p.i., but viral clearance was complete by day 7 (Fig. 1B). In mice coinfected with T. spiralis, there was no alteration in weight loss in the first 4 days, and these animals also lost 20% of their body mass over this time period (Fig. 1A). After day 4, however, the coinfected mice gained weight more rapidly and were significantly heavier than controls from day 7 onward. Nevertheless, the number of infectious viral particles in the lungs was not significantly different on days 2 and 4, and the rate of viral clearance was unaffected. In contrast, coinfection with influenza virus during the later phase of trichinosis (day 60) did not affect the dynamics of weight loss and recovery, and viral replication and clearance were similarly unaffected (Fig. 1C and D). These observations proved to be highly reproducible.
FIG. 1.
Coinfection with enteric-stage T. spiralis infection enhances recovery from influenza infection but has no effect on viral clearance. Mice were infected orally with T. spiralis and at two later distinct time points inoculated intranasally with 50 HA units of influenza A virus (X31). Weight loss was monitored daily and expressed as a percentage of the weight prior to influenza infection (influenza administered at day 7 [A] and at day 60 [C] of parasite infection). Viral load per lung was assessed by plaque assay (influenza administered at day 7 [B] and at day 60 [D] of parasite infection). Points in panels B and D represent viral titers from individual mice. In all panels, data for mice infected with influenza virus alone are shown as filled circles and data for coinfected animals shown as open circles; data represent mean values ± 1 SD. Significance values are shown relative to mice infected solely with influenza. **, P ≤ 0.01; ***, P ≤ 0.001.
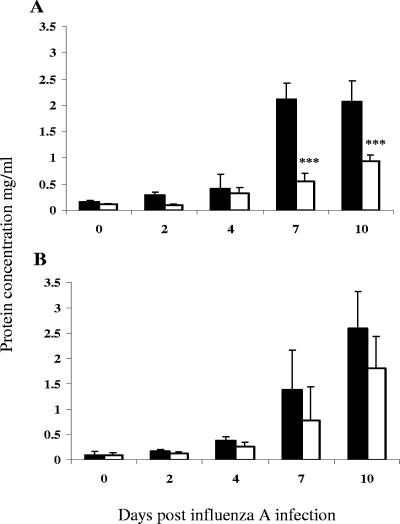
The concentration of serum albumin in BAL fluid reflects vascular integrity in pulmonary tissues and permeability of the epithelial lining of the airways and was thus measured as an indicator of lung pathology. In control mice singly infected with influenza virus, the albumin concentration in BAL fluid remained relatively low in the first 4 days but was then sharply elevated to over 2 mg ml−1 at days 7 and 10 p.i. By contrast, albumin in BAL fluid of mice coinfected during early trichinosis remained under 1 mg ml−1, which was significantly lower than the control group at both of these time points (Fig. 2A). In mice coinfected with influenza virus during the later phase of trichinosis, the concentration of albumin in BAL fluid was slightly reduced at days 7 and 10 p.i. but was not significantly different from that of controls (Fig. 2B).
FIG. 2.
Coinfection results in lower albumin concentrations in BAL fluid. Mice were infected orally with T. spiralis and 7 days (A) or 60 days (B) later inoculated intranasally with influenza A virus. The albumin concentration in lavage fluid was determined at different time points after viral infection. Filled bars indicate data for mice infected with influenza alone and open bars represent data for coinfected animals. Mean values ± 1 SD are shown, and significant differences relative to mice infected solely with influenza are highlighted. ***, P ≤ 0.001.
Altered characteristics of cellular infiltration.
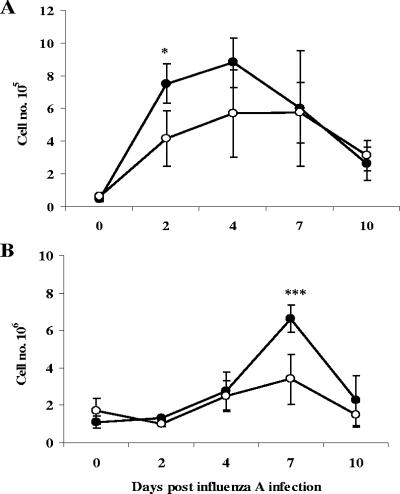
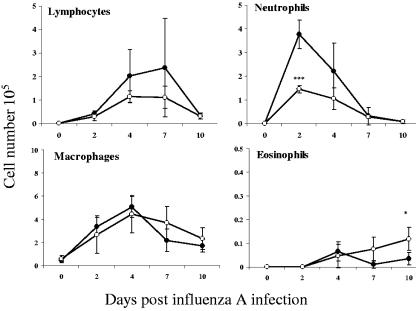
Mice with early-phase T. spiralis infection did not display greatly altered kinetics of cellular infiltration into the pulmonary environment following inoculation with influenza virus but had reduced cell numbers in the BAL fluid at day 2 p.i. and in the lungs at day 7 p.i. (Fig. 3). As with other parameters, there was no significant alteration in cellular infiltration when influenza virus was administered at day 60 of parasite infection, and this again was highly reproducible (data not shown). We therefore focused our attention on phenotypic alterations observed during early-phase trichinosis. Early influx of neutrophils into BAL fluid is a characteristic response during influenza, and this was recapitulated in these experiments, peaking at day 2 but reduced by over 50% as a result of parasite coinfection. Levels of lymphocytes and macrophages were also elevated at later time points, and although the numbers of lymphocytes were lower in coinfected mice, there was no statistically significant difference, whereas macrophage numbers were unaffected. Eosinophil numbers were very low but were slightly enhanced by parasite infection at day 10 (Fig. 4).
FIG. 3.
Cellular infiltration into the lungs is suppressed by T. spiralis. Mice were inoculated with influenza virus 7 days following infection with T. spiralis. Total cells recovered from the BAL fluid (A) and lungs (B) were enumerated over the course of viral infection. Values represent means ± 1 SD, and significant differences to mice infected with influenza virus alone are shown. Filled circles, influenza infection alone; open circles, coinfected animals. *, P ≤ 0.05; ***, P ≤ 0.001.
FIG. 4.
Parasite infection inhibits the early influx of neutrophils. Cells recovered from BAL fluid at different times after influenza infection were stained with hematoxylin and eosin following cytospin preparation. Neutrophils, eosinophils, lymphocytes, and macrophages were enumerated. Data are presented from mice infected solely with influenza virus and from those infected 7 days earlier with T. spiralis. The values shown are means ± 1 SD, and significance values for coinfected mice (open circles) are expressed in comparison to mice infected with influenza virus alone (filled circles). ***, P ≤ 0.001.
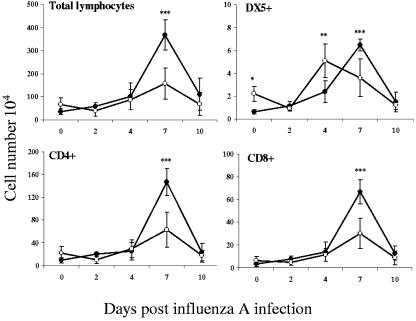
Coinfection did result in reduced numbers of lymphocytes in the lungs at day 7. This was not restricted to a single cell subset, as numbers of CD4+, CD8+, and DX5+ (NK) cells were all significantly lower than controls at this time (Fig. 5). Interestingly, the kinetics of NK cell influx was altered, peaking at day 4, and it was notable that there was already an elevated resident population in parasite-infected mice prior to inoculation with influenza virus. Expression of CD45RB progressively decreased on CD4+ lymphocytes during influenza infection as would be expected during activation, but the kinetics of expression of CD4+ CD45RBhi did not differ between groups. In a similar manner, the proportion of CD8+ CD45RBhi cells was decreased at day 10 after influenza infection in both singly infected and coinfected animals (data not shown).
FIG. 5.
Reduction and altered kinetics of lymphocyte infiltration. Mice were infected with T. spiralis and with influenza A virus 7 days later, or influenza A virus alone, as described. Single-cell suspensions from the lungs were analyzed by flow cytometry, and total numbers of lymphocytes and CD4+, CD8+, and DX5+ cells were determined. Numbers are expressed as means ± 1 SD, and significant differences between singly infected (filled circles) and coinfected (open circles) mice are shown. **, P ≤ 0.01; ***, P ≤ 0.001.
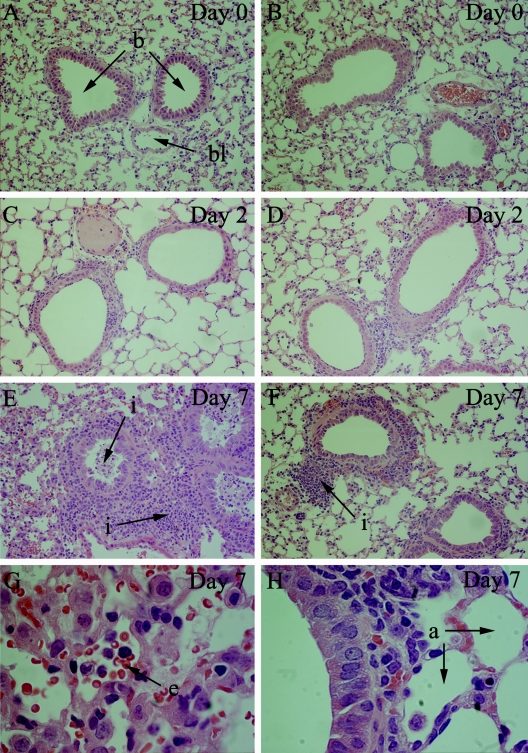
Histological examination confirmed that pulmonary inflammation was ameliorated by coinfection with T. spiralis. Mice which had been infected with parasites for 1 week did not show any noticeable alterations (Fig. 6A and B), and the same applied for animals infected for 2 days with influenza virus (Fig. 6C and D). By day 7 after influenza infection, however, there was an intense infiltration of leukocytes surrounding the airways, and a cellular exudate was evident in the lumen of the bronchioles (Fig. 6E). This was primarily mononuclear at this stage, with some polymorphonuclear cells. Examination at higher magnification revealed that the alveolar spaces frequently contained erythrocytes (Fig. 6G). In contrast, animals coinfected with T. spiralis showed a greatly reduced cellular infiltration in pulmonary tissues (Fig. 6F), with both the bronchial and alveolar spaces remaining largely clear (Fig. 6H).
FIG. 6.
Amelioration of cellular infiltration and airway occlusion. Lungs were fixed, sectioned, and stained with hematoxylin and eosin. (A, C, E, and G) Tissue from mice infected with influenza virus alone is shown; (B, D, F, and H) tissue from mice preinfected with T. spiralis is shown. Representative sections from each group are shown at day 0 (A and B), day 2 (C and D), and day 7 (E and F) after influenza infection at 40× magnification. Sections of lungs at day 7 p.i. are also shown at 100× magnification (G and H). Bronchioles (b), alveoli (a), and blood vessels (bl) are shown. Infiltrating cells (i) are indicated, as are erythrocytes (e) in the alveolar spaces.
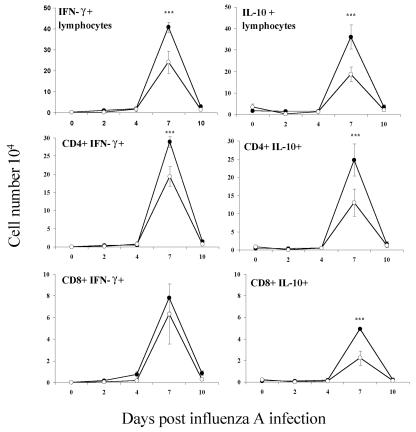
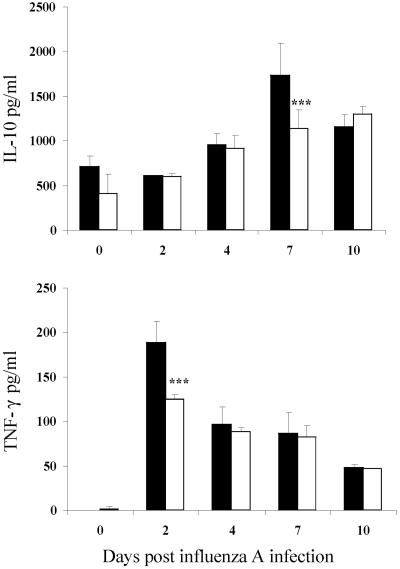
We had previously observed that infection of NIH mice with T. spiralis resulted in high levels of IL-10 produced by splenocytes and mesenteric lymph nodes at day 7 p.i. (15), and thus we examined the phenotype of T cells recruited into the lungs during coinfection by intracellular cytokine staining. There was no alteration in the relative proportion of IL-10- or IFN-γ-positive lymphocytes, however. The numbers of CD4+ cells secreting these cytokines and CD8+ cells secreting IL-10 were reduced (Fig. 7). The concentration of IL-10 in BAL fluid was also significantly lower at this time point (Fig. 8). Early production of tumor necrosis factor alpha (TNF-α) was inhibited (Fig. 8), which may be significant as this has been shown to be a contributory factor in illness caused by influenza (20).
FIG. 7.
Infiltration of both IFN-γ- and IL-10-secreting lymphocytes is suppressed by parasite infection. Samples were analyzed by flow cytometry and the numbers of IFN-γ- and IL-10-positive lymphocytes in the lung determined by intracellular cytokine staining. Numbers are expressed as means ± 1 SD, and significant differences between singly infected (filled circles) and coinfected (open circles) mice are shown. ***, P ≤ 0.001.
FIG. 8.
Prior infection with T. spiralis suppresses IL-10 and TNF-α in BAL fluid. Concentrations of cytokines were determined by enzyme-linked immunosorbent assay. The values shown represent the means ± 1 SD, and significant differences between singly infected (filled bars) and coinfected (open bars) mice are shown. ***, P ≤ 0.001.
Cellular turnover during coinfection.
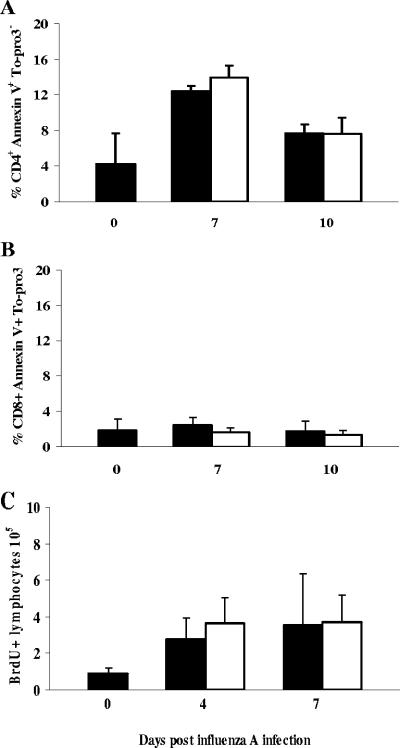
It was clear that the rapid recovery of coinfected mice was associated with lower numbers of a broad spectrum of cells infiltrating pulmonary tissues, but the mechanisms underlying this phenomenon were unclear. We therefore examined the rate of apoptosis and proliferation in cells isolated from the MdLN, the primary site of lymphocyte expansion in influenza infection, as alterations in these parameters had previously been observed to contribute to decreased immunopathology in murine influenza (19). The proportion of apoptotic CD4+ cells was increased in influenza virus-infected mice at 7 and 10 days after inoculation relative to uninfected mice, but it was not significantly different in those animals coinfected with T. spiralis (Fig. 9A). The proportion of apoptotic CD8+ lymphocytes in naive mice was extremely low and was not significantly affected in singly infected or coinfected mice (Fig. 9B). The possibility that decreased cell numbers in coinfected animals might be due to lower levels of lymphocyte proliferation was investigated by administering mice with BrdU in their drinking water. The total numbers of proliferating cells in MdLN were elevated in both singly infected and coinfected groups at day 4 and day 7, but no significant difference between these groups was evident (Fig. 9C).
FIG. 9.
Reduced numbers of T lymphocytes in the lungs are not accounted for by enhanced apoptosis or suppression of proliferation. Mice were infected orally with T. spiralis and 7 days later with influenza A virus as described. Single-cell suspensions from the MdLN were analyzed by flow cytometry and apoptotic cells defined as annexin+ and To-pro-3−. The proportions of apoptotic CD4+ (A) and CD8+ (B) lymphocytes were determined. Cellular proliferation was assessed by incorporation of BrdU and flow cytometry (C). No significant differences were observed between singly infected (filled bars) or coinfected (open bars) animals in any case.
DISCUSSION
This study demonstrates that coinfection of mice with influenza virus during the early phase of trichinosis results in a reduced inflammatory infiltrate in the lungs, decreased concentrations of cytokines in the BAL fluid, and faster recovery of weight, but it does not impede upon the mechanisms of viral clearance. The latter point indicates that generalized pulmonary inflammation does not contribute to viral clearance per se but represents an immunological background within which antiviral immunity operates or that the inflammatory response is magnified severalfold over what is necessary and sufficient for effective clearance (19). With respect to parasite infection, the timing was observed to be crucial for amelioration of pathology, as there was no effect when parasites were encysted in skeletal muscle.
An integral component of the adaptive immune response to viral infection is the generation of specific cytotoxic T cells. It might be predicted that helminth infection would impair their development via inhibition of IL-2 and IFN-γ production, and experimental evidence suggests that this can occur in given situations (1). In the current experiments, viral clearance was unimpaired even though the total numbers of NK+ and CD8+ lymphocytes in the lungs were decreased. NK cells have been shown to play a role in immunity to influenza (4). Multiple mechanisms contribute to viral clearance, however, and even the total absence of IFN-γ or IFN-α/β is not sufficient to prevent eradication of influenza virus in mice (17, 31), indicative of considerable redundancy in the response (19).
For the large part, parasite infection did not induce alterations in the pulmonary cell infiltrate prior to inoculation with influenza virus, although the numbers of DX5+ cells were elevated. This is consistent with previous reports of enhanced NK cell cytolytic activity in the lungs during the period in which newborn larvae are migrating from the intestine to skeletal muscle (2, 30). Although this site is not actively occupied by the parasite, Korten et al. (24) observed that numbers of DX5+/CD3− NK cells and DX5+/CD3+ T cells were expanded systemically during Litomosoides sigmodontis infection, and thus it is possible that the systemic release of parasite products and/or local cytokine and chemokine activity resulting from migration of newborn larvae through the capillary bed lining the lungs invokes recruitment and activation of this population.
The suppression of pulmonary pathology and cellular infiltration observed here could be due to a variety of factors, such as a state of immune exhaustion as a result of ongoing intestinal inflammation, although if this were a contributing factor, then the same effect might have been expected during the systemic phase of T. spiralis infection. Alternatively, lower numbers of cells could have been accounted for by increased apoptosis or reduced levels of cellular proliferation, via interference with lymphocyte activation in the regional lymph nodes. OX40-immunoglobulin fusion proteins block the interaction of OX40 (CD134) on activated T cells with its ligand on antigen-presenting cells. Directed blockade of this costimulatory pathway via administration of OX40-immunoglobulin prevented the loss of weight which accompanies murine influenza without impairing viral clearance and was associated with reduced proliferation and enhanced apoptosis of T cells in the lungs and MdLN (19). The similarities with the current study prompted us to examine these two parameters, and although the proportion of apoptotic CD4+ cells increased following inoculation with influenza virus, this was not perturbed by concomitant parasite infection.
IL-10 is a potent anti-inflammatory cytokine which operates on many levels, including the inhibition of lymphocyte activation and macrophage function. Given that IL-10 is a dominant feature of helminth infection and has been shown to prevent necrosis and inflammation during T. spiralis infection (3, 5), this seemed a likely candidate for amelioration of the lung pathology seen here. Perhaps somewhat surprisingly, reduced numbers of IL-10-secreting lymphocytes were observed in the lungs and lower amounts of cytokine were present in the BAL fluid of coinfected mice. The lower amounts of cytokine could simply be due to reduced numbers of local T cells, although we do not know to what extent other IL-10-producing cells contribute to the situation. Parasite infection did not downregulate the capacity of cells to respond to influenza, as incorporation of BrdU indicated elevated numbers of proliferating cells in the MdLN, with no evident difference in coinfected mice. Other studies have shown that helminth infection can result in suppression of inflammation in an IL-10-independent manner. Thus, H. polygyrus has been documented to inhibit established colitis in IL-10-deficient mice. Resolution of inflammation could be adoptively transferred by T cells from mesenteric lymph nodes, and enhanced expression of Foxp3 in these cells implies that they may display regulatory activity (12). IL-10-deficient mice injected intravenously with T. spiralis newborn larvae show a marked increase in the numbers of inflammatory cells around parasites in skeletal muscle. This peaked at around 20 days but had resolved by 55 days p.i., indicative of an IL-10-independent mechanism for resolution of inflammation during late-stage infection (3).
Production of proinflammatory cytokines contributes to the symptoms observed during acute influenza, which can be ameliorated by the use of blocking antibodies against IFN-α/β, IL-1α, and TNF-α (20, 25). In the current study, TNF-α in BAL fluid peaked early at day 2 p.i., which is consistent with both murine and human studies (14, 28), and it was significantly suppressed in coinfected mice. Depletion of TNF-α during infection with respiratory syncytial virus or influenza A virus abolished illness and weight loss, resulted in decreased inflammatory infiltration in the lungs, and reduced the expression of IFN-γ on CD4+ cells, but it did not alter viral clearance (20). It is therefore possible that the decreased levels of TNF-α observed in this study result in decreased expression of adhesion molecules on the vascular endothelium and/or reduced chemokine and chemokine receptor expression, resulting in a net suppression of inflammation. Alleviation of the general increase in vascular permeability in pulmonary tissues was also evidenced by suppression of serum albumin in BAL fluid and the lack of erythrocytes in the alveolar spaces of coinfected mice.
Trichinella pseudospiralis has been observed to suppress inflammation in coinfection studies and also results in reduced cellular recruitment around subcutaneously implanted material (35). Early downregulation of both the latter response and delayed-type hypersensitivity to trinitrochlorobenzene was followed later during infection by normalization of these parameters relative to uninfected mice, leading to the conclusion that the immunomodulatory effects were associated with the presence of migratory larvae in the host (34). A similar conclusion was reached in a study in which inflammatory demyelination in a rat model for multiple sclerosis was suppressed by T. pseudospiralis (6). The course of influenza infection in our experiments is coincident with the production and dissemination of newborn parasite larvae, and thus, the immunomodulatory effects observed here may have evolved to minimize tissue damage during parasite migration and to protect the relatively vulnerable larval stages when they are most exposed to immune attack. This is conceptually similar to the immunomodulation seen during the late phase of infection with most other helminth parasites, which, due to differences in parasite biology, also coincides with dispersal of eggs and larval stages (27).
Acknowledgments
This work was supported by the Wellcome Trust (no. 059532). R.C.F. is the recipient of a Prize Studentship (no. 064610).
We thank Lorraine Lawrence for histological processing and staining.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Actor, J. K., M. Shirai, M. C. Kullberg, R. M. Buller, A. Sher, and J. A. Berzofsky. 1993. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc. Natl. Acad. Sci. USA 90:948-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bany, J., M. K. Janiak, and W. Budzynski. 1992. Activity of natural killer (NK) cells in the course of experimental trichinellosis in mice. Wiad. Parazytol. 38:117-126. [PubMed] [Google Scholar]
- 3.Beiting, D. P., S. K. Bliss, D. H. Schlafer, V. L. Roberts, and J. A. Appleton. 2004. Interleukin-10 limits local and body cavity inflammation during infection with muscle-stage Trichinella spiralis. Infect. Immun. 72:3129-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biron, C. A., and L. Brossay. 2001. NK cells and NKT cells in innate defense against viral infections. Curr. Opin. Immunol. 13:458-464. [DOI] [PubMed] [Google Scholar]
- 5.Bliss, S. K., A. Alcaraz, and J. A. Appleton. 2003. IL-10 prevents liver necrosis during murine infection with Trichinella spiralis. J. Immunol. 171:3142-3147. [DOI] [PubMed] [Google Scholar]
- 6.Boles, L. H., J. M. Montgomery, J. Morris, M. A. Mann, and G. L. Stewart. 2000. Suppression of multiple sclerosis in the rat during infection with Trichinella pseudospiralis. J. Parasitol. 86:841-844. [DOI] [PubMed] [Google Scholar]
- 7.Borkow, G., Z. Weisman, Q. Leng, M. Stein, A. Kalinkovich, D. Wolday, and Z. Bentwich. 2001. Helminths, human immunodeficiency virus and tuberculosis. Scand. J. Infect. Dis. 33:568-571. [DOI] [PubMed] [Google Scholar]
- 8.Brown, M., P. A. Mawa, S. Joseph, J. Bukusuba, C. Watera, J. A. Whitworth, D. W. Dunne, and A. M. Elliott. 2005. Treatment of Schistosoma mansoni infection increases helminth-specific type 2 cytokine responses and HIV-1 loads in coinfected Ugandan adults. J. Infect. Dis. 191:1648-1657. [DOI] [PubMed] [Google Scholar]
- 9.Chowaniec, W., R. B. Wescott, and L. L. Congdon. 1972. Interaction of Nematospiroides dubius and influenza virus in mice. Exp. Parasitol. 32:33-44. [DOI] [PubMed] [Google Scholar]
- 10.Cooper, P. J., M. Chico, C. Sandoval, I. Espinel, A. Guevara, M. M. Levine, G. E. Griffin, and T. B. Nutman. 2001. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect. Immun. 69:1574-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elias, D., D. Wolday, H. Akuffo, B. Petros, U. Bronner, and S. Britton. 2001. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin. Exp. Immunol. 123:219-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott, D. E., T. Setiawan, A. Metwali, A. Blum, J. F. Urban, Jr., and J. V. Weinstock. 2004. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur. J. Immunol. 34:2690-2698. [DOI] [PubMed] [Google Scholar]
- 13.Erb, K. J., C. Trujillo, M. Fugate, and H. Moll. 2002. Infection with the helminth Nippostrongylus brasiliensis does not interfere with efficient elimination of Mycobacterium bovis BCG from the lungs of mice. Clin. Diagn. Lab. Immunol. 9:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritz, R. S., F. G. Hayden, D. P. Calfee, L. M. Cass, A. W. Peng, W. G. Alvord, W. Strober, and S. E. Straus. 1999. Nasal cytokine and chemokine responses in experimental influenza A virus infection: results of a placebo-controlled trial of intravenous zanamivir treatment. J. Infect. Dis. 180:586-593. [DOI] [PubMed] [Google Scholar]
- 15.Furze, R. C., and M. E. Selkirk. 2005. Comparative dynamics and phenotype of the murine immune response to Trichinella spiralis and Trichinella pseudospiralis. Parasite Immunol. 27:181-188. [DOI] [PubMed] [Google Scholar]
- 16.Graham, A. L., T. J. Lamb, A. F. Read, and J. E. Allen. 2005. Malaria-filaria coinfection in mice makes malarial disease more severe unless filarial infection achieves patency. J. Infect. Dis. 191:410-421. [DOI] [PubMed] [Google Scholar]
- 17.Graham, M. B., D. K. Dalton, D. Giltinan, V. L. Braciale, T. A. Stewart, and T. J. Braciale. 1993. Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J. Exp. Med. 178:1725-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grencis, R. K., L. Hultner, and K. J. Else. 1991. Host protective immunity to Trichinella spiralis in mice: activation of Th cell subsets and lymphokine secretion in mice expressing different response phenotypes. Immunology 74:329-332. [PMC free article] [PubMed] [Google Scholar]
- 19.Humphreys, I. R., G. Walzl, L. Edwards, A. Rae, S. Hill, and T. Hussell. 2003. A critical role for OX40 in T cell-mediated immunopathology during lung viral infection. J. Exp. Med. 198:1237-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hussell, T., A. Pennycook, and P. J. Openshaw. 2001. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 31:2566-2573. [DOI] [PubMed] [Google Scholar]
- 21.Hussell, T., L. C. Spender, A. Georgiou, A. O'Garra, and P. J. Openshaw. 1996. Th1 and Th2 cytokine induction in pulmonary T cells during infection with respiratory syncytial virus. J. Gen. Virol. 77:2447-2455. [DOI] [PubMed] [Google Scholar]
- 22.Kamal, S., M. Madwar, L. Bianchi, A. E. Tawil, R. Fawzy, T. Peters, and J. W. Rasenack. 2000. Clinical, virological and histopathological features: long-term follow-up in patients with chronic hepatitis C co-infected with S. mansoni. Liver 20:281-289. [DOI] [PubMed] [Google Scholar]
- 23.Kelly, E. A., E. S. Cruz, K. M. Hauda, and D. L. Wassom. 1991. IFN-gamma- and IL-5-producing cells compartmentalize to different lymphoid organs in Trichinella spiralis-infected mice. J. Immunol. 147:306-311. [PubMed] [Google Scholar]
- 24.Korten, S., L. Volkmann, M. Saeftel, K. Fischer, M. Taniguchi, B. Fleischer, and A. Hoerauf. 2002. Expansion of NK cells with reduction of their inhibitory Ly-49A, Ly-49C, and Ly-49G2 receptor-expressing subsets in a murine helminth infection: contribution to parasite control. J. Immunol. 168:5199-5206. [DOI] [PubMed] [Google Scholar]
- 25.Kurokawa, M., M. Imakita, C. A. Kumeda, and K. Shiraki. 1996. Cascade of fever production in mice infected with influenza virus. J. Med. Virol. 50:152-158. [DOI] [PubMed] [Google Scholar]
- 26.Liesenfeld, O., I. R. Dunay, and K. J. Erb. 2004. Infection with Toxoplasma gondii reduces established and developing Th2 responses induced by Nippostrongylus brasiliensis infection. Infect. Immun. 72:3812-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maizels, R. M., and M. Yazdanbakhsh. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3:733-744. [DOI] [PubMed] [Google Scholar]
- 28.Monteiro, J. M., C. Harvey, and G. Trinchieri. 1998. Role of interleukin-12 in primary influenza virus infection. J. Virol. 72:4825-4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nacher, M. 2001. Malaria vaccine trials in a wormy world. Trends Parasitol. 17:563-565. [DOI] [PubMed] [Google Scholar]
- 30.Niederkorn, J. Y., G. L. Stewart, S. Ghazizadeh, E. Mayhew, J. Ross, and B. Fischer. 1988. Trichinella pseudospiralis larvae express natural killer (NK) cell-associated asialo-GM1 antigen and stimulate pulmonary NK activity. Infect. Immun. 56:1011-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price, G. E., A. Gaszewska-Mastarlarz, and D. Moskophidis. 2000. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. J. Virol. 74:3996-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rousseau, D., Y. Le Fichoux, X. Stien, I. Suffia, B. Ferrua, and J. Kubar. 1997. Progression of visceral leishmaniasis due to Leishmania infantum in BALB/c mice is markedly slowed by prior infection with Trichinella spiralis. Infect. Immun. 65:4978-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seki, M., K. Yanagihara, Y. Higashiyama, Y. Fukuda, Y. Kaneko, H. Ohno, Y. Miyazaki, Y. Hirakata, K. Tomono, J. Kadota, T. Tashiro, and S. Kohno. 2004. Immunokinetics in severe pneumonia due to influenza virus and bacteria coinfection in mice. Eur. Respir. J. 24:143-149. [DOI] [PubMed] [Google Scholar]
- 34.Stewart, G. L., J. Y. Niederkorn, R. R. Kennedy, and E. Mayhew. 1991. Effect of acute versus chronic Trichinella pseudospiralis infections on systemic cell-mediated immunity. Int. J. Parasitol. 21:935-940. [DOI] [PubMed] [Google Scholar]
- 35.Stewart, G. L., B. Wood, and R. B. Boley. 1985. Modulation of host response by Trichinella pseudospiralis. Parasite Immunol. 7:223-233. [DOI] [PubMed] [Google Scholar]
- 36.Stoicov, C., M. Whary, A. B. Rogers, F. S. Lee, K. Klucevsek, H. Li, X. Cai, R. Saffari, Z. Ge, I. A. Khan, C. Combe, A. Luster, J. G. Fox, and J. Houghton. 2004. Coinfection modulates inflammatory responses and clinical outcome of Helicobacter felis and Toxoplasma gondii infections. J. Immunol. 173:3329-3336. [DOI] [PubMed] [Google Scholar]
- 37.Takamatsu, S., T. Horie, T. Mishima, Y. Minamide, M. Hayashi, and S. Awazu. 1990. Heterogeneity of rat plasma albumin and drug binding. Biochem. Pharmacol. 39:1205-1212. [DOI] [PubMed] [Google Scholar]
- 38.Walzl, G., S. Tafuro, P. Moss, P. J. Openshaw, and T. Hussell. 2000. Influenza virus lung infection protects from respiratory syncytial virus-induced immunopathology. J. Exp. Med. 192:1317-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webby, R. J., and R. G. Webster. 2003. Are we ready for pandemic influenza? Science 302:1519-1522. [DOI] [PubMed] [Google Scholar]
- 40.Williams, B. G., E. Gouws, C. Boschi-Pinto, J. Bryce, and C. Dye. 2002. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect. Dis. 2:25-32. [DOI] [PubMed] [Google Scholar]