Abstract
The bacterial toxin Yersinia outer protein T (YopT) is a cysteine protease that cleaves Rho GTPases immediately upstream of a carboxyl-terminal isoprenylcysteine. By clipping off the lipid anchor, YopT releases Rho GTPases from membranes, resulting in rounding up of mammalian cells in culture. The proteolytic activity of YopT depends on the isoprenylation of the cysteine within the carboxyl-terminal CaaX motif, a reaction carried out by geranylgeranyltransferase type I. The CaaX motif (where “a” indicates aliphatic amino acids) of Rho proteins undergoes two additional processing steps: endoproteolytic removal of the last three amino acids (i.e., -aaX) by Rce1 (Ras-converting enzyme 1) and methylation of the geranylgeranylcysteine by Icmt (isoprenylcysteine carboxyl methyltransferase). In in vitro experiments, RhoA retaining -aaX cannot be cleaved by YopT. Nothing is known, however, about the influence of Rce1-mediated removal of -aaX on the activity of YopT in living cells. We hypothesized that Rce1-deficient mouse fibroblasts, in which the geranylgeranylated Rho proteins are not endoproteolytically processed, would be resistant to YopT. Indeed, this was the case. Microinjection of recombinant YopT into Rce1-deficient fibroblasts had no impact on the subcellular localization of RhoA and no impact on cell morphology. To determine if carboxyl methylation is also required for YopT action, we microinjected YopT into Icmt-deficient fibroblasts. In contrast to the results with Rce1-deficient cells, YopT cleaved RhoA and caused rounding up of the Icmt-deficient cells. Our data demonstrate that Rce1-mediated removal of -aaX from isoprenylated Rho GTPases is required for the proteolytic activity of YopT in living cells, whereas carboxyl methylation by Icmt is not.
Rho proteins are key regulators of a wide variety of cellular functions, including regulation of actin structure, integrin signaling, and phospholipid signaling (12). Rho proteins are also important for the regulation of endocytosis, secretion, control of transcription, cell cycle progression, and cell transformation (for reviews see references 2, 8, 14, and 19). Like all members of the Ras superfamily of small GTPases, Rho GTPases cycle between the GDP-bound inactive and GTP-bound active forms. The exchange of GDP for GTP is catalyzed by guanosine nucleotide exchange factors. Inactivation of Rho results from hydrolysis of bound GTP, a process that is stimulated by GTPase-activating proteins (for a review see reference 13).
Rho proteins are posttranslationally modified by isoprenylation of the CaaX (where “a” indicates aliphatic amino acids) box cysteine at the carboxyl terminus, a reaction catalyzed by geranylgeranyltransferase type I. After geranylgeranylation, the terminal -aaX tripeptide is released by Ras-converting enzyme 1 (Rce1). Finally, the α-carboxylate anion of the newly exposed geranylgeranylcysteine is methylated by isoprenylcysteine carboxyl methyltransferase (Icmt) (20). It is widely accepted that isoprenylation is required for the proper activation and targeting of GTPases to membrane surfaces. Less is known, however, about the physiologic impact of the endoproteolysis and methylation steps. Recent studies, however, have shown that Rce1 or Icmt knockouts in mice cause developmental lethal phenotypes (4, 6) and that the absence of these enzymes leads to the mislocalization of several small GTPases in embryonic fibroblasts (11).
Rho GTPases have attracted interest for studies on bacterial pathogenicity. A wide variety of pathogenic bacteria produce protein toxins that either activate or inactivate Rho GTPases. The Yersinia enterocolitica outer protein YopT cleaves the geranylgeranylated Rho GTPases RhoA, Rac, and Cdc42 directly upstream of the carboxyl-terminal geranylgeranylcysteine. In this way, the lipid anchor of the GTPases is cleaved off and the proteins are released from the membrane into the cytosol (16).
Recently, in vitro experiments revealed that isoprenylation of Rho GTPases is required for the recognition and cleavage of Rho proteins by YopT. Moreover, in vitro-prenylated RhoA that retained -aaX appeared to be resistant to cleavage by YopT (16). In view of the latter finding, we hypothesized that Rce1-deficient fibroblasts would be resistant to YopT-induced Rho inactivation and cell rounding. To test this hypothesis, we cultured mouse embryonic fibroblasts deficient in Rce1 and studied YopT action after microinjection into living cells as well as by incubation of purified membranes with the toxin. We also analyzed the effect of injecting YopT into cells lacking Icmt. We provide evidence that Rce1 is required for the YopT-induced cleavage of RhoA and cell rounding, whereas carboxyl methylation by Icmt is not.
MATERIALS AND METHODS
Rce1- and Icmt-deficient cell lines.
Spontaneously immortalized mouse embryonic fibroblasts lacking Rce1 or Icmt were generated as described previously (5, 9). The genotype of the cells was confirmed by Southern blotting of genomic DNA.
Expression and purification of glutathione S-transferase (GST)-YopT.
The YopT gene was amplified from the virulence plasmid pYV from Y. enterocolitica JB580v and cloned in frame into the expression vector pGEX2TGL (17). The glutathione fusion protein was expressed in Escherichia coli TG1 at an optical density of 0.8 at 29°C by induction with isopropyl-β-d-thiogalactopyranoside (IPTG) at a final concentration of 0.1 mM. After growing overnight, the cells were harvested and lysed by sonication in lysis buffer (20 mM Tris-HCl [pH 7.3], 10 mM NaCl, 5 mM MgCl2, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 5 mM dithiothreitol [DTT]) and purified by affinity chromatography with glutathione-Sepharose (Amersham Biosciences). The beads were washed five times with lysis buffer (without detergent and PMSF) and five times with wash buffer (50 mM Tris-HCl, pH 8.0; 150 mM NaCl). GST-YopT was eluted from the beads with glutathione (10 mM in 50 mM Tris-HCl, pH 8.0) four times for 10 min and concentrated with a 30-kDa Centricon filter (Amicon).
Cell culture and microinjection.
Rce1+/+, Rce1−/−, Icmt+/+, and Icmt−/− cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 1% sodium pyruvate, 1% penicillin, and 1% streptomycin in humidified 5% CO2 at 37°C. For microinjection, cells were seeded at a subconfluent density on glass coverslips (Cellocate; Eppendorf) and cultured overnight. GST-YopT (400 ng/μl in 50 mM Tris-HCl, pH 8.0, with or without Alexa-labeled rabbit immunoglobulin G [IgG] as indicated), buffer, or the catalytically inactive mutant GST-YopT C139A was microinjected into cells with an Eppendorf 5242 microinjector. Thirty minutes after injection, the cells were fixed in a formaldehyde solution (3.7% formaldehyde, 0.1% Tween 20 in phosphate-buffered saline [PBS]) and washed with PBS. Cells were then incubated with rhodamine-conjugated phalloidin (1 U/coverslip) for 1 h at room temperature and washed again with PBS. Actin fibers were visualized by fluorescence microscopy, and images were recorded (AxioCam HRm; Zeiss). Photographs of cells coinjected with a dye were taken 30 min after injection, once under fluorescence and once as phase contrast.
Virus production and transduction.
The plasmid containing the human RCE1 cDNA was a gift of Patrick Casey (Duke University Medical Center, Durham, N.C.). RCE1 was cloned into the retroviral transfer vector pREX. The plasmids pMD-G and pMD-g/p were provided by R. Mulligan (Harvard Medical School, Boston, Mass.). The retroviral vector was produced by cotransfection of HEK-293T cells with pMD-G and pMD-g/p and the retroviral transfer vector using the calcium phosphate method. After 4 days, the supernatant fluid was collected and spun down to remove cellular debris. The virus-containing medium was filtered (0.22 μm) and then ultracentrifuged to concentrate the virus. Rce1−/− cells were infected with the virus in the presence of Polybrene (8 μg/ml). Transduction efficiency was monitored by fluorescence microscopy of the coexpressed green fluorescent protein.
Preparation of cell membranes.
Subconfluent cells on 10-cm culture dishes were washed with ice-cold PBS and then scraped into 1 ml of mammalian lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 1 mM PMSF). Cells were disrupted by sonication (three times on ice), followed by centrifugation for 20 min at 1,000 × g to remove the nuclear fraction. After ultracentrifugation (60 min at 100,000 × g) of the supernatant fluid, the pellet containing the membrane fraction was resuspended in lysis buffer (without PMSF) and used in the membrane release assay.
Membrane release assay and Western blot analysis.
Resuspended membrane fractions (100 μl) were incubated with 1 μM GST-YopT for 30 min at 37°C. After incubation, the sample was separated into membrane and soluble fractions by ultracentrifugation (60 min at 100,000 × g) and proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 12.5% gel and transferred to a polyvinylidene difluoride membrane. After blocking with 5% nonfat milk for 1 h, the blots were incubated overnight with a polyclonal antibody against RhoA (119; Santa Cruz) and then for 1 h with a horseradish peroxidase-conjugated secondary antibody.
RESULTS
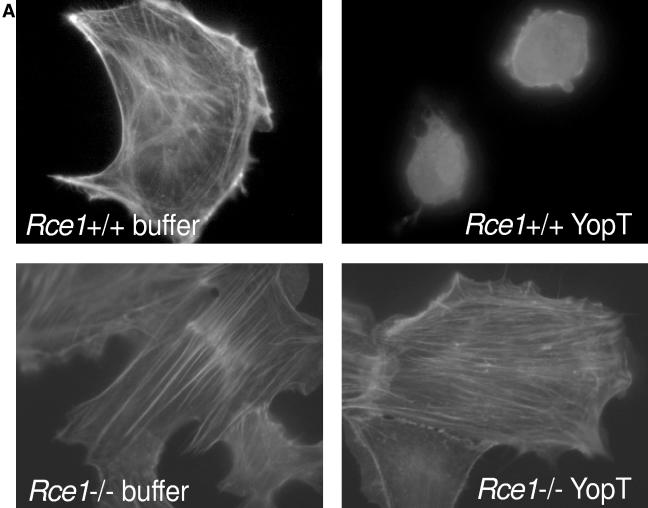
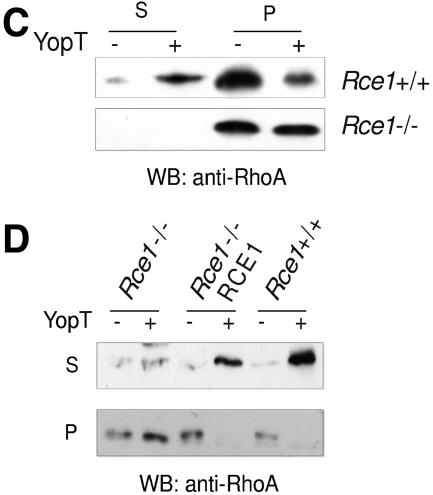
We and others have shown that the isoprenylation of Rho GTPases is required for substrate recognition by YopT (16, 18). Farnesylated as well as geranylgeranylated RhoA proteins are acceptable substrates, whereas V192Y RhoA, which cannot be isoprenylated, is not cleaved by YopT. Moreover, in vitro-prenylated RhoA retaining -aaX (terminating with -LVL and -LIL in human and mouse RhoA, respectively) was resistant to cleavage by YopT (16). To determine if Rce1-mediated cleavage of -aaX is required for substrate recognition in living cells, we microinjected recombinant YopT, buffer, or the catalytically inactive mutant YopT C139A as a negative control into Rce1−/− cells and assessed cell rounding. As expected, Rce1+/+ cells rounded up within 15 min after microinjection of YopT and then detached from the dish. In contrast, the Rce1−/− cells showed no rounding after 15 min and retained normal morphology even 2 h after the injection (Fig. 1A and B). These data indicate that Rho GTPases that are isoprenylated but not further processed cannot be recognized and cleaved by YopT. To verify this result, we assessed whether YopT is capable of releasing RhoA from purified membranes of Rce1+/+ or Rce1−/− cells. Thus, we prepared cell membranes, incubated them with YopT or buffer as a control, and separated soluble and membrane fractions by ultracentrifugation. We then analyzed the release of RhoA from the membranes, and the appearance of RhoA in the soluble fraction, by Western blotting. In line with the microinjection experiments, no RhoA was released by YopT from membranes of Rce1−/− cells, whereas efficient cleavage was observed in membranes from Rce1+/+ cells (Fig. 1C). Further, we asked whether the YopT-catalyzed cleavage of RhoA could be restored by expressing human RCE1 in the Rce1−/− cells with a retroviral system. As expected, the expression of human RCE1 in the Rce1−/− cells resulted in the cleavage of RhoA by YopT (Fig. 1D). Rce1−/− cells expressing human RCE1 also rounded up following microinjection of YopT (data not shown).
FIG. 1.
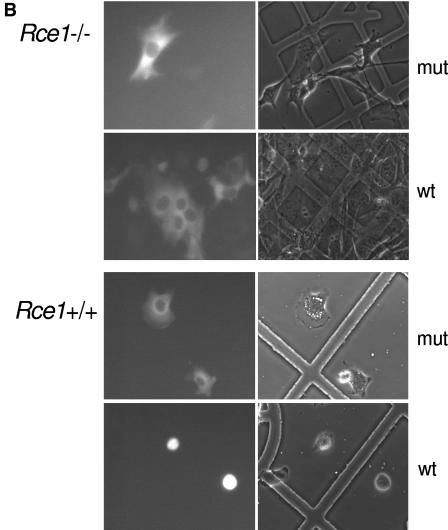
Rce1 deficiency prevents YopT-induced cell rounding and cleavage of RhoA. (A) Rce1+/+ and Rce1−/− cells were injected with buffer or 400 ng/ml recombinant YopT as described in Materials and Methods. Thirty minutes later, the cells were stained with rhodamine-conjugated phalloidin and then viewed and photographed under a fluorescence microscope. (B) Rce1+/+ and Rce1−/− cells were coinjected with an Alexa-labeled rabbit IgG (500 ng/ml) together with GST-YopT or the catalytically inactive mutant GST-YopTC139A (400 ng/ml, respectively) as described in Materials and Methods. Thirty minutes later, photographs of the cells were taken under fluorescence light and as phase contrast. (C) Membrane release assay demonstrating the appearance of YopT-cleaved RhoA in the soluble (S) fraction of Rce1+/+ cells but not in the soluble fraction of Rce1−/− cells. Membranes of Rce1+/+ and Rce1−/− cells were isolated and incubated with GST-YopT. After separation of membrane (P) and soluble (S) fractions by ultracentrifugation, the samples were analyzed for RhoA by Western blotting. Shown is a typical result of four independent experiments. As a consequence of the appearance of RhoA in the soluble fraction of Rce1+/+ cells after incubation with YopT, there was a clear-cut reduction in the amount of RhoA in the membrane fraction. There was no reduction in the amount of RhoA in the membrane fraction of Rce1−/− cells after incubation with YopT. (D) Membrane release assay demonstrating the restoration of the YopT-catalyzed cleavage of RhoA in Rce1−/− cells by retroviral expression of human RCE1. Membranes of Rce1+/+, Rce1−/−, and “Rce1−/−-RCE1” cells (i.e., Rce1−/− cells transfected with a human RCE1 cDNA) were isolated and incubated with GST-YopT as described in Materials and Methods. After separation of membrane (P) and soluble (S) fractions by ultracentrifugation, the samples were analyzed for RhoA by Western blotting. We detected RhoA in the soluble fraction of Rce1+/+ and Rce1−/−-RCE1 cells, whereas RhoA in Rce1−/− cells was exclusively found in the membrane fraction. The experiment was repeated three times with similar results. mut, mutant; wt, wild type; WB, Western blot.
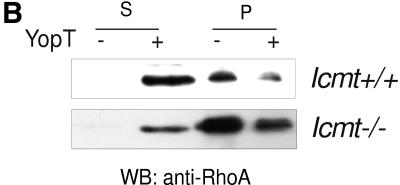
The loss of YopT action in Rce1-deficient cells could be due to -aaX sterically blocking the interaction of RhoA with YopT or, alternatively, the last step of the posttranslational modification, carboxyl methylation, being required for substrate recognition by YopT. To determine whether carboxyl methylation of RhoA is required for cleavage by YopT, we injected recombinant YopT or the catalytically inactive mutant YopT C139A into Icmt−/− cells and assessed cell rounding. When fully attached to the culture dish, Icmt−/− cells are flat and extremely stretched out (5), and the microinjection procedure itself leads to disruption of the cell membrane. We found, however, that it was possible to inject Icmt−/− cells shortly after seeding them (i.e., before they had become fully flattened out). Consequently, we injected the Icmt−/− and control Icmt+/+ cells 2 h after seeding them onto the slides. To visualize the injected cells, an Alexa-labeled rabbit IgG was coinjected with YopT. Notably, Icmt−/− cells injected with the inactive mutant continued to flatten, whereas the YopT-injected cells rounded up and detached (Fig. 2A). These data indicate that the endoproteolytically processed but unmethylated Rho GTPases in Icmt−/− cells are cleaved by YopT. In line with these microinjection experiments, when purified membranes from Icmt−/− cells were incubated with YopT, RhoA was readily released into the soluble fraction (Fig. 2B). We conclude that carboxyl methylation of the geranylgeranylcysteine is not required for substrate recognition and cleavage by YopT.
FIG. 2.
Carboxyl methylation of RhoA is not required for cleavage by YopT. (A) The Icmt−/− cells are very flat and stretched out and are sensitive to the microinjection procedure when they are fully attached to the coverslip. To circumvent this issue, the Icmt−/− cells were injected before they were fully flattened out, 2 h after seeding Icmt+/+, and Icmt−/− cells were coinjected with an Alexa-labeled rabbit IgG (500 ng/ml) together with GST-YopT or the catalytically inactive mutant GST-YopT C139A (400 ng/ml, respectively), as described in Materials and Methods. Thirty minutes later, photographs of the cells were taken once under fluorescence light and once as phase contrast. Note that the magnification of the objective used for Icmt+/+ and Icmt−/− cells was 40× in all cases. Thirty minutes after injection of GST-YopT, the Icmt+/+ cells have a rounded morphology. Injection of the catalytically inactive mutant GST-YopT C139A has no effect. YopT-injected Icmt−/− cells round up again after 30 min, whereas the control cells injected with the inactive mutant continue to attach and attain a flattened morphology. (B) Membrane release assay demonstrating YopT-cleaved RhoA in the soluble fraction (S) in both Icmt+/+ cells (top panel) and Icmt−/− cells (lower panel). Membranes of Icmt+/+ and Icmt−/− cells were isolated and incubated with GST-YopT as indicated. Following separation of membrane (P) and soluble (S) fractions by ultracentrifugation, samples were analyzed for RhoA by Western blotting. We detected RhoA from both Icmt+/+ and Icmt−/− cells in the soluble fraction. The appearance of RhoA in the soluble fraction (S) was accompanied by a reduction in membrane-associated RhoA (P). All experiments were repeated five times with similar results. mut, mutant; wt, wild type; WB, Western blot.
DISCUSSION
This study demonstrates that Rce1-mediated release of the -aaX tripeptide from isoprenylated RhoA is required for substrate recognition by YopT, whereas carboxyl methylation by Icmt is not.
The Yersinia outer protein T (YopT) is a cysteine protease that cleaves Rho GTPases at their carboxyl terminus. It removes the geranylgeranyl lipid that anchors the GTPase to the plasma membrane. Recently, we and others showed that isoprenylation of the GTPase is absolutely required for cleavage by YopT (16, 18). It was further shown that the 13 carboxyl-terminal residues of RhoA are sufficient for cleavage by YopT. These data suggest that YopT recognizes both a sequence of basic amino acids and the isoprenylated cysteine. Moreover, it has been shown that YopT is targeted to the plasma membrane following its injection into cells by the bacteria. These data suggest that YopT is quite capable of reaching the substrate RhoA at the plasma membrane.
Using Rce1−/− fibroblasts, we showed that isoprenylated RhoA that has not undergone the Rce1-mediated removal of -aaX is not a substrate for YopT in living cells and, importantly, YopT-injected Rce1−/− cells do not round up and detach from the dish. We prepared membrane and cytosolic fractions from Rce1−/− cells and showed that RhoA containing -aaX is at least partly localized in the membrane fraction. (This result is entirely consistent with recent results from Michaelson et al. [11], which showed that Rce1-mediated processing affects the localization of farnesylated CaaX proteins but has little effect on the membrane targeting of geranylgeranylated CaaX proteins.) Even though RhoA was appropriately localized, the membrane-bound RhoA was not cleaved by YopT, indicating that it is not some mislocalization of the GTPase that blocks YopT action in these cells.
The loss of YopT action in Rce1-deficient cells could potentially be due to two factors: that -aaX sterically blocks the interaction with the toxin or that the last step of RhoA posttranslational modification, carboxyl methylation, is required for substrate recognition.
To determine if carboxyl methylation of RhoA is required for the recognition by YopT, we injected recombinant YopT into Icmt−/− cells and studied cell rounding. Using this assay, and by studying the membrane release of RhoA, we documented that carboxyl methylation is not required for YopT recognition and cleavage of RhoA. Thus, it seems likely that the inability of YopT to cleave RhoA in Rce1−/− cells is due to the retention of the -aaX motif.
In view of the growing number of bacteria with antibiotic resistance, studying the exact action of bacterial toxins like YopT is potentially relevant to the treatment of bacterial infections. One of the pathogenic mechanisms in Yersinia infections is cleavage of Rho proteins by YopT (7). The influence of posttranslational modifications on other Rho-modifying toxins from Yersinia is unknown and should be studied. Thus, our studies with YopT provide a general example for future possibilities of directly counteracting the action of bacterial toxins in a patient with an acute bacterial infection. We propose that the toxicity of YopT with specific RCE1 inhibitor drugs (10, 15) might be quite feasible and without untoward side effects. Our previous experiments suggest that there is minimal toxicity when Rce1 is inactivated. Previously, we generated a conditional allele for Rce1 in mice and showed that Cre-mediated inactivation of Rce1 had only barely perceptible effects on the growth of fibroblasts in culture (3). Rce1 deficiency has no detectable effects on hematopoiesis (1), and Cre-mediated inactivation of Rce1 has no detectable effect on the liver (8). Inactivation of Rce1 adversely affected the heart, but that effect required months to be apparent (8). Thus, exemplified for YopT and an RCE1 inhibitor, future drugs might directly prevent the action of toxins by modifying substrate susceptibility in acute bacterial infections.
Acknowledgments
This study was supported by the DFG, SPP 1150 (to F.F. and G.S.), a grant from the Swedish Cancer Society (to M.O.B.), NIH grants CA103999, CA099506, and AR050200 (to S.G.Y.), and a grant from the Progeria Research Foundation.
Editor: J. T. Barbieri
REFERENCES
- 1.Aiyagari, A. L., B. R. Taylor, V. Aurora, S. G. Young, and K. M. Shannon. 2003. Hematologic effects of inactivating the Ras processing enzyme Rce1. Blood 101:2250-2252. [DOI] [PubMed] [Google Scholar]
- 2.Aspenstrom, P. 1999. Effectors for the Rho GTPases. Curr. Opin. Cell Biol. 11:95-102. [DOI] [PubMed] [Google Scholar]
- 3.Bergo, M. O., P. Ambroziak, C. Gregory, A. George, J. C. Otto, E. Kim, H. Nagase, P. J. Casey, A. Balmain, and S. G. Young. 2002. Absence of the CaaX endoprotease Rce1: effects on cell growth and transformation. Mol. Cell. Biol. 22:171-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergo, M. O., B. J. Gavino, C. Hong, A. P. Beigneux, M. McMahon, P. J. Casey, and S. G. Young. 2004. Inactivation of Icmt inhibits transformation by oncogenic K-Ras and B-Raf. J. Clin. Investig. 113:539-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergo, M. O., G. K. Leung, P. Ambroziak, J. C. Otto, P. J. Casey, A. Q. Gomes, M. C. Seabra, and S. G. Young. 2001. Isoprenylcysteine carboxyl methyltransferase deficiency in mice. J. Biol. Chem. 276:5841-5845. [DOI] [PubMed] [Google Scholar]
- 6.Bergo, M. O., H. D. Lieu, B. J. Gavino, P. Ambroziak, J. C. Otto, P. J. Casey, Q. M. Walker, and S. G. Young. 2004. On the physiological importance of endoproteolysis of CaaX proteins. J. Biol. Chem. 279:4729-4736. [DOI] [PubMed] [Google Scholar]
- 7.Cornelis, G. R. 2002. The Yersinia Ysc-Yop ‘type III’ weaponry. Nat. Rev. Mol. Cell. Biol. 3:742-752. [DOI] [PubMed] [Google Scholar]
- 8.Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature 420:629-635. [DOI] [PubMed] [Google Scholar]
- 9.Kim, E., P. Ambroziak, J. C. Otto, B. Taylor, B. Ashby, K. Shannon, P. J. Casey, and S. G. Young. 1999. Disruption of the mouse Rce1 gene results in defective Ras processing and mislocalization of Ras within cells. J. Biol. Chem. 274:8383-8390. [DOI] [PubMed] [Google Scholar]
- 10.Ma, Y. T., B. A. Gilbert, and R. R. Rando. 1993. Inhibitors of the isoprenylated protein endoprotease. Biochemistry 32:2386-2393. [DOI] [PubMed] [Google Scholar]
- 11.Michaelson, D., W. Ali, V. K. Chiu, M. Bergo, J. Silletti, L. Wright, S. G. Young, and M. Philips. 2005. Postprenylation CAAX processing is required for proper localization of Ras but not Rho GTPases. Mol. Biol. Cell 16:1606-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Provost, J. J., J. Fudge, S. Israelit, A. R. Siddiqi, and J. H. Exton. 1996. Tissue-specific distribution and subcellular distribution of phospholipase D in rat: evidence for distinct RhoA- and ADP-ribosylation factor (ARF)-regulated isoenzymes. Biochem. J. 319:285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridley, A. J. 2001. Rho family proteins: coordinating cell responses. Trends Cell Biol. 11:471-477. [DOI] [PubMed] [Google Scholar]
- 14.Ridley, A. J. 1996. Rho: theme and variations. Curr. Biol. 6:1256-1264. [DOI] [PubMed] [Google Scholar]
- 15.Schlitzer, M., A. Winter-Vann, and P. J. Casey. 2001. Non-peptidic, non-prenylic inhibitors of the prenyl protein-specific protease Rce1. Bioorg. Med. Chem. Lett. 11:425-427. [DOI] [PubMed] [Google Scholar]
- 16.Shao, F., P. O. Vacratsis, Z. Bao, K. E. Bowers, C. A. Fierke, and J. E. Dixon. 2003. Biochemical characterization of the Yersinia YopT protease: cleavage site and recognition elements in Rho GTPases. Proc. Natl. Acad. Sci. USA 100:904-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorg, I., U.-M. Goehring, K. Aktories, and G. Schmidt. 2001. Recombinant Yersinia YopT leads to uncoupling of RhoA-effector interaction. Infect. Immun. 69:7535-7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorg, I., C. Hoffmann, J. Dumbach, K. Aktories, and G. Schmidt. 2003. The C terminus of YopT is crucial for activity and the N terminus is crucial for substrate binding. Infect. Immun. 71:4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takai, Y., T. Sasaki, and T. Matozaki. 2001. Small GTP-binding proteins. Physiol. Rev. 81:153-208. [DOI] [PubMed] [Google Scholar]
- 20.Young, S. G., P. Ambroziak, E. Kim, and S. Clarke. 2000. Postisoprenylation protein processing: CXXX (CaaX) endoproteases and isoprenylcysteine carboxyl methyltransferase, p. 155-213. In F. Tamanoi and D. S. Sigman (ed.), The enzymes, vol. 21. Academic Press, San Diego, Calif. [Google Scholar]