Abstract
Eosinophils are frequently found in increased numbers in a variety of chronic fibrotic diseases; however, their role in the development of hepatic fibrosis has not been dissected in vivo. Here, we used interleukin-5 (IL-5) knockout (KO) mice to determine whether eosinophils contribute to the progressive liver fibrosis that develops in response to chronic Schistosoma mansoni infection. Although infection intensities were similar in C57BL/6 and IL-5 KO mice, the average size of granulomas was significantly smaller in both acutely and chronically infected IL-5 KO mice. Their granulomas were also completely devoid of eosinophils. In addition, the knockout mice displayed over a 40% reduction in hepatic fibrosis by week 16 postinfection. The reduced fibrosis was associated with increased production of the antifibrotic cytokine gamma interferon. Moreover, although IL-13 production did not decrease consistently in the absence of IL-5, IL-13-triggered responses were substantially reduced in the granulomatous tissues. This was confirmed by analyzing the expression of several genes associated with alternative macrophage activation, including arginase 1, Fizz-1, and YM-1. Importantly, all of these IL-13-regulated genes have been linked with the mechanisms of wound healing and fibrosis. In addition to IL-5 polarizing the antigen-specific CD4+ Th2 cell response, we found that granuloma eosinophils were themselves a significant source of IL-13. Thus, by producing profibrotic mediators and polarizing the Th2 response, these findings illustrate both direct and indirect roles for eosinophils and IL-5 in the pathogenesis of schistosomiasis-induced liver fibrosis. Thus, inhibiting the activity of IL-5 or eosinophils may prove effective for a variety of chronic fibrotic diseases.
The helminth parasites Schistosoma mansoni and Schistosoma japonicum are a major cause of hepatic fibrosis in tropical countries. Together, over 200 million people are infected with these parasites. Upon penetration through the skin, S. mansoni cercariae mature to schistosomula and migrate to the liver and mesenteric veins, where the adult parasites lay eggs. Some eggs migrate to the lumen of the bowel and are excreted, while others are trapped in the liver and induce a strong Th2-polarized cytokine response, which triggers the formation of eosinophil-rich granulomas. Pathological sequelae of infection include severe hepatic fibrosis that can lead to portal obstruction, liver malfunction, and, ultimately, fatal hematemesis (27, 37).
In mice, S. mansoni worms induce an early Th1-type cytokine response. However, pathological fibrosis is due to the chronic production of Th2 cytokines in response to the parasite eggs that are produced in large numbers. Interleukin-4 (IL-4) is important for the initiation of Th2-type cytokine responses during schistosomiasis (6), while IL-13 is the key mediator of fibrosis (7, 8). It is well established that IL-5 is an important mediator of eosinophil progenitor expansion in the bone marrow and survival in the periphery (2, 10, 20, 29, 30). They are also the most abundant cell type within S. mansoni-induced granulomas (23). Interestingly, mice with a targeted deletion of the IL-5 gene (IL-5 knockout [KO] mice) have normal levels of eosinophils in the blood and bone marrow and have no defects in T-cell-dependent antibody responses or in cytotoxic T-cell development (19). In contrast, transgenic mice that overexpress IL-5 have increased serum levels of immunoglobulin, increased numbers of B-1 cells, autoantibody production, and persistent eosinophilia (11, 33). Surprisingly, despite their abundance, the contribution of eosinophils and IL-5 to the progression of S. mansoni-induced liver fibrosis remains unclear.
In the present study, we investigated whether IL-5 and/or eosinophils regulate the development of egg-induced pathology in the liver. To do this, we exposed IL-5-deficient mice to S. mansoni cercariae and examined their immune responses and pathologies at both acute and chronic time points postinfection. We also performed granuloma studies in the lung to determine whether there were any anatomical or model-specific differences in the activity of IL-5.
MATERIALS AND METHODS
Animals, parasite infections, and antigen preparations.
Six- to 8-week-old female mice deficient in IL-5 on a C57BL/6 background were bred at the NIH. Age- and sex-matched C57BL/6 mice were obtained from Taconic. All animals received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals (NIH publication 86-23, revised 1985) (24a). S. mansoni eggs for intravenous injection were extracted from the livers of infected mice (Biomedical Research Institute, Rockville, MD). Mice for examination of hepatic pathology were infected percutaneously through the tail with 25 to 30 cercariae of a Puerto Rican strain of S. mansoni (Naval Medical Research Institute) obtained from infected Biomphalaria glabrata snails (Biomedical Research Institute). Soluble egg antigen (SEA) was purified from homogenized S. mansoni eggs as described previously (4). All animals were perfused at the time of sacrifice to determine worm and tissue egg burdens as described previously (4).
Histopathology and fibrosis.
The sizes of pulmonary and hepatic granulomas were determined in histologic sections stained by Wright's Giemsa stain (Histopath of America, Clinton, MD). The percentages of eosinophils, mast cells, and other cell types were evaluated microscopically in the same sections. The numbers of schistosome eggs in the liver, eggs per worm pair, and total worm pairs and collagen content of the liver, determined as hydroxyproline, were measured as described previously (4). The same individual scored all histologic features without knowledge of the experimental design.
Purification and analysis of eosinophils.
Eosinophils were isolated from the livers of S. mansoni-infected C57BL/6 mice. Single-cell suspensions were prepared from liver homogenates following a collagenase I (40 μg/ml) and DNase I (2 μg/ml) digestion at 37°C for 45 min. The digested mixture was passed through a 100-μm cell sieve and washed in phosphate-buffered saline, and the leukocyte fraction was enriched over 38% isotonic Percoll. After the Percoll step, ammonium chloride and potassium bicarbonate lysing buffer was used to remove any contaminating red blood cells. Eosinophils were subsequently isolated with a MiniMACS column using the Siglec-F-phycoerythrin monoclonal antibody (MAb) (BD Biosciences-Pharmingen, San Diego, CA) (32). The purity of the eosinophil fraction (>95%) was determined by flow cytometry and confirmed microscopically by fast green staining (Sigma) of cytospin preparations using standard protocols. Nonfractionated cells and Siglec-F-negative and Siglec-F-positive fractions were used to prepare total RNA for real-time PCR analysis according to the RNeasy animal cell I protocol provided by the manufacturer or placed in culture for 72 h to examine cytokine production.
Lymphocyte culture and cytokine detection by ELISA.
Spleen, lung-associated lymph nodes (LALN) (periaortic and mediastinal), and mesenteric lymph nodes (infection model) were removed aseptically, and single-cell suspensions were prepared (4). Cultures were incubated at 37°C in a humidified atmosphere of 5% CO2. Cells were stimulated with SEA (20 μg/ml), concanavalin A (ConA) (1 μg/ml), soluble worm antigen preparation (50 μg/ml), or medium alone. Supernatant fluids were harvested at 72 h and assayed for cytokine production. Gamma interferon (IFN-γ), IL-4, and IL-13 were measured by two-site enzyme-linked immunosorbent assay (ELISA) as previously described (4). IFN-γ levels were measured using purified anti-mouse IFN-γ capture (catalog no. 551216; Pharmingen) and biotin-labeled detection antibodies (catalog no. 554410; Pharmingen). IL-4 levels were determined using DUOSET reagents supplied by Genzyme (Cambridge, MA), and IL-13 levels were measured using a capture ELISA kit supplied by R&D Systems (Minneapolis, MN) according to the manufacturer's instructions. Cytokine levels were tested in duplicate, and standard curves were generated using recombinant murine cytokines. The same culturing conditions and stimuli were used for the purified granuloma-associated eosinophil preparations.
Isolation and purification of RNA and real-time PCR.
Lung and liver tissues were homogenized in Trizol reagent (Invitrogen, Carlsbad, CA), and total RNA was extracted according to the recommendations of the manufacturer. The purified eosinophil preparations were directly lysed in RLT buffer provided with the RNeasy kit (QIAGEN). Real-time reverse transcription-PCR (ABI Prism 7900 sequence detection system; Applied Biosystems) was used to determine relative quantities of mRNA for several cytokine, cytokine receptor, and fibrosis genes using SYBR green PCR master mix (ABI) after reverse transcription of 1 μg of RNA. The amount of PCR product was determined by the comparative cycle threshold method as described by Applied Biosystems for the ABI Prism 7700/7900 sequence detection systems, in which each sample was normalized to hypoxanthine phosphoribosyltransferase and expressed as a severalfold increase or decrease versus the average value for naïve controls.
Statistics.
Although total hepatic fibrosis increases with infection intensity, hepatic fibrosis per egg or per worm pair decreases with increasing intensity of infection. Therefore, these variables were compared by analysis of covariance using the log of total liver eggs as the covariate and the log of hydroxyproline per egg. Variables that did not change with infection intensity were compared by one-way analysis of variance or by Student's t test. Changes in cytokine mRNA and protein expression and granuloma size were evaluated by Student's t test. Results were considered significant if the P value was <0.05.
RESULTS
Pulmonary granulomatous inflammation is reduced in the absence of IL-5.
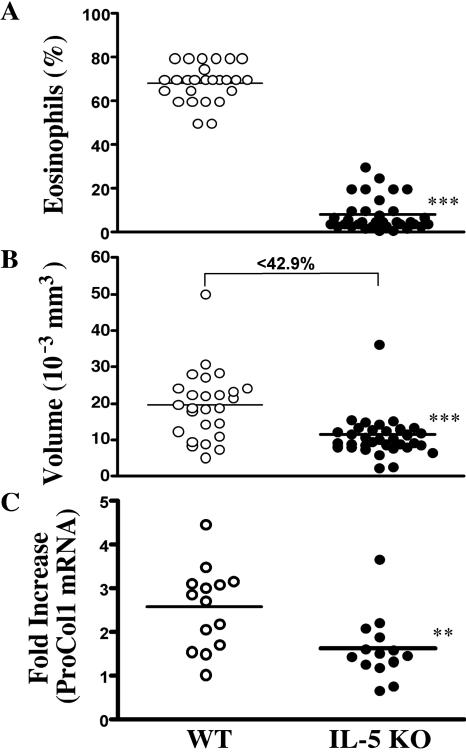
Our initial experiments examined whether schistosome egg-induced inflammation was altered in the lungs of IL-5 KO mice. We also examined whether the pulmonary eosinophil response was regulating IL-13 production, as was recently suggested in a related mouse asthma study (21). Mice were sensitized by intraperitoneal injection of 5,000 mature and viable schistosome eggs and then challenged intravenously 2 weeks later with the same dose of eggs. Eggs lodge in the lung and induce a vigorous inflammatory response that peaks around day 8 postchallenge (36). As expected, eosinophils were the predominant cell type found in the granulomas of wild-type (WT) mice, with percentages ranging between 60 and 80% (Fig. 1A). In marked contrast, granulomas that formed in most IL-5 KO mice were almost completely devoid of eosinophils (P < 0.001). These observations were somewhat unexpected, since other studies have suggested that the deletion of both IL-5 and eotaxin is required to ablate tissue eosinophil responses in the lung (21). The average size of granulomas was also significantly decreased in the IL-5 KO mice (40% smaller; P < 0.001) (Fig. 1B). In the absence of IL-5, the predominant granuloma-associated cell type was the macrophage, which displayed a 20 to 25% increase over the percentages found in WT granulomas (data not shown).
FIG. 1.
Granuloma formation is reduced in the lungs of IL-5 KO mice. WT C57BL/6 and IL-5 KO mice were sensitized with S. mansoni eggs and then challenged intravenously 2 weeks later. (A) The animals were sacrificed on day 8, and the average percentage of eosinophils in pulmonary granulomas was assessed in individual WT (n = 25) and IL-5 KO (n = 36) mice. (B) The average granuloma size was also determined. The data shown are the individual averages of 30 granulomas/mouse, and the bar denotes the average of all mice. (C) Real-time PCR was used to quantify the expression of procollagen 1 mRNA in the granulomatous tissues (n = 14 mice per group). The data shown are severalfold increases over naïve mice. Asterisks indicate a significant difference (**, P < 0.01; ***, P < 0.001) between WT and IL-5 KO animals.
IL-5 and eosinophils polarize type 2 responses in the lung.
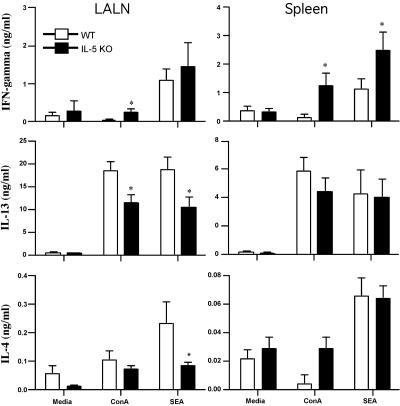
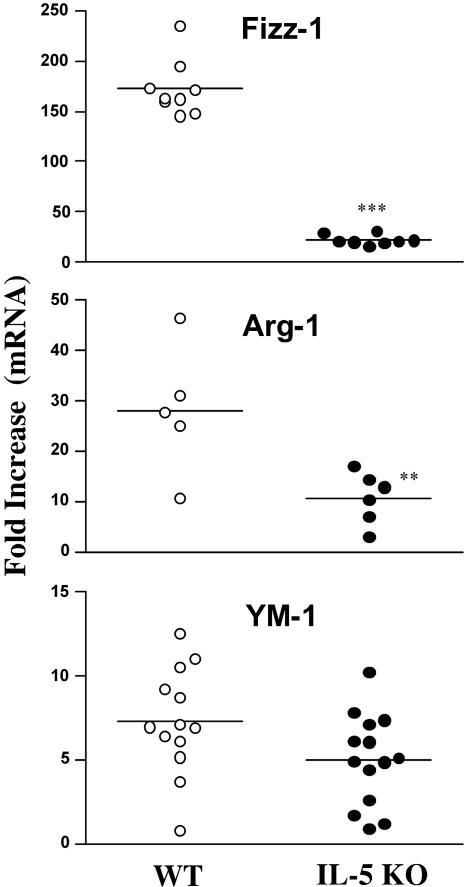
LALN cells and splenocytes were isolated from the egg-challenged mice and stimulated in vitro with mitogen or soluble egg antigen. IFN-γ, IL-13, and IL-4 were assayed in the culture supernatants as markers of type 1 and type 2 responses. Significant quantities of IL-4 and IL-13 were detected in both groups following stimulation with ConA or SEA (Fig. 2). However, there was a slight but significant decrease in IL-4 and IL-13 in the LALN cultures prepared from IL-5 KO mice. This was also associated with a modest but consistent increase in IFN-γ production in both the spleen and draining lymph nodes. Consequently, when their overall cytokine responses were analyzed as a ratio of IL-13 to IFN-γ or IL-4 to IFN-γ, the IL-5 KO mice developed more of a mixed type 1/type 2 cytokine response. Real-time PCR analysis of granulomatous lung tissue supported this conclusion, although the most consistent finding in the tissues was a small increase in IFN-γ mRNA production (Fig. 3). The decreased ratio of IL-13 to IFN-γ in the tissues was also associated with a significant decrease in procollagen 1 mRNA expression (Fig. 1C), which was consistent with the known collagen-inducing and -suppressive activities of IL-13 and IFN-γ, respectively (34). We also examined the expression of several markers of “alternative macrophage activation,” which are tightly regulated by the type 1/type 2 cytokine balance (16, 24). Consistent with the overall reduction in type 2 cytokine dominance, Fizz-1 (Relmα), arginase 1 (Arg-1), and, to a lesser extent, YM-1 mRNAs were found at reduced levels in intravenously egg-challenged IL-5 KO mice (Fig. 4).
FIG. 2.
IL-5 KO mice develop unpolarized type 1/type 2 cytokine responses. WT C57BL/6 and IL-5 KO mice were sensitized with S. mansoni eggs and then challenged intravenously 2 weeks later. The animals were sacrificed on day 8, and the LALN and spleens were extracted, processed for in vitro culture, and restimulated with medium alone, ConA, or SEA. Seventy-two-hour culture supernatants were assayed by ELISA for IFN-γ, IL-4, and IL-13. The data shown are averages ± standard deviations of three groups with three mice per group and are representative of several experiments performed. Asterisks denote significant differences (*, P < 0.05) between WT and IL-5 KO mice.
FIG. 3.
IFN-γ mRNA increases in the lungs of egg-challenged IL-5 KO mice. WT C57BL/6 and IL-5 KO mice were sensitized with S. mansoni eggs and then challenged intravenously 2 weeks later. The animals were sacrificed on day 8, and real-time PCR was used to analyze the cytokine response in the lungs. mRNA expression is displayed for individual mice as a severalfold increase compared to the respective naïve control group.
FIG. 4.
Markers of “alternative macrophage activation” are reduced in the lungs of egg-challenged IL-5 KO mice. WT C57BL/6 and IL-5 KO mice were sensitized with S. mansoni eggs and then challenged intravenously 2 weeks later. The animals were sacrificed on day 8, and real-time PCR was used to quantify the levels of Fizz-1 (Relmα), arginase 1, and YM-1 in the lung. The data shown are severalfold increases over the respective naïve control groups for individual mice. The asterisks indicate a significant difference (**, P < 0.01; ***, P < 0.001) between WT and IL-5 KO animals.
Liver inflammation and fibrosis are reduced in the absence of IL-5.
Many of the genes associated with Th2 responses and alternative macrophage activation have been implicated in the mechanisms of wound healing and fibrosis (14, 24, 35). Therefore, the results from the pulmonary granuloma model suggested that IL-5 and eosinophils might play a more important role in the pathogenesis of schistosomiasis than previously thought (1, 31). To investigate the role of IL-5 during both the acute and chronic stages of infection, WT and IL-5 KO mice were exposed to S. mansoni cercariae percutaneously and then sacrificed 9 weeks [acute] and 16 weeks (chronic) postinfection. No differences in parasite or tissue egg burdens were noted (Table 1) on week 9, indicating that IL-5 plays no role in the establishment of infection in mice, which was consistent with results from previous studies (1, 31). However, we did observe a small increase in worm burdens in some but not all studies of chronically infected IL-5 KO mice. Nevertheless, there was no evidence of increased morbidity or mortality in the IL-5 KO mice throughout the 16 weeks of study.
TABLE 1.
Parasite recovery
| Wk | Mean recovery ± SEM (no. of mice)
|
|||||
|---|---|---|---|---|---|---|
| Group | Worm pairs | Total worms | Eggs/liver (1,000) | Eggs/worm pair (1,000) | Total tissue eggs (1,000) | |
| 9 | C57BL/6 | 5.583 ± 0.399 (24) | 13.33 ± 0.963 (24) | 38.69 ± 2.660 (24) | 9.729 ± 1.291 (24) | 45.52 ± 3.308 (24) |
| IL-5 KO | 5.433 ± 0.331 (20) | 12.67 ± 0.819 (30) | 36.62 ± 2.950 (30) | 7.992 ± 0.794 (24) | 49.25 ± 3.556 (20) | |
| 16 | C57BL/6 | 3.85 ± 0.617 (20) | 10.10 ± 1.231 (20) | 68.40 ± 7.523 (20) | 24.65 ± 2.237 (20) | 78.86 ± 10.45 (15) |
| IL-5 KO | 5.741 ± 0.499 (27) | 14.33 ± 1.299 (27) | 79.69 ± 7.845 (25) | 20.69 ± 1.823 (25) | 85.59 ± 11.73 (17) | |
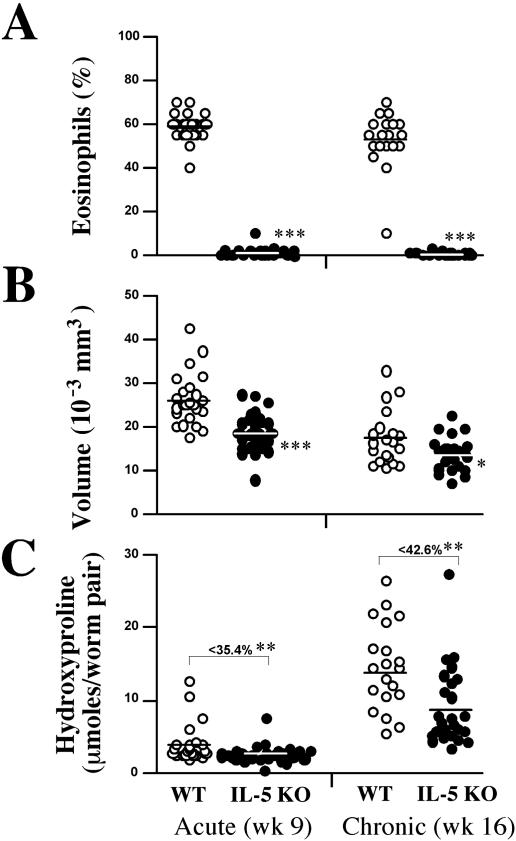
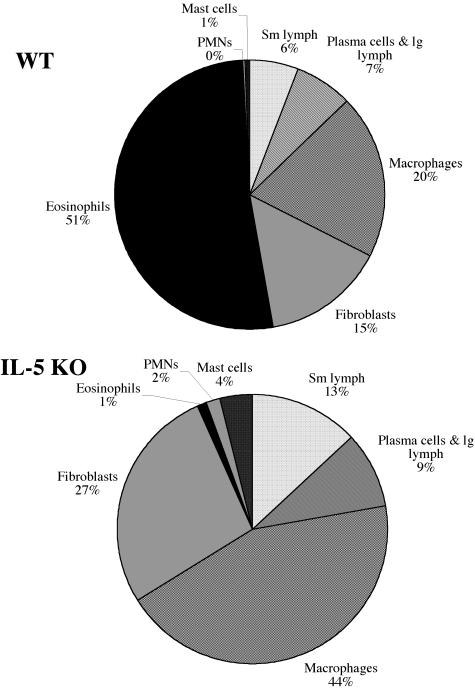
As observed in the lung (Fig. 1A), few, if any, eosinophils were found in the livers of acutely infected IL-5 KO mice (Fig. 5A). Strikingly, this was also true following chronic infection, suggesting that IL-5 deficiency alone is sufficient to completely ablate the tissue eosinophil response in the liver. The average size of granulomas in the IL-5 KO mice was also significantly reduced at both the acute and chronic time points (Fig. 5B) compared with WT animals, likely reflecting the absence of eosinophils. Nevertheless, because both groups developed smaller granulomas at the chronic time point than at the acute time point, IL-5 and eosinophils do not appear to regulate the mechanism of immune down-modulation (27). When the cellular composition of the granuloma was examined in detail (Fig. 6), the percentage of macrophages and fibroblasts increased in the IL-5 KO mice. The percentage of both populations nearly doubled in the absence of IL-5, presumably because eosinophils were absent. This contrasted greatly with WT granulomas, which were dominated by eosinophils. Finally, hepatic fibrosis, assessed by the hydroxyproline assay, was significantly reduced in the IL-5 KO mice at both time points, demonstrating that IL-5 plays a significant role in the pathogenesis of schistosomiasis (Fig. 5C).
FIG. 5.
Inflammation and fibrosis are reduced in infected IL-5 KO mice. WT C57BL/6 and IL-5 KO mice were infected with 25 to 30 S. mansoni cercariae. (A) Groups of animals were sacrificed 9 [acute] and 16 (chronic) weeks (wk) later, and the percentage of eosinophils in liver granulomas was assessed in individual WT and IL-5 KO mice (at least 20 mice per group). (B) The average size of the granulomas was also determined. The data shown are for individual mice and represent the averages of 30 granulomas measured/mouse. The bar denotes the average of all mice in that group. (C) Hepatic fibrosis was quantified with the hydroxyproline assay. Asterisks indicate a significant difference (*, P < 0.05; **, P < 0.01; ***, P < 0.001) between WT and IL-5 KO animals, and the values shown in C represent the average decrease in hydroxyproline levels in IL-5 KO versus WT mice shown as a percentage.
FIG. 6.
Eosinophils are replaced with macrophages and fibroblasts in infected IL-5 KO mice. The cellular composition of hepatic granulomas was compared in WT and IL-5 KO mice at 9 weeks postinfection. The data shown in the pie graph represent the averages of 20 IL-5 KO mice and 19 C57BL/6 mice pooled from two separate experiments. PMNs, polymorphonuclear leukocytes; lg, large; Sm, small.
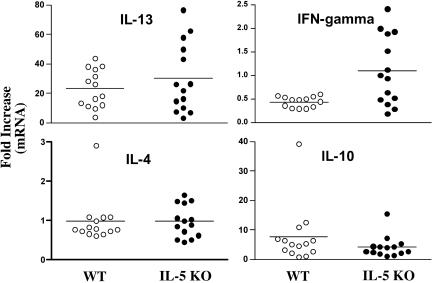
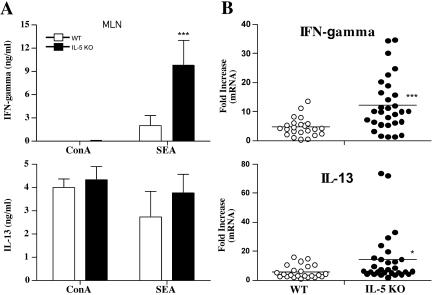
The mesenteric lymph nodes were extracted from both groups and restimulated in vitro with mitogen or SEA. The culture supernatants were assayed for IFN-γ and IL-13. WT and IL-5 KO mice produced similar quantities of IL-13 in response to ConA or SEA (Fig. 7A). IL-13 mRNA expression also increased in the granulomatous tissues of both groups, suggesting no significant impairment in type 2 response generation in the absence of IL-5 (Fig. 7B). Nevertheless, consistent with the lung granuloma studies, we observed marked increases in IFN-γ protein (Fig. 7A) and mRNA expression (Fig. 7B) in infected IL-5 KO mice, indicating that they were developing more of a mixed type 1/type 2 cytokine response.
FIG. 7.
Infected IL-5 KO mice fail to polarize the type 2 cytokine response. WT C57BL/6 and IL-5 KO mice were infected with 25 to 30 S. mansoni cercariae. (A) Groups of animals were sacrificed on week 9, and the mesenteric lymph nodes (MLN) were extracted, processed for in vitro culture, and restimulated with ConA or SEA. Seventy-two-hour culture supernatants were assayed by ELISA for IFN-γ and IL-13. The data shown are the averages ± standard deviations of three separate groups with three mice included in each group (9 to 10 mice total). The data are representative of several separate experiments. (B) Real-time PCR was used to analyze the cytokine response in the liver. mRNA expression is displayed for individual mice as severalfold increases over the respective naïve control group.
Eosinophils contribute to hepatic fibrosis by producing the profibrotic cytokine IL-13.
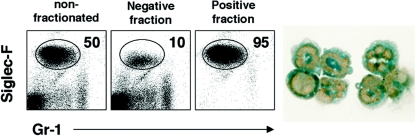
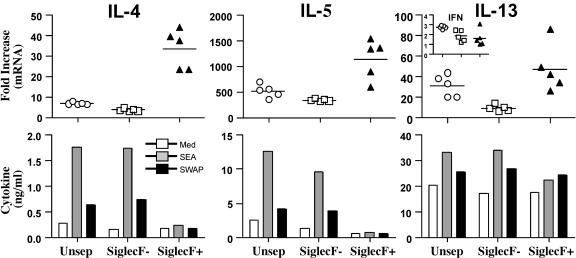
To determine if eosinophils were regulating tissue fibrogenesis directly, we isolated eosinophils from the granulomatous livers of infected mice and examined whether they were producing profibrotic mediators like IL-13. Lymphocytes isolated from the granulomatous tissues were separated into eosinophil-positive and -negative fractions by column purification. Flow cytometry demonstrated that the purity of the positive fraction was approximately 95% eosinophils (Fig. 8). Morphological analysis with the fast green stain further confirmed the identity of the purified population as eosinophils (cytoplasmic green eosinophilic granules) (Fig. 8, right). mRNA was isolated from eosinophil-negative and -positive fractions and then subjected to real-time PCR analysis to determine whether the granuloma-associated eosinophils were expressing type 2 cytokines. As expected, IL-4, IL-5, and IL-13 mRNAs were easily detected in the nonfractionated population obtained from infected mice (Fig. 9, top). Strikingly, however, the quantities of all three mRNAs increased in the purified eosinophil fraction and decreased in the eosinophil-negative population (relative to naïve liver tissue), suggesting that eosinophils are the dominant source of type 2 cytokine mRNA in the granulomatous tissues. IFN-γ mRNA showed an opposite pattern (Fig. 9, inset with IL-13), indicating that eosinophils were displaying a polarized type 2 phenotype. The isolated fractions were also cultured in vitro in the presence of soluble egg or adult parasite antigens so that the production of type 2 cytokines could be examined by ELISA. Only the unseparated and eosinophil-negative fractions responded to antigen, confirming the presence of antigen-specific CD4+ Th2 cells in those fractions (Fig. 9, bottom). Importantly, however, type 2 cytokines were also detected at significant levels in all three groups without additional stimulation. Most impressive, though, was the marked production of IL-13, which approached 20 ng/ml in the eosinophil-positive fraction.
FIG. 8.
Eosinophils are highly purified from the livers of infected WT mice. WT C57BL/6 mice were infected with 35 S. mansoni cercariae and sacrificed on week 9. Eosinophils were purified from perfused livers by positive selection using the Siglec-F antibody. The cells were separated into nonfractionated, eosinophil-negative, and eosinophil-positive fractions, respectively (left panels). The numbers in the boxes indicate the percentage of Siglec-F-positive eosinophils. Cytospin cells were stained with fast green to document the presence of cytoplasmic green eosinophilic granules (right panel). Magnification, ×400. Gr-1, granulocyte cell surface marker.
FIG. 9.
Granuloma eosinophils produce large quantities of IL-13. WT C57BL/6 mice were infected with 35 S. mansoni cercariae and sacrificed on week 9. Eosinophils were purified from perfused livers by positive selection using the Siglec-F antibody. The cells were separated into nonfractionated (Unsep) (open circles), eosinophil-negative (open squares), and eosinophil-positive (filled triangles) fractions. Real-time PCR was used to quantify the amounts of IL-4, IL-5, IL-13, and IFN-γ (inset) mRNA in each fraction. The data shown in the top panels represent the severalfold increases over naïve, uninfected control liver tissue. Each fraction was also placed in culture so that type 2 cytokine production could be determined by ELISA. Some cells were stimulated for 72 h with the parasite antigen preparations SEA and soluble worm antigen preparation. All of these experiments were repeated, with similar results.
DISCUSSION
Granuloma formation is significantly impaired in IL-4Rα-, Stat6-, and IL-4/IL-13-deficient mice (8, 17, 18, 22), illustrating the important role of type 2 cytokines in the pathogenesis of schistosomiasis. Deletion of both IL-4 and IL-13 is necessary to markedly inhibit granuloma formation in infected animals (8, 9), while IL-13 deficiency alone significantly blocks the progression of hepatic fibrosis (7). IL-13-deficient mice also survive chronic infections better than similarly infected wild-type animals (13), further emphasizing the pathogenic role of IL-13. Thus, understanding how IL-13 production is regulated in this disease as well as in other type 2-driven inflammatory disorders has become an important area of research (35). A recent study by Mattes et al. suggested that eosinophils can either directly or indirectly modulate the production of IL-13 in the lung (21). This was demonstrated in IL-5−/−/eotaxin−/− mice, which developed significantly impaired pulmonary eosinophil responses. Those researchers concluded that IL-5 and eosinophils contribute to the pathogenesis of allergic lung disease by promoting the production of IL-13 in CD4+ Th2 cells.
Given the apparent link between eosinophils and IL-13, we examined whether a similar IL-5/eosinophil-dependent mechanism was contributing to the pathogenesis of schistosomiasis. Surprisingly, the results from our studies revealed little to no impairment in antigen-specific IL-13 production in IL-5/eosinophil-deficient mice. However, the knockout animals consistently produced more IFN-γ, indicating that they were less capable of developing a “polarized” type 2 response. We also found that granuloma-associated eosinophils were a significant source of profibrotic mediators including IL-13, suggesting a direct role for these cells in the mechanisms of tissue remodeling and fibrosis. Thus, instead of simply modulating IL-13 production in T cells (21), our studies suggest a more complex and indispensable role for IL-5 and eosinophils in the pathogenesis of schistosomiasis.
In our studies, deletion of IL-5 alone was sufficient to remove nearly 100% of the eosinophils from the granulomatous tissues. This contrasts with other studies that suggested that the removal of both IL-5 and specific chemotactic factors might be required to suppress eosinophil accumulation in tissues (21, 39). We observed the same effect in the lung and liver, so organ-specific differences are unlikely to explain the nearly complete ablation of granuloma-associated eosinophils in our IL-5 KO mice. Eotaxin 1 mRNA expression was also similarly increased in the lungs of egg-injected WT and IL-5 KO mice (approximately 15-fold in both) (data not shown), further suggesting that the removal of IL-5 is sufficient to eliminate the tissue eosinophils. Strikingly, the deletion of IL-5 and eosinophils had a much larger effect on the development of granulomatous pathology than was originally predicted (1, 31). The sizes of pulmonary and hepatic granulomas decreased approximately 30 to 40% in the absence of IL-5, while liver fibrosis was reduced 40 to 50% by week 16 postinfection. We also noted a marked reduction in procollagen 1 mRNA expression in the lungs of egg-injected mice, further confirming the profibrotic role of IL-5 and eosinophils. Importantly, these data are the first to directly demonstrate a pathogenic role for IL-5 and eosinophils in an experimental model of liver fibrosis.
Although the role of IL-5 in granuloma formation was investigated previously, the contribution of IL-5 and eosinophils to the progression of hepatic fibrosis in chronically infected mice was not examined (1, 3, 12, 28, 31). The specific role of granuloma-associated eosinophils was also unexplored in those studies. In one report, a neutralizing anti-IL-5 MAb was administered to mice during the acute phase of infection. In these studies, granuloma size was only marginally decreased in the anti-IL-5 MAb-treated mice, and there was no effect on fibrosis (31). Because rat antibodies were used, the effects on long-term chronic infections could not be easily examined (5). In a subsequent report using IL-5-deficient mice, apart from the relative absence of eosinophils in the lesions, it was concluded that IL-5 has no impact on granuloma formation (1). The development of hepatic fibrosis was not examined in the latter study. Because of the absence of functional information on the role of IL-5/eosinophils in hepatic fibrosis, we focused on this question in our studies. As seen in the study reported previously by Brunet et al. (1), the IL-5 KO mice downregulated their granulomas at the chronic stage of infection as efficiently as WT mice (granulomas were smaller at the chronic time point than at the acute time point) (27). However, our IL-5 KO mice clearly developed significantly smaller granulomas at both time points. More importantly, however, there was solid evidence that IL-5 contributes to the progression of hepatic fibrosis. The reduction in fibrosis in the IL-5 KO mice was more apparent by week 16 (Fig. 5C), which could explain why the anti-IL-5 MAb had no obvious effect at the acute time point (31). Thus, our data demonstrate that IL-5 and eosinophils play a more important role in the pathogenesis of schistosomiasis than was previously concluded.
In agreement with the study reported previously by Brunet et al. (1), we observed little to no impairment in Th2 cytokine production following infection. However, we consistently observed an increase in IFN-γ production in the mesenteric lymph nodes and livers, suggesting that the IL-5 KO mice were developing more of a mixed Th1/Th2-type response. This was not observed in the study by Brunet et al., although their experiments focused on systemic splenocyte responses. Because type 1-associated cytokines are known to antagonize IL-13 effector functions (35), the increase in IFN-γ in the granulomatous tissues may in part explain the reduction in granuloma formation and fibrosis in our infected IL-5 KO mice. IFN-γ mRNA was similarly increased in the lungs of egg-injected animals. Thus, an important function of IL-5/eosinophils revealed in our studies appears to be the polarization and stabilization of helminth-induced type 2 responses.
The decreased expression of several markers of “alternative macrophage activation” in the granulomatous tissues, including Arg-1, YM-1, and Fizz-1 (Relmα), provided further evidence of reduced IL-13 effector function. These data were somewhat surprising because the large population of eosinophils that dominate wild-type granulomas was replaced by macrophages and fibroblasts in the IL-5 KO mice. Thus, although the percentage of granuloma-associated macrophages increased in the IL-5 KO mice, the gene expression data suggested that many fewer of these cells were alternatively activated. Because alternatively activated macrophages have been shown to play important roles in the pathogenesis of schistosomiasis (15), reduced development of these cells could provide an additional explanation for the decrease in fibrosis in the IL-5 KO mice (16).
We also found that granuloma-associated eosinophils are a significant source of type 2 cytokines. When the noneosinophil and purified eosinophil fractions were compared, the eosinophils clearly expressed significant quantities of IL-4, IL-5, and IL-13 mRNA. In contrast, there was little IFN-γ, indicating that that the granuloma eosinophils were expressing a type 2-polarized phenotype. When placed in culture, the eosinophils also secreted type 2 cytokines, with IL-13 approaching the levels detected in antigen-stimulated CD4+ Th2 cells. Thus, in addition to promoting a polarized CD4+ Th2 cell response, granuloma-associated eosinophils are themselves an important source of profibrotic mediators, including IL-13. Consequently, they contribute to the pathogenesis of chronic schistosomiasis through both indirect and direct mechanisms. Rumbley et al. also found granuloma eosinophils to be an important source of IL-4 (28). However, in their studies, Rumbley et al. did not examine the IL-13 response or the possible involvement of IL-5 and eosinophils in the progression of fibrosis. Thus, our study nicely complements and extends their findings.
In summary, these data illustrate that IL-5 and eosinophils regulate the pathogenesis of schistosomiasis-induced fibrosis through multiple mechanisms. (i) By suppressing production ofthe antifibrotic cytokine IFN-γ, IL-5/eosinophils help polarize the profibrotic CD4+ Th2 cell response. (ii) By increasing the ratio of IL-13 to IFN-γ in the tissues, eosinophils increase the number of “alternatively activated” macrophages and fibroblasts, which are important mediators of tissue remodeling and fibrosis. (iii) By producing profibrotic mediators like IL-13, eosinophils also contribute directly to the mechanism of fibrosis. Finally, these studies show that the progression of hepatic fibrosis is significantly attenuated in the absence of IL-5, thus revealing an important role for IL-5 and eosinophils in the pathogenesis of schistosomiasis. Eosinophilic infiltration is seen in a large number of fibrotic diseases, including viral liver diseases (hepatitis C), adverse drug reactions (such as in halothane hepatitis), liver allograft rejection, graft-versus-host disease, primary biliary cirrhosis, endomyocardial fibrosis, idiopathic retroperitoneal fibrosis, sclerosing mediastinitis, sclerosing cholangitis, and pulmonary fibrosis (25, 26, 38). Thus, targeting the activity of IL-5 and/or eosinophils may prove to be beneficial in a wide variety of chronic fibrotic disorders.
Acknowledgments
We thank Fred Lewis and his colleagues at the Biomedical Research Institute for providing the parasite materials used in these studies. We also thank Sandy White and the NIH animal care facility for excellent technical assistance and Margaret Mentink-Kane, Thiru Ramalingam, John Pesce, and Mark Wilson for their comments and many helpful discussions.
This research was supported by the Intramural Research Program of the NIAID, NIH.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Brunet, L. R., E. A. Sabin, A. W. Cheever, M. A. Kopf, and E. J. Pearce. 1999. Interleukin 5 (IL-5) is not required for expression of a Th2 response or host resistance mechanisms during murine schistosomiasis mansoni but does play a role in development of IL-4-producing non-T, non-B cells. Infect. Immun. 67:3014-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bystrom, J., T. A. Wynn, J. B. Domachowske, and H. F. Rosenberg. 2004. Gene microarray analysis reveals interleukin-5-dependent transcriptional targets in mouse bone marrow. Blood 103:868-877. [Online.] [DOI] [PubMed] [Google Scholar]
- 3.Capron, M. 1992. Dual function of eosinophils in pathogenesis and protective immunity against parasites. Mem. Inst. Oswaldo Cruz 87:83-89. [DOI] [PubMed] [Google Scholar]
- 4.Cheever, A. W., M. E. Williams, T. A. Wynn, F. D. Finkelman, R. A. Seder, T. M. Cox, S. Hieny, P. Caspar, and A. Sher. 1994. Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J. Immunol. 153:753-759. [PubMed] [Google Scholar]
- 5.Cheever, A. W., Y. Xu, J. G. Macedonia, T. Cox, S. Hieny, and A. Sher. 1992. The role of cytokines in the pathogenesis of hepatic granulomatous disease in Schistosoma mansoni infected mice. Mem. Inst. Oswaldo Cruz 87:81-85. [DOI] [PubMed] [Google Scholar]
- 6.Chensue, S. W., P. D. Terebuh, K. S. Warmington, S. D. Hershey, H. L. Evanoff, S. L. Kunkel, and G. I. Higashi. 1992. Role of IL-4 and IFN-gamma in Schistosoma mansoni egg-induced hypersensitivity granuloma formation. Orchestration, relative contribution, and relationship to macrophage function. J. Immunol. 148:900-906. [PubMed] [Google Scholar]
- 7.Chiaramonte, M. G., A. W. Cheever, J. D. Malley, D. D. Donaldson, and T. A. Wynn. 2001. Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatology 34:273-282. [DOI] [PubMed] [Google Scholar]
- 8.Chiaramonte, M. G., D. D. Donaldson, A. W. Cheever, and T. A. Wynn. 1999. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J. Clin. Investig. 104:777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiaramonte, M. G., L. R. Schopf, T. Y. Neben, A. W. Cheever, D. D. Donaldson, and T. A. Wynn. 1999. IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs. J. Immunol. 162:920-930. [PubMed] [Google Scholar]
- 10.Coffman, R. L., B. W. Seymour, S. Hudak, J. Jackson, and D. Rennick. 1989. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science 245:308-310. [DOI] [PubMed] [Google Scholar]
- 11.Dent, L. A., M. Strath, A. L. Mellor, and C. J. Sanderson. 1990. Eosinophilia in transgenic mice expressing interleukin 5. J. Exp. Med. 172:1425-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fallon, P. G., H. E. Jolin, P. Smith, C. L. Emson, M. J. Townsend, R. Fallon, P. Smith, and A. N. McKenzie. 2002. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity 17:7-17. [DOI] [PubMed] [Google Scholar]
- 13.Fallon, P. G., E. J. Richardson, G. J. McKenzie, and A. N. McKenzie. 2000. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J. Immunol. 164:2585-2591. [DOI] [PubMed] [Google Scholar]
- 14.Gordon, S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23-35. [DOI] [PubMed] [Google Scholar]
- 15.Herbert, D. R., C. Holscher, M. Mohrs, B. Arendse, A. Schwegmann, M. Radwanska, M. Leeto, R. Kirsch, P. Hall, H. Mossmann, B. Claussen, I. Forster, and F. Brombacher. 2004. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20:623-635. [DOI] [PubMed] [Google Scholar]
- 16.Hesse, M., M. Modolell, A. C. La Flamme, M. Schito, J. M. Fuentes, A. W. Cheever, E. J. Pearce, and T. A. Wynn. 2001. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J. Immunol. 167:6533-6544. [DOI] [PubMed] [Google Scholar]
- 17.Jankovic, D., M. C. Kullberg, N. Noben-Trauth, P. Caspar, J. M. Ward, A. W. Cheever, W. E. Paul, and A. Sher. 1999. Schistosome-infected IL-4 receptor knockout (KO) mice, in contrast to IL-4 KO mice, fail to develop granulomatous pathology while maintaining the same lymphokine expression profile. J. Immunol. 163:337-342. [PubMed] [Google Scholar]
- 18.Kaplan, M. H., J. R. Whitfield, D. L. Boros, and M. J. Grusby. 1998. Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J. Immunol. 160:1850-1856. [PubMed] [Google Scholar]
- 19.Kopf, M., F. Brombacher, P. D. Hodgkin, A. J. Ramsay, E. A. Milbourne, W. J. Dai, K. S. Ovington, C. A. Behm, G. Kohler, I. G. Young, and K. I. Matthaei. 1996. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 4:15-24. [DOI] [PubMed] [Google Scholar]
- 20.Lee, J. J., M. P. McGarry, S. C. Farmer, K. L. Denzler, K. A. Larson, P. E. Carrigan, I. E. Brenneise, M. A. Horton, A. Haczku, E. W. Gelfand, G. D. Leikauf, and N. A. Lee. 1997. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J. Exp. Med. 185:2143-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattes, J., M. Yang, S. Mahalingam, J. Kuehr, D. C. Webb, L. Simson, S. P. Hogan, A. Koskinen, A. N. McKenzie, L. A. Dent, M. E. Rothenberg, K. I. Matthaei, I. G. Young, and P. S. Foster. 2002. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J. Exp. Med. 195:1433-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenzie, G. J., P. G. Fallon, C. L. Emson, R. K. Grencis, and A. N. McKenzie. 1999. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J. Exp. Med. 189:1565-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore, D. L., D. I. Grove, and K. S. Warren. 1977. The Schistosoma mansoni egg granuloma: quantitation of cell populations. J. Pathol. 121:41-50. [DOI] [PubMed] [Google Scholar]
- 24.Nair, M. G., I. J. Gallagher, M. D. Taylor, P. Loke, P. S. Coulson, R. A. Wilson, R. M. Maizels, and J. E. Allen. 2005. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect. Immun. 73:385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.National Academy of Sciences. 1985. Guide for the care and use of laboratory animals. NIH publication 86-23, revised 1985. National Institutes of Health, Bethesda, Md.
- 25.Neuberger, J. 1999. Eosinophils and primary biliary cirrhosis—stoking the fire? Hepatology 30:335-337. [DOI] [PubMed] [Google Scholar]
- 26.Noguchi, H., G. M. Kephart, T. V. Colby, and G. J. Gleich. 1992. Tissue eosinophilia and eosinophil degranulation in syndromes associated with fibrosis. Am. J. Pathol. 140:521-528. [PMC free article] [PubMed] [Google Scholar]
- 27.Pearce, E. J., and A. S. MacDonald. 2002. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2:499-511. [DOI] [PubMed] [Google Scholar]
- 28.Rumbley, C. A., H. Sugaya, S. A. Zekavat, M. El Refaei, P. J. Perrin, and S. M. Phillips. 1999. Activated eosinophils are the major source of Th2-associated cytokines in the schistosome granuloma. J. Immunol. 162:1003-1009. [PubMed] [Google Scholar]
- 29.Sanderson, C. J. 1992. Interleukin-5, eosinophils, and disease. Blood 79:3101-3109. [PubMed] [Google Scholar]
- 30.Sanderson, C. J., H. D. Campbell, and I. G. Young. 1988. Molecular and cellular biology of eosinophil differentiation factor (interleukin-5) and its effects on human and mouse B cells. Immunol. Rev. 102:29-50. [DOI] [PubMed] [Google Scholar]
- 31.Sher, A., R. L. Coffman, S. Hieny, P. Scott, and A. W. Cheever. 1990. Interleukin 5 is required for the blood and tissue eosinophilia but not granuloma formation induced by infection with Schistosoma mansoni. Proc. Natl. Acad. Sci. USA 87:61-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tateno, H., P. R. Crocker, and J. C. Paulson. 2005. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6′-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology 15:1125-1135. [DOI] [PubMed] [Google Scholar]
- 33.Tominaga, A., S. Takaki, N. Koyama, S. Katoh, R. Matsumoto, M. Migita, Y. Hitoshi, Y. Hosoya, S. Yamauchi, Y. Kanai, et al. 1991. Transgenic mice expressing a B cell growth and differentiation factor gene (interleukin 5) develop eosinophilia and autoantibody production. J. Exp. Med. 173:429-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wynn, T. A. 2004. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 4:583-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wynn, T. A. 2003. IL-13 effector functions. Annu. Rev. Immunol. 21:425-456. [DOI] [PubMed] [Google Scholar]
- 36.Wynn, T. A., I. Eltoum, I. P. Oswald, A. W. Cheever, and A. Sher. 1994. Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J. Exp. Med. 179:1551-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wynn, T. A., R. W. Thompson, A. W. Cheever, and M. M. Mentink-Kane. 2004. Immunopathogenesis of schistosomiasis. Immunol. Rev. 201:156-167. [DOI] [PubMed] [Google Scholar]
- 38.Yamazaki, K., K. Suzuki, A. Nakamura, S. Sato, K. D. Lindor, K. P. Batts, J. E. Tarara, G. M. Kephart, H. Kita, and G. J. Gleich. 1999. Ursodeoxycholic acid inhibits eosinophil degranulation in patients with primary biliary cirrhosis. Hepatology 30:71-78. [DOI] [PubMed] [Google Scholar]
- 39.Yang, M., S. P. Hogan, S. Mahalingam, S. M. Pope, N. Zimmermann, P. Fulkerson, L. A. Dent, I. G. Young, K. I. Matthaei, M. E. Rothenberg, and P. S. Foster. 2003. Eotaxin-2 and IL-5 cooperate in the lung to regulate IL-13 production and airway eosinophilia and hyperreactivity. J. Allergy Clin. Immunol. 112:935-943. [DOI] [PubMed] [Google Scholar]