Abstract
We previously reported the cloning and characterization of leptospiral immunoglobulin-like proteins LigA and LigB of Leptospira interrogans. LigA and LigB are conserved at the amino-terminal region but are variable at the carboxyl-terminal region. Here, we evaluate the potential of recombinant LigA (rLigA) as a vaccine candidate against infection by L. interrogans serovar Pomona in a hamster model. rLigA was truncated into conserved (rLigAcon) and variable (rLigAvar) regions and expressed in Escherichia coli as a fusion protein with glutathione-S-transferase (rLigA). Golden Syrian hamsters were immunized at 3 and 6 weeks of age with rLigA (rLigAcon and rLigAvar) with aluminum hydroxide as an adjuvant. Hamsters given recombinant glutathione-S-transferase (rGST)-adjuvant and phosphate-buffered saline-adjuvant served as nonvaccinated controls. Three weeks after the last vaccination, all animals were challenged intraperitoneally with 108 L. interrogans serovar Pomona bacteria (NVSL 1427-35-093002). All hamsters immunized with recombinant LigA survived after challenge and had no significant histopathological changes. In contrast, nonimmunized and rGST-immunized hamsters were subjected to lethal doses, and the hamsters that survived showed severe tubulointerstitial nephritis. All vaccinated animals showed a rise in antibody titers against rLigA. Results from this study indicate that rLigA is a potential vaccine candidate against L. interrogans serovar Pomona infection.
Leptospirosis is a serious worldwide zoonotic disease caused by infection with Leptospira spp., gram-negative spirochetes that comprise 24 serogroups and more than 250 serovars (3, 25). Infection of animals or people occurs through direct or indirect contact with contaminated urine or, less frequently, by exposure to infected animal tissues. An infected animal can remain asymptomatic and shed infectious organisms in the urine throughout its life (18). In most cases of human leptospirosis, patients develop an influenza-like illness, while diarrhea, vomiting, meningitis, or uveitis may occur in some cases (11, 12). In 5 to 15% of cases, severe multisystemic complications may develop, including renal failure, jaundice, and occasionally pulmonary failure (3). Recently, a case of acute respiratory failure with lethal pulmonary hemorrhage has been reported (5). In animals, leptospiral infection is a frequent cause of kidney and liver failure (dogs), abortion, stillbirth, infertility (cattle, pigs, and horses), uveitis (horses), hemolytic anemia (sheep and cattle), and occasionally death (15-17, 37).
The worldwide distribution of this potentially fatal zoonotic infection and its association with autoimmune disease (12, 23, 35) provide the impetus to develop an effective and safe vaccine. Prevention of leptospirosis in dogs is accomplished to some extent by inoculation with bacterins that contain the most commonly encountered serovars. Although leptospiral bacterins may protect dogs from developing clinical signs of the disease, they are ineffective in preventing leptospiremia and renal shedding (2). In contrast, a monovalent leptospiral vaccine can prevent renal colonization and urinary shedding in cattle challenged with Leptospira borgpetersenii serovar Hardjo, but with minor interstitial nephritis (4). Immunity in vaccinated cattle is reportedly mediated by a type 1 (Th1) cell-mediated immune response to serovar Hardjo infection (8, 29, 30). Comparison of different bacterial extracts indicates that only the protein fraction of L. interrogans can provide cross-protection against heterologous challenge (18). Efforts to develop recombinant leptospiral vaccines have therefore focused on the outer membrane proteins of the spirochetes. Despite the identification of leptospiral antigens such as OmpL1, LipL41, LipL36, LipL32, and LipL21 (13, 14, 20, 21, 36), only a few attempts have been made to utilize these leptospiral antigens in a recombinant vaccine (22). Most studies exploring the pathogenicity of Leptospira have employed hamsters or guinea pigs as animal models because the mouse is not considered an ideal model (28). However, Lig protein has been reported to protect mice against L. interrogans serovar Manila infection (24). Recently, 3- or 6-week-old C3H/HeJ and C3H/SCID mice have been employed to study the lethality with L. interrogans serovar Copenhageni, where acclimatization and immunization including a booster require at least a 6- to 7-week period (31). However, L. interrogans serovar Pomona infection is not lethal to mice (1). Therefore, the hamster is an ideal alternative animal model for a leptospiral vaccine trial against L. interrogans serovar Pomona infection.
Leptospires survive both in the environment and inside the host. Thus, it seems likely that spirochetes adapt to diverse environments by selective gene expression (19). Therefore, identification of leptospiral antigens that are expressed only during infection may provide new strategies for construction of novel vaccines. We recently described two closely related outer surface proteins, termed leptospiral immunoglobulin-like proteins LigA and LigB, from L. interrogans serovar Pomona that are upregulated during infection (33, 34). LigA and LigB are identical at the amino terminus but vary at the carboxyl terminus (33). These leptospiral immunoglobulin-like proteins are not present in the nonpathogenic L. biflexa serovar Patoc (33). The lack of lethality of leptospiral infection in hamsters by high-passage L. interrogans and L. kirschneri strain RM52 and culture-attenuated strains is associated with loss of LigA and LigB expression (26, 27). Although these homologous proteins are expressed at very low levels in low-passage strains, expression could not be detected in rats immunized with a killed virulent strain and whole-cell preparations from the low-passage strain (26, 27). Therefore, most vaccines, which rely on whole-cell lysates or bacterins, likely do not contain the Lig proteins. In this study, we report that vaccination with recombinant LigA (rLigA) induced protective immunity against challenge in a hamster model. Thus, recombinant Lig protein is a promising candidate for an effective vaccine to prevent leptospirosis.
MATERIALS AND METHODS
Bacterial strains, media, and plasmids.
L. interrogans serovar Pomona was isolated from naturally infected dogs (34). Leptospires were maintained on EMJH medium at 30°C (34). To isolate low-passage cultures of leptospires, hamsters were experimentally infected with a sublethal dose of L. interrogans serovar Pomona (NVSL 1427-35-093002). Infected hamster tissues were harvested aseptically and homogenized with sterile phosphate-buffered saline (PBS), and the lysates were inoculated into EMJH medium. Growth was monitored using dark-field microscopy.
Fifty-percent lethal dose (LD50) value of L. interrogans in hamster.
Forty-eight 8- to 10-week-old Golden Syrian hamsters (Harlan Sprague-Dawley) were divided into six equal groups. Each group was infected intraperitoneally with 109 to 105 low-passage (three to four passages) L. interrogans serovar Pomona (NVSL 1427-35-093002). One group of hamsters received only PBS (control). The animals were monitored daily. Tissues were collected aseptically from animals that died due to infection and subjected to culture and histopathological analysis.
Cloning, expression, and purification of LigA as a GST fusion protein.
Attempts to express the entire open reading frame of LigA (with/without signal sequences) led to toxicity in Escherichia coli, resulting in weak expression. Therefore, LigA was expressed as conserved and variable regions of LigA. Cloning and expression of LigA was carried out as previously described (33, 34). Briefly, LigA was truncated into conserved (Con) (the N-terminal 599 amino acids without the signal sequences) and variable (VarA) (the C-terminal 595 amino acids) regions. The regions were amplified using PCR with the following primers: ligConF, TCCCCCGGGGCTGGCAAAAGA; ligConR, CCCTCGAGAATATCCGTATTAGA; VariAF, CCCCCGGGCTTACCGTTCC; and VariAR, CCCTCGAGTGGCTCCGTTTTAAT. The underlined nucleotides indicate the restriction site. Genomic DNA from Leptospira interrogans serovar Pomona was amplified using PCR with AccuPrime Taq polymerase (Invitrogen, CA), and the other reagents were added as outlined by the manufacturer's instructions (Invitrogen, CA). The amplified PCR products were cloned into the pCR2.1 vector (Invitrogen, CA) and subcloned into pGEX4T-2 (Pharmacia, NJ). The resulting recombinant plasmids were transformed into BL21(DE3), and expression was induced with 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside). The purification of glutathione S-transferase (GST) fusion protein was carried out as previously described (33). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as previously described (33).
Preparation of anti-rLigA antiserum in rabbits.
Antisera to the conserved and variable regions of Lig proteins were prepared as described previously (33).
Immunization and challenge experiments.
Four-week-old Golden Syrian hamsters (Harlan Sprague-Dawley) were divided into three equal groups. The groups were immunized twice at 3-week intervals by subcutaneous injection of rLigA (50 μg of rCon-GST and 50 μg of rVarA-GST), recombinant GST (rGST) (50 μg), or PBS, respectively. Prior to immunization, the recombinant proteins and/or PBS were mixed with an equal volume of aluminum hydroxide (Alhydrogel; Accurate Chemical & Scientific Corp., Westbury, NY). Blood for serum separation was collected on days 0 (prevaccination), 21, and 42 directly from the saphenous vein.
The vaccinated hamsters were challenged intraperitoneally with the estimated LD50 value of 108 leptospires on day 42. The animals were monitored twice daily and sacrificed on day 71 (29 days after challenge), and blood was collected by cardiac puncture. The tissues from infected animals were collected aseptically for histopathology and culture. The experiments were repeated three times. The protocol of this study was approved by IACUC (Institutional Animal Care and Use Committee) at Cornell University to comply with federal law (PL99-198). All work was conducted in compliance with regulations, policies, and principles of the Animal Welfare Act; the Public Health Service for Policy on Humane Care and Use of Laboratory Animals used in Testing, Research, and Training; the NIH Guide for the Care and Use of Laboratory Animals; and the New York State Department of Public Health regulations.
Culture.
Hamster tissues, including liver, kidney, spleen, lungs, urinary bladder, and blood, were collected aseptically from each animal, homogenized in 0.5 ml EMJH medium, transferred into 20 ml EMJH medium, and maintained at 30°C for 4 weeks. Growth was monitored using dark-field microscopy.
Histopathology.
Hamster tissues were collected and fixed in 10% neutral buffered formalin. The fixed tissues were sectioned at 5 μm, stained with hematoxylin and eosin, and examined by light microscopy. The renal lesions for hamsters were graded on a scale with 0 as normal, 1 as mild, 2 as moderate, and 3 as severe. Statistical analysis was performed based on the scores for the overall experiments between control and rLigA-immunized animals by use of Statistix 7.0 software.
KELA with recombinant antigens.
Sera from hamsters given rLigA, rGST, and PBS were evaluated for the presence of specific immunoglobulin G (IgG) by use of a kinetic enzyme-linked immunosorbent assay (KELA) with recombinant antigens (33). A checkerboard titration was followed to optimize the concentrations of reagents for the KELA as previously described (27, 29, 30). Briefly, the optimum concentrations of recombinant antigens (rLigAcon-GST, rLigAvar-GST, and rGST) were diluted in 0.1 M bicarbonate buffer, coated onto a 96-well microtiter plate (Nunc, Denmark), rocked for 1 h, and then incubated overnight at 4°C. The plates were washed three times with 0.1 M PBS containing 0.05% Tween 20 (PBST). Next, 100 μl of hamster serum (primary antibody) diluted 1:100 in PBST was added to each well and incubated for 1 h at 37°C in a humid chamber. The plates were washed three times as described above with PBST and incubated with 100 μl of a 1:1,000 dilution of goat anti-hamster IgG conjugated to horseradish peroxidase (Cappel, Durham, NC) for 30 min at room temperature. The plates were washed again three times with PBST, and 100 μl of tetramethylbenzidine (Kirkegaard, MD) was added to each well. Each plate was read three times at 1-min intervals at an optical density at 650 nm (EL-312; Bio-Tek, Winooski, VT). The results were calculated by the KELA computer program and expressed as the slope of the reaction between the enzyme and the substrate to the amount of antibody bound (9, 10).
Passive-protection assay using rabbit anti-rLigA antiserum.
Polyclonal antibodies to rLigAcon and rLigAvar were prepared as previously described (33). The titer was determined using the KELA, and the serum had a titer of approximately 200 KELA units. Equal volumes of polyclonal antibody to rLigAcon and rLigAvar were mixed together, and 50, 100, 200, and 300 μl were injected intraperitoneally to three animals per group. After 1 h, the hamsters were challenged with 108 L. interrogans serovar Pomona organisms given intraperitoneally. Animals given similar volumes of preimmune serum served as the controls.
RESULTS
Expression and purification of LigA as truncated GST fusion proteins.
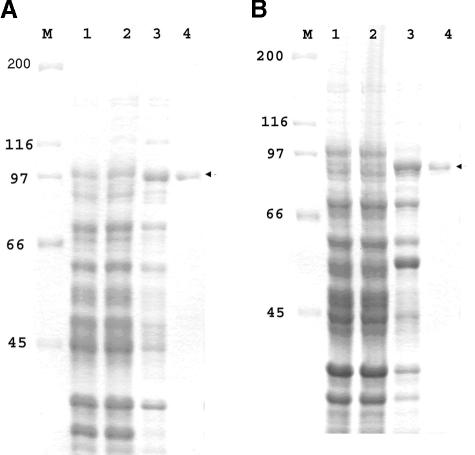
We truncated LigA into conserved (rLigAcon) and variable (rLigAvar) regions and expressed them as GST fusion proteins in E. coli. The purified GST fusion proteins (rCon-GST and VarA-GST) appeared as a single band by SDS-PAGE analysis (Fig. 1A and B).
FIG. 1.
Expression and purification of conserved and variable regions of LigA. The conserved and variable regions of LigA were cloned into pGEX4T-2 and expressed as GST fusion proteins as described in Materials and Methods. GST fusion protein was purified by affinity chromatography and subjected to SDS-PAGE followed by Coomassie blue staining. (A) Expression of the conserved region of LigA. (B) Expression of the variable region of LigA. Lane 1, E. coli with vector and pGEX4T-2 only (control); lane 2, uninduced E. coli with recombinant construct; lane 3, IPTG-induced E. coli with recombinant construct; lane 4, affinity chromatography-purified GST fusion proteins (∼1.5 μg). M represents the molecular size marker in kilodaltons (Bio-Rad).
LD50 value for L. interrogans serovar Pomona in a hamster model.
A dose of 109 leptospires was uniformly lethal, whereas five of eight hamsters that received a dose of 108 leptospires survived. Thus, the LD50 for L. interrogans serovar Pomona infection was approximately 108.
Antibody responses to recombinant antigens.
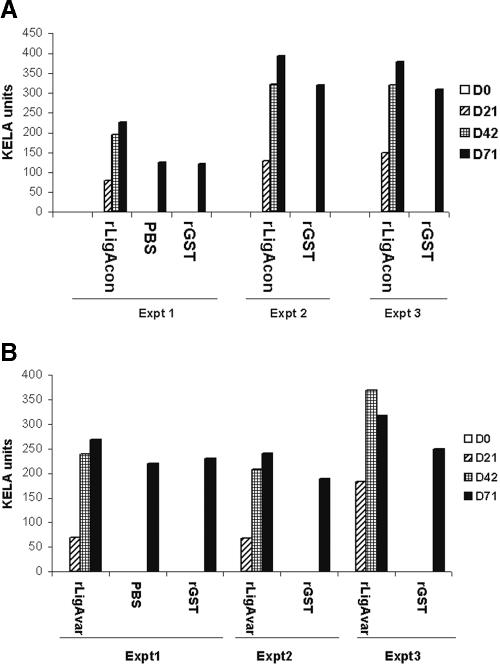
In order to assess whether hamsters given rLigA (rCon-GST and rVarA-GST), rGST, and PBS (control) developed IgG antibody response against the antigens, sera collected from the animals on days 0, 21, 42, and 71 (29 days after challenge) were tested by KELA using rCon-GST, rVarA-GST, and rGST. The results indicate that hamsters vaccinated with rLigA developed IgG antibodies to rCon and rVarA (Fig. 2A and B). Hamsters also developed antibodies to GST, which suggests that hamsters mounted an immune response to both the GST portion and the recombinant protein. Reactivity of rCon and rVarA was determined by subtracting GST activity. No detectable IgG response was found with the group of hamsters administered PBS-adjuvant.
FIG. 2.
Serum IgG responses in hamsters after two subcutaneous injections at 3-week intervals with recombinant proteins. Sera from hamsters immunized with 50 μg of recombinant protein (rCon-GST and rVarA-GST), rGST, or PBS were diluted 1:100 and subjected to KELA with rCon-GST (A), rVarA-GST (B), and rGST. The reactivity of hamsters to rLigA was determined by subtracting the reactivity of rGST. Day 0 (D0) represents preimmune sera, D21 and D42 indicate serum samples after the first (D21) and second (D42) immunizations, and D71 represents serum samples after challenge (29 days postinfection). Expt, experiment.
After challenge with leptospires (day 79), sera collected from all hamsters contained IgG antibodies to rLigA. These results support previous observations that leptospires upregulate Lig expression only upon infection.
rLigA confers protective immunity in a hamster model.
Control and rLigA-immunized hamsters were challenged using a single LD50 inoculum. As shown in Table 1, 13 of 16, 4 of 5, and 4 of 7 challenged control animals survived in three separate experiments, respectively. By comparison, all animals immunized with rLigA survived after challenge, indicating the immunoprotective potential of LigA (Table 1). The difference between survival rates of rLigA-immunized and control animals for overall experiments was statistically significant (P = 0.01), indicating the immunoprotection of rLigA after challenge. The effectiveness of immunoprotection was further evaluated by histopathology and culture.
TABLE 1.
Immunoprotective potential of rLigA based on lethality in a hamster modela
| Expt and inoculum | Total no. of animals | No. of animals that survivedb |
|---|---|---|
| Expt 1 | ||
| rLigA | 8 | 8* |
| rGST | 8 | 7 |
| PBS | 8 | 6 |
| Expt 2 | ||
| rLigA | 5 | 5* |
| rGST | 5 | 4 |
| Expt 3 | ||
| rLigA | 8 | 8* |
| rGST | 7 | 4 |
A chi-square test was performed with Statistix 7.0 software to determine if there was a statistically significant difference in mortality between the control and rLigA-immunized animals from all experiments. A significant difference (P = 0.01) was found, indicating the protective efficacy of rLigA against challenge.
*, no histopathological lesions were found in the rLigA-immunized animals, which is in contrast to results for control groups.
Histopathological analysis.
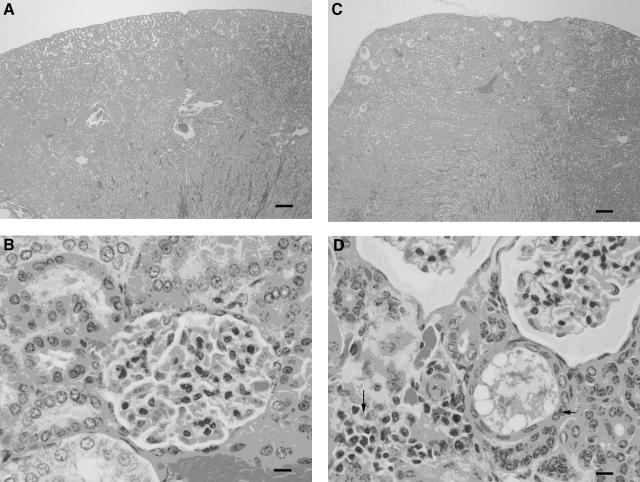
Except for insignificant interstitial lymphocytic infiltrates in the kidneys of three hamsters, no changes were detected in any tissues obtained from hamsters immunized with rLigA (Fig. 3A and B). In contrast, chronic tubulointerstitial nephritis was found in hamsters given GST-adjuvant and PBS-adjuvant (Fig. 3C and D). Prominent interstitial infiltrates of lymphocytes were accompanied by a few heterophils and occasional macrophages mixed with lower numbers of plasma cells. Occasionally, tubules were mildly dilated and distended with hyaline material that contained rare sloughed epithelial cells. The lining epithelium was also moderately attenuated. Rarely, desquamated epithelial cells and heterophils mixed with hyaline material formed casts within the lumina of proximal convoluted tubules. In some cases, prominent hyaline casts were accompanied by widespread moderate interstitial fibrosis. Glomerular changes were generally mild and considered to be secondary to the tubulointerstitial nephritis. Occasional glomeruli had shrunken glomerular tufts, while others had patchy increases in the amount of mesangial matrix and mild hypertrophy of the parietal epithelium lining Bowman's capsules. Bowman's capsules of some glomeruli in regions of severe tubular atrophy and fibrosis were dilated, and the uriniferous spaces were filled with a faintly eosinophilic lacy material. The renal lesions of hamsters were graded, and statistical analysis of results for control and rLigA-immunized animals was performed. The difference in graded renal lesions between control and rLigA-immunized animals was statistically significant, indicating the protective efficacy of rLigA (Table 2).
FIG. 3.
Histopathological analysis of hamster tissues. (A) Kidney from hamster vaccinated with rLigA and challenged with 108 L. interrogans serovar Pomona organisms by intraperitoneal injection. Note the normal renal architecture. Bar = 200 μm. (B) High-magnification view of cortex from panel A. Bar = 10 μm. (C) Kidney from hamster vaccinated with rGST and challenged with 108 L. interrogans serovar Pomona organisms by intraperitoneal injection. The renal profile is distorted due to atrophy of proximal convoluted tubules and interstitial fibrosis. Bar = 200 μm. (D) High-magnification view of cortex from panel C. The interstitium is infiltrated by small numbers of lymphocytes and plasma cells (long arrow). Occasionally, tubules are dilated and lined by a moderately attenuated epithelium (short arrow). Bowman's capsules of glomeruli are dilated, and the uriniferous spaces are filled with a faintly eosinophilic lacy material (upper right corner). Bar = 10 μm. All tissues were stained with hematoxylin and eosin.
TABLE 2.
Immunoprotective potential of rLigA based on histopathological analysisa
| Score | No. of control animals | No. of rLigA- immunized animals |
|---|---|---|
| 0 | 0 | 17 |
| 1 | 0 | 3 |
| 2 | 4 | 0 |
| 3 | 17 | 0 |
The renal lesions in hamsters were graded on a scale of severity, with 0 as normal, 1 as mild, 2 as moderate, and 3 as severe. Statistical analysis of results for control and rLigA-immunized animals was performed using the Wilcoxon rank sum test with Statistix 7.0 software. The difference between overall results for control and rLigA-immunized animals was statistically significant (P < 0.001), indicating the protective efficacy of rLigA.
Leptospira culture from tissues.
Kidney, liver, urinary bladder, lungs, and spleen were evaluated for the presence of leptospires by culture of tissues from both immunized and control groups. Except for the kidneys from two rLigA-immunized animals from the third experiment, all tissues from rLigA-immunized hamsters were negative. In contrast, all tissue samples collected from the control groups were culture positive.
Passive-immunization assay.
Although the hamsters that received 300 μl of rabbit serum (group D) to LigA survived after challenge, all had severe kidney lesions, similar to results for control groups, as revealed by histopathological analysis. The results indicate that passive immunization with rabbit serum failed to provide protection against leptospiral infection (Table 3).
TABLE 3.
Passive-protection study with polyclonal antibodies to rLigA
| Group | Amt of anti-rLigA (μl) | Total no. of animals | No. of animals that surviveda |
|---|---|---|---|
| A | 50 | 3 | 1 |
| B | 100 | 3 | 1 |
| C | 200 | 3 | 2 |
| D | 300 | 3 | 3 |
| Control | 300 | 3 | 2 |
All animals that survived had severe histopathological lesions, as seen with the control animals.
DISCUSSION
The main focus of our research is to identify leptospiral antigens that can be used for the development of more-effective vaccines for leptospirosis. Since outer membrane and surface proteins of bacteria mediate the primary interaction with the host, efforts to develop recombinant vaccines based on these proteins hold great promise. The outer membrane proteins of Leptospira are of special interest because Leptospira survives outside (contaminated water or soil) as well as inside the host and expression of some of these proteins is regulated by temperature (32). Currently available vaccines provide only short-term immunity and afford little cross-protection against different serovars (6, 7, 38). Therefore, identification of leptospiral antigens that are uniquely expressed during infection may not only help in the development of an ideal vaccine but also aid in studies about the pathogenesis of leptospirosis.
E. coli containing a plasmid with full-length ligA expressed rLigA only at very low levels because of its high toxicity (34). Attempts to express recombinant LigA in a pET system with a His tag fusion protein also failed (our unpublished data). Since GST represents one of the carrier systems for vaccination (33), we truncated Lig proteins and utilized the conserved regions of LigA and LigB as GST fusion proteins. Recently, the utility of recombinant LigA and LigB proteins for serodiagnosis was established (33, 34). Based on the NCBI database, Lig proteins (NCBI accession no. AF368236, AF219120, and AY327260) show homology to cell binding proteins such as invasin of Yersinia spp. (23% identity and 40% similarity) and invasin of Clostridium spp. (31% identity and 45% similarity). These Lig proteins contain an immunoglobulin-like domain as found in intimin of E. coli and invasin of Yersinia spp. (34). However, the role of Lig proteins in adhesion still needs to be characterized. Since the attachment of bacteria to host cells triggers various virulence genes, the use of surface proteins such as Lig proteins as vaccine candidates may prevent attachment and abrogate colonization.
In order to develop an effective vaccine, immunization with the recombinant LipL41 and OmpL1 provided only partial immunoprotection in a hamster model (22). Adenovirus-mediated vaccination with Hap1, a hemolysis-associated protein of Leptospira that shows 93% homology with LipL32, provides approximately 87% protection against challenge in a gerbil model (6, 7). However, recombinant Hap1 or LipL32 purified from E. coli failed to induce protective immunity (7). Therefore, rLigA is the first leptospiral protein that confers protective immunity in a hamster model. Although there is variability in the lethality rates between experiments, the overall difference in survival rate between control and rLigA-immunized animals was statistically significant, indicating the protective efficacy of rLigA. Further, all of the surviving control animals showed severe tubulointerstitial nephritis, indicating the end stage of infection. In contrast, all of the rLigA-immunized animals showed no signs of lesion, which was also statistically significant, indicating the protective efficacy of recombinant LigA. We demonstrated earlier that sera collected from animals given commercial vaccines have no reactivity to Lig proteins (33). Since Lig proteins are not present in culture-attenuated strains and high-passage cultures of Leptospira (26), it may be important to include the Lig proteins in the vaccine preparation. Hamsters and guinea pigs are good models for studying leptospirosis, but susceptibility to infection depends on age and weight of the animal. Koizumi and Watanabe indicated that ligA from L. interrogans serovar Manila is very similar to that of L. interrogans Pomona (97% and 68% similarity with conserved and variable regions of LigA, respectively), and the recombinant Lig proteins have been shown to confer protective immunity in a mouse model (24, 34). Since McBride et al. indicated that the mouse is not an ideal model for leptospirosis (28), we studied the protective immunity of Lig proteins in a hamster model. Further, a number of different serovars of Leptospira are pathogenic in hamsters and, therefore, cross-protective efficacy can be studied with the hamster model. The immunogenicity of Lig proteins and their effective use in diagnosis of canine and equine leptospirosis is evident (33, 34). This confirms that Lig proteins are expressed or upregulated during infection. We previously showed that a single recombinant outer surface protein, OspA of Borrelia burgdorferi, protects dogs and horses against infection (9, 10). However, the immunoprotective potential of rLigA in domestic animals or humans needs to be studied.
Passive protection with rLigA antiserum failed to provide hamsters with complete protection against leptospiral infection. Although it has recently been reported that a monovalent leptospiral vaccine could induce a type 1 protective immune response (30), the immunological mechanism responsible for protective immunity in hamsters immunized with rLigA is not known. Since leptospires are considered invasive and not facultative intracellular pathogens (39), cell-mediated immunity may play an important role in protection against leptospiral infection. The present study indicates that vaccination with rLigA in a hamster model not only prevented fatalities but also prevented histopathological lesions in the kidney and prevented growth of the organisms significantly in vivo. Thus, recombinant LigA represents a strong candidate for an effective expanded-spectrum vaccine to prevent leptospirosis. Although LD50 is generally used to evaluate leptospiral vaccine efficacy, our results clearly demonstrate severe renal disease in the control group survivors. Thus, it is crucial to include renal histopathology when evaluating vaccine efficacy against leptospiral challenge. Further studies to understand the mechanism of immunoprotection and/or cross-protection of rLigA against various serovars are in progress.
Acknowledgments
This work was supported by the Harry M. Zweig Memorial Fund for Equine Research, the New York State Science and Technology Foundation, and the Jack Lowe Equine Research Fund.
Editor: D. L. Burns
REFERENCES
- 1.Adler, B., and S. Faine. 1976. Susceptibility of mice treated with cyclophosphamide to lethal infection with Leptospira interrogans serovar Pomona. Infect. Immun. 14:703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andre-Fontaine, G., C. Branger, A. W. Gray, and H. L. Klaasen. 2003. Comparison of the efficacy of three commercial bacterins in preventing canine leptospirosis. Vet. Rec. 153:165-169. [DOI] [PubMed] [Google Scholar]
- 3.Bharti, A. R., J. E. Nally, J. N. Ricaldi, M. A. Matthias, M. M. Diaz, M. A. Lovett, P. N. Levett, R. H. Gilman, M. R. Willig, E. Gotuzzo, and J. M. Vinetz. 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 3:757-771. [DOI] [PubMed] [Google Scholar]
- 4.Bolin, C. A., and D. P. Alt. 2001. Use of a monovalent leptospiral vaccine to prevent renal colonization and urinary shedding in cattle exposed to Leptospira borgpetersenii serovar Hardjo. Am. J. Vet. Res. 62:995-1000. [DOI] [PubMed] [Google Scholar]
- 5.Borer, A., I. Metz, J. Gilad, K. Riesenberg, N. Weksler, G. Weber, M. Alkan, and J. Horowitz. 1999. Massive pulmonary haemorrhage caused by leptospirosis successfully treated with nitric oxide inhalation and haemofiltration. J. Infect. 38:42-45. [DOI] [PubMed] [Google Scholar]
- 6.Branger, C., B. Chatrenet, A. Gauvrit, F. Aviat, A. Aubert, J. M. Bach, and G. Andre-Fontaine. 2005. Protection against Leptospira interrogans sensu lato challenge by DNA immunization with the gene encoding hemolysin-associated protein 1. Infect. Immun. 73:4062-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Branger, C., C. Sonrier, B. Chatrenet, B. Klonjkowski, N. Ruvoen-Clouet, A. Aubert, G. Andre-Fontaine, and M. Eloit. 2001. Identification of the hemolysis-associated protein 1 as a cross-protective immunogen of Leptospira interrogans by adenovirus-mediated vaccination. Infect. Immun. 69:6831-6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, R. A., S. Blumerman, C. Gay, C. Bolin, R. Duby, and C. L. Baldwin. 2003. Comparison of three different leptospiral vaccines for induction of a type 1 immune response to Leptospira borgpetersenii serovar Hardjo. Vaccine 21:4448-4458. [DOI] [PubMed] [Google Scholar]
- 9.Chang, Y., V. Novosol, S. P. McDonough, C. F. Chang, R. H. Jacobson, T. Divers, F. W. Quimby, S. Shin, and D. H. Lein. 1999. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface protein A (rOspA) in horses. Vaccine 18:540-548. [DOI] [PubMed] [Google Scholar]
- 10.Chang, Y. F., M. J. Appel, R. H. Jacobson, S. J. Shin, P. Harpending, R. Straubinger, L. A. Patrican, H. Mohammed, and B. A. Summers. 1995. Recombinant OspA protects dogs against infection and disease caused by Borrelia burgdorferi. Infect. Immun. 63:3543-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciceroni, L., P. D'Aniello, N. Russo, D. Picarella, D. Nese, F. Lauria, A. Pinto, and B. Cacciapuoti. 1995. Prevalence of leptospire infections in buffalo herds in Italy. Vet. Rec. 137:192-193. [DOI] [PubMed] [Google Scholar]
- 12.Ciceroni, L., A. Pinto, E. Benedetti, P. Pizzocaro, R. Lupidi, M. Cinco, L. Gelosa, R. Grillo, V. Rondinella, L. Marcuccio, et al. 1995. Human leptospirosis in Italy, 1986-1993. Eur. J. Epidemiol. 11:707-710. [DOI] [PubMed] [Google Scholar]
- 13.Cullen, P. A., D. A. Haake, D. M. Bulach, R. L. Zuerner, and B. Adler. 2003. LipL21 is a novel surface-exposed lipoprotein of pathogenic Leptospira species. Infect. Immun. 71:2414-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cullen, P. A., X. Xu, J. Matsunaga, Y. Sanchez, A. I. Ko, D. A. Haake, and B. Adler. 2005. Surfaceome of Leptospira spp. Infect. Immun. 73:4853-4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson, M. G., M. P. Nasisse, and S. M. Roberts. 1987. Immunodiagnosis of leptospiral uveitis in two horses. Equine Vet. J. 19:155-157. [DOI] [PubMed] [Google Scholar]
- 16.Donahue, J. M., B. J. Smith, K. B. Poonacha, J. K. Donahoe, and C. L. Rigsby. 1995. Prevalence and serovars of leptospira involved in equine abortions in central Kentucky during the 1991-1993 foaling seasons. J. Vet. Diagn. Investig. 7:87-91. [DOI] [PubMed] [Google Scholar]
- 17.Donahue, J. M., B. J. Smith, K. J. Redmon, and J. K. Donahue. 1991. Diagnosis and prevalence of leptospira infection in aborted and stillborn horses. J. Vet. Diagn. Investig. 3:148-151. [DOI] [PubMed] [Google Scholar]
- 18.Faine, S., B. Adher, C. Bloin, and P. Perolat. 1999. Leptospira and leptospirosis, 2nd ed. MedSci, Melbourne, Australia.
- 19.Haake, D. A. 2000. Spirochaetal lipoproteins and pathogenesis. Microbiology 146:1491-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haake, D. A., G. Chao, R. L. Zuerner, J. K. Barnett, D. Barnett, M. Mazel, J. Matsunaga, P. N. Levett, and C. A. Bolin. 2000. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect. Immun. 68:2276-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haake, D. A., C. Martinich, T. A. Summers, E. S. Shang, J. D. Pruetz, A. M. McCoy, M. K. Mazel, and C. A. Bolin. 1998. Characterization of leptospiral outer membrane lipoprotein LipL36: downregulation associated with late-log-phase growth and mammalian infection. Infect. Immun. 66:1579-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haake, D. A., M. K. Mazel, A. M. McCoy, F. Milward, G. Chao, J. Matsunaga, and E. A. Wagar. 1999. Leptospiral outer membrane proteins OmpL1 and LipL41 exhibit synergistic immunoprotection. Infect. Immun. 67:6572-6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalsow, C. M., and A. E. Dwyer. 1998. Retinal immunopathology in horses with uveitis. Ocul. Immunol. Inflamm. 6:239-251. [DOI] [PubMed] [Google Scholar]
- 24.Koizumi, N., and H. Watanabe. 2004. Leptospiral immunoglobulin-like proteins elicit protective immunity. Vaccine 22:1545-1552. [DOI] [PubMed] [Google Scholar]
- 25.Levett, P. N. 2001. Leptospirosis. Clin. Microbiol. Rev. 14:296-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsunaga, J., M. A. Barocchi, J. Croda, T. A. Young, Y. Sanchez, I. Siqueira, C. A. Bolin, M. G. Reis, L. W. Riley, D. A. Haake, and A. I. Ko. 2003. Pathogenic Leptospira species express surface-exposed proteins belonging to the bacterial immunoglobulin superfamily. Mol. Microbiol. 49:929-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsunaga, J., Y. Sanchez, X. Xu, and D. A. Haake. 2005. Osmolarity, a key environmental signal controlling expression of leptospiral proteins LigA and LigB and the extracellular release of LigA. Infect. Immun. 73:70-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McBride, A. J., D. A. Athanazio, M. G. Reis, and A. I. Ko. 2005. Leptospirosis. Curr. Opin. Infect. Dis. 18:376-386. [DOI] [PubMed] [Google Scholar]
- 29.Naiman, B. M., D. Alt, C. A. Bolin, R. Zuerner, and C. L. Baldwin. 2001. Protective killed Leptospira borgpetersenii vaccine induces potent Th1 immunity comprising responses by CD4 and γδ T lymphocytes. Infect. Immun. 69:7550-7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naiman, B. M., S. Blumerman, D. Alt, C. A. Bolin, R. Brown, R. Zuerner, and C. L. Baldwin. 2002. Evaluation of type 1 immune response in naïve and vaccinated animals following challenge with Leptospira borgpetersenii serovar Hardjo: involvement of WC1+ γδ and CD4 T cells. Infect. Immun. 70:6147-6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nally, J. E., M. C. Fishbein, D. R. Blanco, and M. A. Lovett. 2005. Lethal infection of C3H/HeJ and C3H/SCID mice with an isolate of Leptospira interrogans serovar Copenhageni. Infect. Immun. 73:7014-7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nally, J. E., J. F. Timoney, and B. Stevenson. 2001. Temperature-regulated protein synthesis by Leptospira interrogans. Infect. Immun. 69:400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palaniappan, R. U., Y. F. Chang, F. Hassan, S. P. McDonough, M. Pough, S. C. Barr, K. W. Simpson, H. O. Mohammed, S. Shin, P. McDonough, R. L. Zuerner, J. Qu, and B. Roe. 2004. Expression of leptospiral immunoglobulin-like protein by Leptospira interrogans and evaluation of its diagnostic potential in a kinetic ELISA. J. Med. Microbiol. 53:975-984. [DOI] [PubMed] [Google Scholar]
- 34.Palaniappan, R. U., Y. F. Chang, S. S. Jusuf, S. Artiushin, J. F. Timoney, S. P. McDonough, S. C. Barr, T. J. Divers, K. W. Simpson, P. L. McDonough, and H. O. Mohammed. 2002. Cloning and molecular characterization of an immunogenic LigA protein of Leptospira interrogans. Infect. Immun. 70:5924-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parma, A. E., M. E. Sanz, P. M. Lucchesi, J. Mazzonelli, and M. A. Petruccelli. 1997. Detection of an antigenic protein of Leptospira interrogans which shares epitopes with the equine cornea and lens. Vet. J. 153:75-79. [DOI] [PubMed] [Google Scholar]
- 36.Shang, E. S., M. M. Exner, T. A. Summers, C. Martinich, C. I. Champion, R. E. Hancock, and D. A. Haake. 1995. The rare outer membrane protein, OmpL1, of pathogenic Leptospira species is a heat-modifiable porin. Infect. Immun. 63:3174-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smyth, J. A., D. A. Fitzpatrick, and W. A. Ellis. 1999. Stillbirth/perinatal weak calf syndrome: a study of calves infected with Leptospira. Vet. Rec. 145:539-542. [DOI] [PubMed] [Google Scholar]
- 38.Thiermann, A. B. 1984. Leptospirosis: current developments and trends. J. Am. Vet. Med. Assoc. 184:722-725. [PubMed] [Google Scholar]
- 39.Yip, Y. L., G. Smith, and R. L. Ward. 2001. Comparison of phage pIII, pVIII and GST as carrier proteins for peptide immunisation in BALB/c mice. Immunol. Lett. 79:197-202. [DOI] [PubMed] [Google Scholar]