Abstract
In the present work, we show that intracellular Trypanosoma cruzi is rarely found in the livers of acutely infected mice, but inflammation is commonly observed. The presence of numerous intrahepatic amastigotes in infected gamma interferon (IFN-γ)-deficient mice corroborates the notion that the liver is protected by an efficient local immunity. The contribution of different cell populations was suggested by data showing that CD4- and CD8-deficient mice were able to restrain liver parasite growth. Therefore, we have characterized the liver-infiltrating lymphocytes and determined the sources of IFN-γ during acute T. cruzi infection. We observed that natural killer (NK) cells increased by day 7, while T and B cells increased by day 14. Among CD3+ cells, CD4+, CD8+, and CD4− CD8− cell populations were greatly expanded. A large fraction of CD3+ cells were positive for PanNK, a β1 integrin expressed by NK and NK T cells. However, these lymphocytes were not classic NK T cells because they did not express NK1.1 and showed no preferential usage of Vβ8. Otherwise, liver NK T (CD3+ NK1.1+) cells were not increased in acutely infected mice. The majority of PanNK+ CD4+ and PanNK+ CD8+ cells expressed T-cell receptor αβ (TCRαβ), whereas PanNK+ CD4− CD8− cells were positive for TCRγδ. In fact, γδ T cells showed the most remarkable increase (40- to 100-fold) among liver lymphocytes. Most importantly, intracellular analysis revealed high levels of IFN-γ production at day 7 by NK cells and at day 14 by CD4+, CD8+, and CD4− CD8− TCRγδ+ cells. We concluded that NK cells are a precocious source of IFN-γ in the livers of acutely infected mice, and, as the disease progresses, conventional CD4+ and CD8+ T cells and γδ T cells, but not classic NK-T cells, may provide the IFN-γ required for liver protection against T. cruzi.
During acute infection by Trypanosoma cruzi, the causative agent of Chagas' disease, a hepatic affection has been demonstrated both in human patients (7, 53) and experimental animals (4, 9, 47), i.e., hepatomegaly, one of the clinical features mentioned by Carlos Chagas in the original description of the illness that bears his name (12). A priori, macrophages, Kupffer cells, and hepatocytes (41) can be colonized by T. cruzi parasites. Moreover, because it contains cells from the mononuclear phagocytic system, the liver plays a major role in the clearance of blood trypomastigotes, a function that gains special relevance after the generation of specific antibodies (51).
A preliminary histological study by our research group of the livers of acutely infected mice revealed the presence of inflammatory infiltration, but amastigote nests were rare, a surprising result since these animals had high levels of parasitemia. The paucity of liver T. cruzi organisms could be due to an efficient control of the parasite by the local immune system. This is an attractive hypothesis, considering that the liver is a peculiar organ, which guarantees both an effective first line of protection against pathogens (52) and a tolerogenic response to gut-derived antigens that come through the portal vein (64).
Many different cell types and soluble molecules have been shown to participate in the systemic control of T. cruzi parasites. Mice lacking B cells or helper or cytotoxic T cells (46, 57, 60) and mice deficient in gamma interferon (IFN-γ), interleukin-12 (IL-12), tumor necrosis factor α, or granulocyte-macrophage colony-stimulating factor are highly susceptible to infection (2, 33, 42, 59). The major protective role of IFN-γ suggests that parasite control is dependent on the TH1 pathway of immune response. IFN-γ not only activates macrophages to destroy ingested parasites (63) but also recruits mononuclear cells through chemokine production, induces immunoglobulin isotype switch to cytophilic and complement-fixing immunoglobulin G2a antibodies (54), and up-regulates the expression of high-affinity Fc receptors (FcγRI) on macrophages, which increase parasite clearance (61).
Natural killer (NK) cells also seem to play a protective role at the early stages of infection (48). The role of NK T cells is, nevertheless, controversial, since opposite conclusions can be taken from reports that evaluated the course of T. cruzi infection in CD1d knockout (CD1dKO) and Jα281KO mice, two strains known to be deficient in the NK T cell population (18, 19, 38, 44). These discrepancies could be attributed to the source of T. cruzi used in these studies or to the differences between the two KO strains, inasmuch as besides classic NK T cells, which express the invariant T-cell receptor ([TCR] Jα281), CD1dKO mice lack nonclassic NK T cells that use other TCRs (18).
The phenotypic and functional characterization of the different lymphocyte populations infiltrating the liver during the acute T. cruzi infection has not been thoroughly investigated. This analysis could be of great value to unravel the elements involved in the local control of the parasite. Besides that, it will permit evaluation from the beginning of the infection of the anti-T. cruzi effector mechanisms in a nonlymphoid tissue, a perspective ignored by most of ex vivo analyses that focus either the blood or the secondary lymphoid organs. Moreover, as the liver is an organ that selectively recruits NK and NK T cells (17), this study could contribute to elucidating the role of these cell populations in the immune response to the parasite.
In this paper, we have approached the phenotypic characterization of IFN-γ-producing cells in the liver in acutely infected mice. In order not to draw conclusions that may reflect the peculiarities of a particular mouse strain, this study was carried out with four different mouse strains that are known to differ in their susceptibility to T. cruzi.
MATERIALS AND METHODS
Mice.
Six to eight-week-old C57BL/6, BALB/c, A/J, C3H/HePAS (lipopolysaccharide-responsive) (55), C57BL/6 IFN-γ-ΚΟ (16), C57BL/6 CD4KO, and C57BL/6 CD8KO female mice were bred under specific pathogen-free conditions at the Isogenic Mice Facility, Instituto de Ciências Biomédicas, Universidade de São Paulo, Brazil.
Parasites and infection.
T. cruzi from the Y strain was maintained by weekly passages in A/J mice. Mice were infected with 1,000 trypomastigote blood forms. Parasitemia levels were determined by microscopic examination of 5-μl blood samples obtained from the tail vein.
Isolation of intrahepatic leukocytes.
Intrahepatic leukocytes were isolated as previously described (15). Briefly, after perfusion with phosphate-buffered saline, the liver was removed, and a cellular suspension was prepared, treated with collagenase 0.2% (Invitrogen, Carlsbad, CA), washed, admixed with 40% metrizamide (Sigma, St. Louis, MO) solution in phosphate-buffered saline, and gently overlaid with RPMI 1640 medium supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), 2-mercaptoethanol (50 μM), l-glutamine (2 mM), sodium pyruvate (1 mM), and 1% heat-inactivated fetal calf serum. Culture medium and supplements were purchased from Invitrogen. After centrifugation at 1,500 × g and 4°C, leukocytes were harvested from the medium-metrizamide interphase.
Phenotypic characterization of intrahepatic leukocyte populations.
The phenotype of intrahepatic leukocytes was determined using a three-color FACSCalibur cytometer (Becton-Dickinson, San Jose, CA), after cells were stained with fluorescein isothiocyanate-, phycoerythrin-, Cy-Chrome-, or biotin-conjugated monoclonal antibodies (MAbs) to CD4 (clone H129.19), CD8 (clone 53-6.7), B220 (clone RA3-6B2), CD3 (clone 145-2C11), TCRαβ (clone H57-597), TCRγδ (clone GL3), Vβ8.1 and 8.2 (clone F23.1), CD11b (Mac-1; clone M1/70), CD11c (clone HL-3), Ly6G (clone RB6-8C5), NK1.1 (clone PK136), and PanNK (clone DX5) purchased from PharMingen (San Diego, CA). When biotin-conjugated MAbs were used, fluorochrome-labeled streptavidin (PharMingen) was added as a second-step reagent. The number of each cell population per liver was determined by multiplying its respective frequency among liver leukocytes by the total number of leukocytes per liver estimated in a Neubauer chamber.
Intracellular detection of IFN-γ.
Intrahepatic leukocytes were cultured overnight with Golgistop at 37°C in a 5% CO2 atmosphere, according to the manufacturer's instructions, in the presence or absence of plate-bound anti-CD3 (10 μg/ml; clone 145-2C11) and soluble anti-CD28 (2 μg/ml; clone 37.51) MAbs. After being washed, cells were surface stained with fluorescein isothiocyanate- or Cy-Chrome-conjugated MAbs to CD4, CD8, NK1.1, and TCRγδ. Cells were then fixed with the Cytofix/Cytoperm buffer and incubated with phycoerythrin-labeled MAb to IFN-γ (XMG-1.2) diluted in Perm/Wash buffer. The analysis was done in a FACSCalibur cytometer. All reagents were purchased from PharMingen.
Histopathological analysis.
Liver and heart tissue specimens were collected and fixed in paraformaldehyde (Merck, La Jolla, CA) for further processing. Paraffin-embedded tissue sections were stained with hematoxylin-eosin and analyzed by optical microscopy. The hepatic inflammatory infiltrates were photographed using an image analysis system (Image Pro Plus Media Cybernetics, Silver Spring, Md.).
Statistics.
Statistical analysis was performed by analysis of variance and Tukey's multiple comparison tests, using GraphPad PRISM 4 software. Differences between two groups were considered significant at P values of <0.05.
RESULTS
T. cruzi parasitism in the liver of acutely infected mice of different strains.
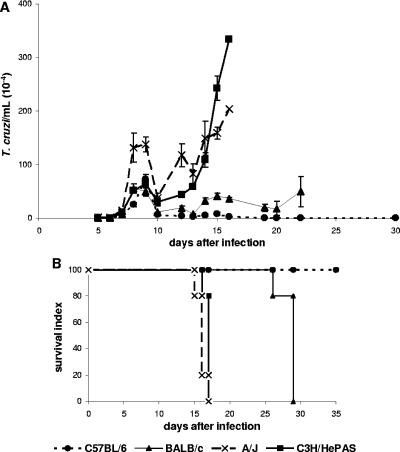
To evaluate whether the paucity of T. cruzi cells in the liver is a general phenomenon, mice of four different isogenic strains were infected with 1,000 blood forms of the Y strain, a virulent and reticulotropic parasite isolate (1). According to parasitemia curves and survival indexes (Fig. 1), C57BL/6 mice displayed a resistant phenotype, with a low first parasitemia peak, a negligible second peak, and absence of mortality. A/J and C3H/HePAS mice were highly susceptible, with 100% of the animals dying during an abrupt second parasitemia peak. BALB/c mice exhibited an intermediate phenotype, with a low second parasitemia peak and increased survival time in relation to A/J and C3H/HePAS mice.
FIG. 1.
Parasitemia curves and survival index in acutely T. cruzi-infected C57BL/6, BALB/c, A/J, and C3H/HePAS mice. Mice were infected with 1,000 blood forms of T. cruzi, and the parasitemias were screened by microscopic observation of 5-μl blood aliquots at different time points after infection. In panel A, results represent the mean ± SD of parasitemia levels (n = 5). In panel B, results represent the survival index (n = 5). A representative experiment (out of three experiments) is shown.
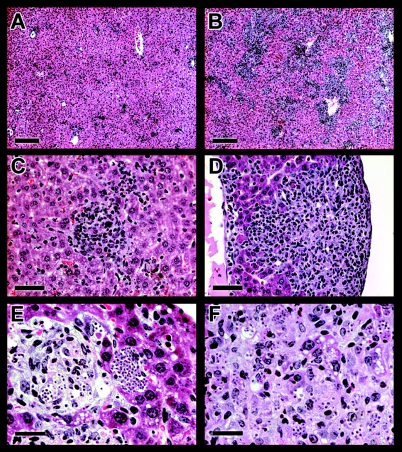
Analysis of liver sections up to days 14 to 16 postinfection (p.i.) revealed the presence of cell infiltrates, which was more pronounced in C57BL/6 (Fig. 2A) and BALB/c mice than in C3H/HePAS and A/J mice (data not shown). Liver lesions consisted of focal infiltrates all over the hepatic lobules. Because of their intrasinusoid location and the presence of hyaline secretion, cell aggregates often had a pseudo-granuloma appearance (Fig. 2C). Mononuclear cells, a few neutrophils, and, rarely, eosinophils constitute these focal infiltrates. Liver inflammation was discrete at day 7 (focal infiltrates occupied less than 2% of the hepatic tissue), but it increased by days 12 to 14 p.i., both as intrasinusoidal focal aggregates and as a diffuse infiltration of mononuclear cells scattered along the sinusoids.
FIG. 2.
Histopathological analysis of the liver in acutely T. cruzi-infected C57BL/6 and IFN-γ-KO mice. Representative photographs of C57BL/6 (A and C) and IFN-γ-KO (B, D, E, and F) mice at days 14 and 12 p.i., respectively. Parasite nests (E) and scattered amastigotes (F) amid infiltrates in the livers of infected IFN-γ-KO mice are shown. Scale bars, 200 μm (A and B), 50 μm (C and D), and 25 μm (E and F).
In spite of this intense inflammatory scenario and of the presence of blood parasites, we were unable to visualize liver T. cruzi nests in most of these mice, though at least six nonconsecutive slides were carefully screened. Moreover, the few amastigote nests showed signs of degeneration. These results were in sharp contrast to those of the heart, where T. cruzi nests were found in the myocardium of all mice of the four strains, in most cases without surrounding infiltrates or degenerative signs (data not shown). Taken together, these results indicate that the difficulty in visualizing T. cruzi parasites in the liver during acute infection is a general phenomenon that occurs in mice with different levels of parasitemia.
Contribution of IFN-γ and CD4+ and CD8+ T cells for liver protection against T. cruzi.
The scarcity of T. cruzi nests found in the livers of acutely infected mice could be due to low invasion of hepatic cells and/or to efficient control of the parasite by local immunity. To evaluate the latter possibility, we did a histopathological analysis of the livers of infected mice lacking IFN-γ, a key mediator of the anti-T. cruzi immune response (24, 59). At day 10 p.i., liver sections of IFN-γ-KO mice revealed the presence of parasite nests, as well as extracellular amastigotes scattered among the inflamed tissue, a picture that dramatically increased by day 12 p.i. (Fig. 2E and F). At this time, liver inflammatory infiltrates in IFN-γ-KO mice were considerably more intense (Fig. 2B and D) and, as reported in mycobacteria infections (20), contained higher numbers of eosinophils (Fig. 2F) than C57BL/6 mice (Fig. 2A and C) or than mice of the other nondeficient strains (data not shown). Multiple necrotic foci were observed in the livers of acutely infected IFN-γ-KO mice. In accordance with its susceptible phenotype, IFN-γ-KO mice showed high levels of parasitemia, with the majority of them dying around day 14 p.i. (data not shown). These results indicate that T. cruzi replication inside liver cells is tightly controlled by the immune system and, most importantly, that IFN-γ has a major role in this process.
To evaluate the contribution of CD4+ and CD8+ T cells for liver protection against T. cruzi, we then analyzed the parasitemia and the hepatic lesions in acutely infected CD4KO and CD8KO mice. Liver inflammation in these mice was notably less intense than in infected IFN-γ-KO mice but slightly higher than in infected C57BL/6 mice, with maximum values attained from days 13 to 20 p.i. (data not shown). Unlike results in IFN-γ-KO mice, parasites were not detected in liver sections of CD4KO and CD8KO mice until day 13 of infection (Table 1). At day 13 p.i., 9 to 12 amastigote nests were found per liver slide of these mice, which contrasted with both the scarcity of parasites detected in C57BL/6 mice and the massive T. cruzi replication observed in IFN-γ-KO mice. Interestingly, by day 16 p.i., the number of amastigote nests in the livers of CD4KO and CD8KO mice declined to 0.3 ± 0.5 (mean ± standard deviation [SD]) and 1.2 ± 2.1, respectively, and remained at this low level up to day 20 p.i. The apparent control of liver parasites in CD4KO or CD8KO mice at the late acute phase contrasted with their parasitemia curves, which, although not different from those of C57BL/6 mice up to day 13 p.i., steadily increased from this time point until the death of mice around day 20 p.i. (data not shown). Moreover, in infected CD4KO and CD8KO mice, the evolution of tissue parasitism in the liver differed from that observed in the heart, where the number of amastigote nests persisted at similarly high levels from days 13 to 20 p.i. (data not shown). From these experiments, we concluded that neither CD4+ T cells nor CD8+ T cells are essential for liver protection against T. cruzi, even though the presence of both T cell populations ensures an earlier parasite control.
TABLE 1.
Liver parasitism in the acute phase of T. cruzi infectiona
| No. of days p.i. | No. of amastigote nests/liver slide in mouse strain:
|
|||
|---|---|---|---|---|
| WT | IFN-γ KO | CD4 KO | CD8 KO | |
| 9 | ND | 1.4 ± 1.2 | ND | ND |
| 13 | 0.2 ± 0.3 | >50.0 | 9.8 ± 3.8 | 12.3 ± 3.0 |
| 16 | ND | 0.3 ± 0.5 | 1.2 ± 2.1 | |
| 20 | ND | 0.3 ± 0.5 | 1.2 ± 1.3 | |
Wild-type and deficient mice infected by Y strain T. cruzi parasites were sacrificed at the indicated days, and their livers were analyzed for the presence of amastigote nests. Results represent the mean (±SD) amastigote nests of six non-consecutive hematoxilin-eosin stained slides, for each mouse, for five mice from each group and time point. Results from a representative experiment out of two experiments are shown. ND, not detected.
Analysis of leukocyte populations in the liver of acutely infected mice.
To analyze the leukocyte populations involved in liver protection against T. cruzi, we have characterized by flow cytometry the infiltrating cells of perfused livers at days 7 and 14 of infection. In C57BL/6, C3H/HePAS, BALB/c and A/J mice, the numbers of cells isolated from the liver progressively increased in parallel with acute infection (data not shown). Moreover, confirming the histological analysis, acutely infected C57BL/6 mice showed higher numbers of infiltrating cells, followed by BALB/c mice, compared with the other mouse strains.
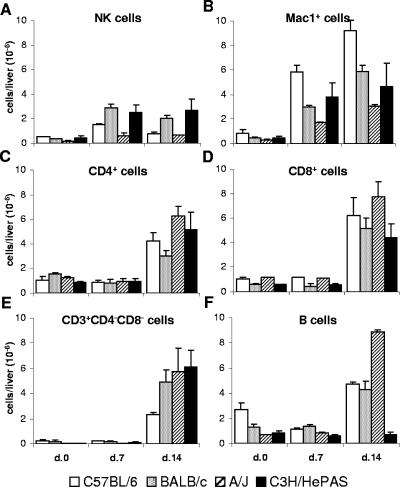
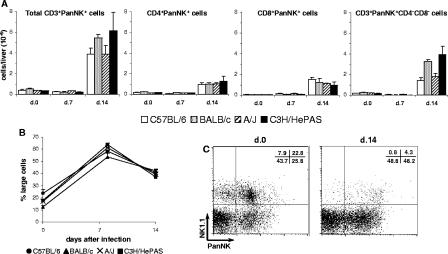
The overall picture of lymphocyte subsets was similar in the four mouse strains and consisted of an early increase in NK (PanNK+ CD3−) cells and a late increase in T and B cells (Fig. 3). At day 7 p.i., PanNK+ CD3− cells predominated among liver lymphocytes in the four mouse strains, with total numbers reaching three to eight times those of noninfected controls (Fig. 3A). In infected C3H/HePAS and BALB/c mice, PanNK+ CD3− cells attained remarkable frequencies of up to 30% of liver leukocytes. PanNK+ CD3− cells were already increased at day 4 p.i, with their frequency among liver leukocytes, but not their total numbers per liver, even higher than at day 7 p.i. (data not shown). At day 14 p.i., total numbers of liver PanNK+ CD3− cells remained high (Fig. 3A), but their percentages decreased due to a sharp increase in T- and B-cell populations (Fig. 3C to F). Liver-infiltrating T cells included CD4+, CD8+, and CD3+ CD4− CD8− cells. Interestingly, in the different mouse strains, the increase in liver lymphocyte populations was always higher for CD8+ cells than for CD4+ cells. Thus, while in noninfected livers the CD4+/CD8+ cell ratio ranged from 1.3 to 3.5 at days 7 and 14 p.i., it dropped to 0.9 to 1.8 and 0.6 to 1.2, respectively. The number of B (B220HIGH) cells also increased in the livers of acutely infected mice; for T cells, the increase was only evident by day 14 of infection. Concerning the peculiarities of the different mice, it is worth noting the low numbers of CD3+ CD4− CD8− cells in C57BL/6 mice (P < 0.05) and B cells in C3H/HePAS mice (P < 0.001)at day 14 p.i., compared to mice from the other strains. A complementary observation was that infected C3H/HePAS mice presented low numbers of B cells not only in the liver but also in the spleen (data not shown), indicating that the B cell response to T. cruzi is impaired in these mice.
FIG. 3.
Total numbers per liver of the different cell populations in acutely T. cruzi-infected C57BL/6, BALB/c, A/J, and C3H/HePAS mice. Seven and fourteen days after infection, intrahepatic leukocytes were isolated from perfused livers. The total numbers of NK (PanNK+ CD3− Mac1LOW) cells (A), macrophages and PMN cells (Mac1HIGH PanNK− CD3− B220−) (B), CD4+ (CD4+ CD8− CD3+) T cells (C), CD8+ (CD4− CD8+ CD3+) T cells (D), double-negative (CD4− CD8− CD3+) T cells (E), and B (B220HIGH) cells (F) were estimated as described in Material and Methods. Noninfected animals were used as controls. Results represent the mean ± SD (n = 3) of a representative experiment (out of three experiments).
Besides lymphocytes, for the four mouse strains, a notable increase in the number of Mac1HIGH PanNK− CD3− B220− liver cells was observed at day 7 p.i., with a little increment at day 14 p.i. (Fig. 3B). These nonlymphocytic hepatic leukocytes, which reached higher numbers in infected C57BL/6 mice (P < 0.05, at day 14 p.i.), included cells with size and granularity typical of macrophages and polymorphonuclear (PMN) cells. Moreover, a fraction of these cells expressed high levels of Ly6G (Gr-1, a PMN cell marker), both in controls (21 to 33% for the different strains) and in infected mice (53 to 57% at day 7 p.i. and 46 to 61% at day 14 p.i.). Microscopic analysis of Giemsa-stained slides of liver leukocyte suspensions confirmed the increase of PMN cells during the acute infection (data not shown).
Characterization of NK cells in the liver of acutely infected mice.
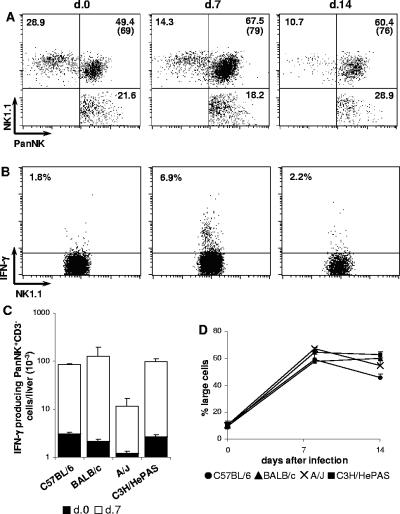
NK cells constitute an early source of IFN-γ in infections by diverse pathogens, including T. cruzi (10). In Fig. 3A, NK cells were characterized by the absence of CD3 and the presence of VLA-2 α chain (CD49b, recognized by PanNK MAb), a β1 integrin mostly expressed by NK and NK T cells (6). To confirm that PanNK+ CD3− cells were indeed NK cells, CD3− cells were analyzed for the concomitant expression of PanNK and NK1.1. This study was done in C57BL/6 mice once the NK1.1 epitope detected by PK136 MAb was restricted to this strain. As shown in Fig. 4A, at days 7 and 14 p.i., 79% and 76% of PanNK+ CD3− cells, respectively, coexpressed NK1.1, confirming that this population is mainly composed by NK cells. In the CD3− population, we also observed small percentages of NK1.1+ PanNK− and NK1.1− PanNK+ cells. Most importantly, after T. cruzi infection, a large fraction of liver NK cells spontaneously produced IFN-γ, a phenomenon that attained its maximum at day 7 p.i. In Fig. 4B, we show the data from a representative experiment in which 6.9% ± 1.5% of NK cells from C57BL/6 mice at day 7 p.i. produced IFN-γ. Indeed, at this time of infection, NK cells comprised around 90% of all IFN-γ-producing cells in the liver. Considering the total numbers of IFN-γ-producing NK cells, an 8- to 58-fold increase was observed at day 7 p.i. in relation to noninfected controls, although A/J mice presented significantly lower numbers compared to the other mouse strains (P < 0.01) (Fig. 4C). From these results, we concluded that NK cells are an early source of IFN-γ in the livers of T. cruzi-infected mice. The notable accumulation of PanNK+ CD3− large cells in the four mouse strains (Fig. 4D) confirmed that activation/proliferation of NK cells is a general phenomenon in a liver response to T. cruzi parasites.
FIG. 4.
Characterization of NK cells in the livers of acutely T. cruzi-infected C57BL/6, BALB/c, A/J and C3H/HePAS mice. Seven and fourteen days after infection, intrahepatic lymphocytes were isolated from perfused livers and analyzed by fluorescence-activated cell sorting. Noninfected animals were used as controls. In panel A, dot plots of a representative C57BL/6 mouse show the coexpression of PanNK and NK1.1 among gated CD3− cells (expressing either one or both these markers). Numbers represent the mean of cell percentages in each region from mice (n = 3) of the same experiment (out of three experiments). Numbers in parenthesis indicate the percentage of PanNK+CD3− cells coexpressing NK1.1. In panel B, dot plots of a representative C57BL/6 mouse show the spontaneous IFN-γ production by gated NK1.1+ CD3− cells, estimated by intracellular staining. Numbers represent the mean of the percentages of IFN-γ-producing NK1.1+ CD3− cells from mice (n = 3) of the same experiment (out of three experiments). In panel C, total numbers of IFN-γ-producing PanNK+CD3− cells in the livers of mice of the four strains are given. Bars represent the mean ± SD (n = 3) of a representative experiment (out of two experiments). In panel D, the percentages of large cells among PanNK+CD3−Mac1LOW cells in the livers of the four mouse strains are given. Results represent the mean ± SD (n = 3) of a representative experiment (out of three experiments).
Characterization of NK T cells in the liver of acutely infected mice.
NK T cells are involved in liver protection against infections by diverse intracellular parasites (3, 21). To investigate the participation of this subset in a liver response to T. cruzi, we analyzed the CD3+ cell population according to the expression of NK cell markers. At first, we noticed that a considerable fraction of T cells in the livers of noninfected mice was positive for PanNK, ranging from 15 to 30% of CD3+ cells in the different mouse strains (data not shown). According to CD4 and CD8 expression, liver PanNK+ CD3+ cells had a CD4+, CD8+, or CD4− CD8− phenotype. At day 7 p.i., even though the numbers of PanNK+ CD3+ cells per liver were similar to those of noninfected controls (Fig. 5A), these cells seemed to be activated and proliferating since 50 to 60% of them were large compared to 20% in noninfected controls (Fig. 5B). Moreover, 1 week later, a drastic increase in three subsets was observed in the four mouse strains (Fig. 5A). At that time, no major difference was observed among the strains in terms of PanNK+ CD4+ and PanNK+ CD8+ cells. For PanNK+ CD3+ CD4− CD8− cells, however, infected C3H/HePAS and BALB/c mice presented the highest cell numbers per liver (P < 0.05), resulting in a 12- and 30-fold expansion in relation to noninfected controls, respectively.
FIG. 5.
Characterization of PanNK+ T cells in the livers of acutely T. cruzi-infected C57BL/6, BALB/c, A/J, and C3H/HePAS mice. Seven and fourteen days after infection, intrahepatic lymphocytes were isolated from perfused livers and analyzed by fluorescence-activated cell sorting. Noninfected animals were used as controls. In panel A, total numbers per liver of CD3+, CD4+, CD8+, and CD4− CD8− cells expressing PanNK in the four mouse strains are given. Results represent the mean ± SD (n = 3) of a representative experiment (out of three experiments). In panel B, percentages of large cells among PanNK+ CD3+ cells in the four mouse strains are given. Results represent the mean ± SD (n = 3) of a representative experiment (out of three experiments). In panel C, dot plots of a representative C57BL/6 mouse show the coexpression of PanNK and NK1.1 among gated CD3+ cells. Mean percentages (n = 3) of cells in each region from the same experiment (out of three experiments) are shown.
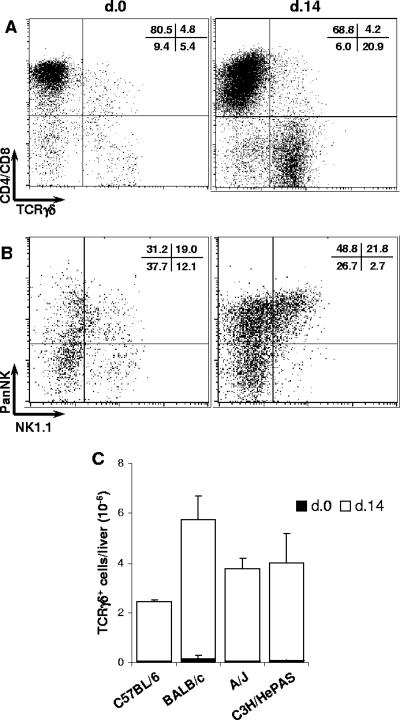
Analysis of the concomitant expression of CD3, PanNK, and NK1.1 by liver lymphocytes of C57BL/6 mice revealed that nearly half of the PanNK+ CD3+ cell population in noninfected mice was positive for NK1.1. Unexpectedly, however, less than 10% of PanNK+ CD3+ cells expressed NK1.1 at day 14 p.i. (Fig. 5C). In addition, considering the total numbers of cells per liver bearing the classic NK T cell phenotype (PanNK+ NK1.1+ CD3+), no significant change was observed in relation to noninfected controls (from [17.8 ± 9.4] × 104 cells in controls to [21.2 ± 4.1] × 104 cells at day 7 p.i. and [27.5 ± 13.1] × 104 cells at day 14 p.i.). To rule out the possibility that an increase in NK T cells occurred earlier than day 7 p.i., additional experiments were carried out in C57BL/6 mice at day 4 p.i. These studies (data not shown) confirmed that the PanNK+ NK1.1+ CD3+ cell population was not expanded in the livers of acutely infected mice, even though a huge fraction of infiltrating lymphocytes expressed the PanNK cell marker. Analysis of IFN-γ production by liver NK T cells of infected C57BL/6 mice revealed that 6.7% ± 2.9% and 3.9% ± 1.4% of CD3+ NK1.1+ cells produced IFN-γ at days 7 and 14 p.i., respectively, frequencies not significantly different from those found in controls (4.1% ± 1.9%). In terms of the total number of IFN-γ-producing cells, the contribution of NK-T cells at day 7 p.i. was approximately one-seventh of that of NK cells.
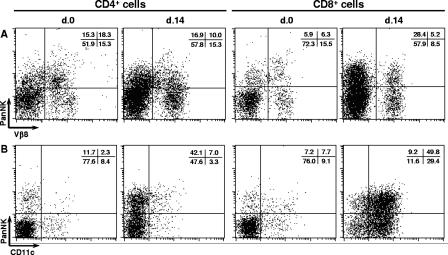
As deduced from data shown in Fig. 3 and 5, at day 14 p.i., around 30 to 60% of both CD4+ and CD8+ cells in the different mouse strains expressed PanNK, but the great majority was negative for NK1.1. To investigate the possibility that CD4+ PanNK+ and CD8+ PanNK+ cells were NK T cells that had lost NK1.1 expression following activation (43), we analyzed Vβ8 usage by these populations. This analysis was done because classic NK T cells preferentially use the Vβ8 segment of the TCR β chain together with an invariant α chain (32). First, we confirmed that liver CD4+ and CD8+ cells of acutely infected mice expressed TCRαβ (>95%) (data not shown). In noninfected mice, around 50% of both PanNK+ CD4+ cells and PanNK+ CD8+ cells were positive for Vβ8, compared to 15% and 25% in PanNK− CD4+ and PanNK− CD8+ cells, respectively (Fig. 6A). At day 14 p.i., Vβ8 usage within the PanNK− CD4+ and PanNK− CD8+ cell populations remained practically the same. However, in the PanNK+ CD4+ and PanNK+ CD8+ cell populations, the frequency of Vβ8+ cells was slightly (from 54.3% to 36.9%) or drastically (from 51.6% to 15.4%) reduced, respectively. These data indicate that after T. cruzi infection, a selective loss of Vβ8 usage occurs in both PanNK+ CD4+ and PanNK+ CD8+ cells, a result that argues against the notion that classic NK T cells predominate in these cell populations.
FIG. 6.
Expression of Vβ8 and CD11c by CD4+ or CD8+ T cells in the livers of acutely T. cruzi-infected C57BL/6 mice. Fourteen days after infection, intrahepatic lymphocytes were isolated from perfused livers and analyzed by fluorescence-activated cell sorting. Noninfected animals were used as controls. Dot plots of a representative C57BL/6 mouse show the coexpression of PanNK and Vβ8 (A) or CD11c (B) among gated CD4+ or CD8+ cells. Mean percentages (n = 3) of cells in each region from the same experiment (out of three experiments) are shown.
Because the molecule recognized by PanNK MAb is a β1 integrin, its expression in liver infiltrating lymphocytes of acutely infected mice could indicate that these cells have adopted a tissue migratory phenotype. To investigate further in this direction, we evaluated two β2 integrins, CD11c found in dendritic cells and other cell types and CD11b expressed in PMN cells, macrophages, NK cells, and migration-prone T cells (34). At day 14 p.i., while the percentage of CD4+ cells expressing CD11c was similar to that of controls (from 10.7% ± 2.1% to 10.3% ± 1.7%), a huge increase in the percentage of CD11c+ cells was found among CD8+ cells (from 16.8% ± 0.1% to 79.2% ± 2.9%) (Fig. 6B). Interestingly, the increase of CD11c+ cells affected both the CD8+ PanNK+ (from 51.6% ± 3.4% to 84.4% ± 2.4%) and CD8+ PanNK− (from 10.7% ± 1.3% to 71.7% ± 4.6%) cell subsets. The great majority of CD11c+ CD8+ cells was positive for CD3, and their size and granularity were characteristic of lymphocytes, confirming that these cells were not dendritic cells (data not shown). Additionally, at day 14 p.i., we also observed moderate increases in the percentages of CD4+ CD11b+ cells (from 10.8% ± 3.4% to 29.7% ± 6.9%) and CD8+ CD11b+ cells (from 9.5% ± 2.1% to 47.9% ± 10.0%). Taken together, our results show that T cells infiltrating the liver of acutely infected mice express β1 and β2 integrins, which can be involved in their homing to the inflamed hepatic tissue.
Characterization of γδ T cells in the liver of acutely infected mice.
Besides NK T cells, lymphocytes bearing TCRγδ (γδ T cells) frequently display a CD4− CD8− phenotype. γδ T cells can be found in very different locations, including the liver, where they can be involved in the protective response against infections (23). As liver CD3+ CD4− CD8− cells showed the most striking increase during acute T. cruzi infection (Fig. 3), we evaluated the expression of TCRγδ within the CD3+ cell population. As shown in Fig. 7A, 10% of CD3+ cells in the livers of noninfected C57BL/6 mice were positive for TCRγδ, a percentage that increased to 25% at day 14 of infection. Interestingly, 83.0% ± 1.9% of the TCRγδ+ cells in acutely infected C57BL/6 mice showed a double-negative phenotype. The increase in TCRγδ+ cells was a general feature of a liver response to T. cruzi. Thus, at day 14 p.i., considering the different mouse strains, a 40- to 100-fold increase in the total number of γδ T cells per liver was observed in relation to noninfected controls (Fig. 7C). As for other lymphocyte populations, TCRγδ+ cells seemed to be activated and proliferating, since 20 to 25% of them were large compared to 5 to 8% in noninfected controls (data not shown). On the other hand, it is worth noticing that, in acutely infected C57BL/6 mice, 71.2% ± 6.2% of TCRγδ+ cells were positive for PanNK and 22.3% ± 4.1% of them coexpressed NK1.1 (Fig. 7B). These results suggest that γδ T cells bearing NK cell markers participate in a liver response against T. cruzi during the acute phase of the disease.
FIG. 7.
Characterization of CD4− CD8− γδ T cells in the livers of acutely T. cruzi-infected C57BL/6 mice. Fourteen days after infection, intrahepatic lymphocytes were isolated from perfused livers and analyzed by fluorescence-activated cell sorting. Noninfected animals were used as controls. In panel A, dot plots of a representative C57BL/6 mouse show the coexpression of CD4/CD8 and TCRγδ among gated CD3+ cells. Mean percentages (n = 3) of cells in each region from the same experiment (out of three experiments) are shown. In panel B, dot plots of a representative C57BL/6 mouse show the coexpression of PanNK and NK1.1 among gated TCRγδ+ cells. Mean percentages (n = 3) of cells in each region from the same experiment (out of three experiments) are shown. In panel C, total numbers of TCRγδ+ cells in the livers of mice of the four strains. Bars represent the mean ± SD (n = 3) of a representative experiment (out of two experiments).
IFN-γ production by CD4+, CD8+, and TCRγδ+ CD4− CD8− liver cells of acutely infected mice.
During T. cruzi infection, both NK and T cells from the spleen and blood have been shown to produce IFN-γ (25, 27, 62), a cytokine that plays a crucial role in defense against this parasite (24, 59). To evaluate the contribution of the different T cell subsets for IFN-γ production in the livers of acutely infected mice, the production of this cytokine was examined by intracellular staining in C57BL/6 and C3H/HePAS mice that are opposite strains in terms of disease susceptibility.
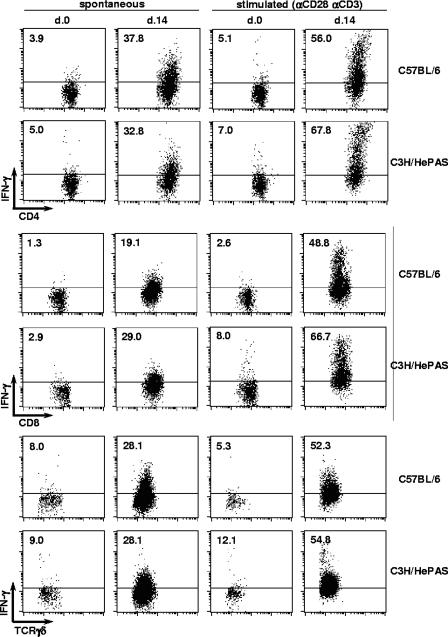
Spontaneous IFN-γ production by liver CD4+ and CD8+ T cells of both mouse strains was dramatically increased at day 14 p.i, with percentages of 37.8% ± 0.3% and 32.8% ± 2.5% for CD4+ cells and of 19.1% ± 2.5% and 29.0% ± 3.4% for CD8+ cells (Fig. 8). A small increase in IFN-γ production was already observed at day 7 p.i., with frequencies around 8% for both CD4+ and CD8+ cells (data not shown). At day 14 p.i., liver CD4− CD8− TCRγδ+ cells spontaneously producing IFN-γ were also enhanced in acutely infected C57BL/6 and C3H/HePAS mice, attaining percentages of 28.1% ± 0.2% and 28.1% ± 5.1%, respectively, while control values varied from 8.0% ± 0.9% to 9.0% ± 2.4% (Fig. 8). Moreover, in terms of total cells per liver, the numbers of IFN-γ producing CD4+, CD8+, and TCRγδ+ CD4− CD8− cells by day 14 p.i. were (3.1 ± 1.0) × 106, (2.5 ± 0.1) × 106, and (1.9 ± 0.4) × 106 in C57BL/6 mice and (2.9 ± 0.7) × 106, (3.4 ± 1.6) × 106 and (4.1 ± 1.4) × 106 in C3H/HePAS mice. It is worth remarking that, at this time of infection, CD4+, CD8+, and TCRγδ+ CD4− CD8− liver cells comprised the great majority of IFN-γ producing cells (data not shown).
FIG. 8.
Production of IFN-γ by CD4+, CD8+, and CD4− CD8− γδ T cells in the livers of acutely T. cruzi-infected C57BL/6 and C3H/HePAS mice. Fourteen days after infection, intrahepatic lymphocytes were isolated from perfused livers and analyzed by fluorescence-activated cell sorting. Noninfected animals were used as controls. Dot plots of a representative C57BL/6 or C3H/HePAS mouse show the spontaneous and anti-CD3/CD28-stimulated IFN-γ production by gated CD4+, CD8+, and γδ+ cells, estimated by intracellular staining. Mean percentages (n = 3) of IFN-γ-producing cells from the same experiment (out of three experiments) are shown.
To estimate the potential capacity of liver lymphocytes to produce IFN-γ, cells stimulated overnight with plate-bound anti-CD3/anti-CD28 MAbs were also analyzed. Following stimulation, the great majority of liver CD4+, CD8+, and γδ T liver cells from mice at day 14 p.i. became IFN-γ producers (Fig. 8), confirming their differentiation toward a TH1/TC1 phenotype. These data sharply contrasted with those of liver cells from noninfected mice that presented little IFN-γ production after anti-CD3/anti-CD28 stimulation, suggesting that other T-cell phenotypes exist in controls. In conclusion, during acute T. cruzi infection, similar levels of IFN-γ are produced by CD4+, CD8+, and CD4− CD8− TCRγδ+ T cells of resistant and susceptible mice.
DISCUSSION
In the present work, we have shown that the presence of inflammation associated with rare T. cruzi nests observed in the livers of acutely infected mice is a general phenomenon, occurring in C57BL/6, BALB/c, A/J, and C3H/HePAS mice, which differ in terms of systemic susceptibility to the parasite. This observation corroborates previous findings showing the existence of a hepatic inflammation (4, 7, 28, 53) with scarce presence of amastigote nests (26, 37, 58) in both chagasic patients and T. cruzi-infected mice. Interestingly, however, in our experiments, the lack of hepatic amastigote nests in more than half of the acutely infected mice should not be taken as an indication of the absence of T. cruzi, since, by using a sensitive amplification method (36), we were able to detect living parasites in perfused livers of mice of the four strains (data not shown).
The presence of low numbers of amastigote nests in the livers of resistant (C57BL/6), intermediate (BALB/c), and susceptible (C3H/HePAS and A/J) mice, in clear dissonance with parasitemia levels and heart parasitism, indicates that local factors may inhibit T. cruzi replication inside liver cells. IFN-γ seems to play a major role in liver protection against T. cruzi since acutely infected IFN-γ-KO mice presented a high level of hepatic parasitism. The relevance of IFN-γ for controlling hepatic parasitism was also indirectly suggested by our experiments in acutely infected CD4KO and CD8KO mice. Thus, the fact that both groups of deficient mice were able to restrain the parasite growth in the liver, but not systemically, supports the idea that the effector mechanism responsible for liver protection against T. cruzi is not peculiar to either CD4+ T cells or CD8+ T cells. IFN-γ-mediated mechanisms are an attractive possibility for this effector activity, since IFN-γ can be produced by several lymphocyte populations infiltrating the liver, including CD4+ and CD8+ T cells. Activation of Kupffer cells or even hepatocytes (30) and recruitment of macrophages are examples of effector mechanisms mediated by IFN-γ that could operate in local parasite control.
The precocious source of IFN-γ in the livers of acutely infected mice seems to be NK cells, a concept supported by the finding that an IFN-γ-producing PanNK+ CD3− cell population coexpressing NK1.1 in C57BL/6 mice drastically increases by day 7 of infection. The involvement of NK cells in the initial phase of the disease is also supported by the fact that, in the four mouse strains, a great proportion of liver PanNK+ CD3− cells showed an activated cell phenotype. These results extend to the liver previous observations suggesting that NK cells are early participants of the anti-T. cruzi response. Thus, acutely infected mice undergo an increase in spleen NK cell activity against tumor targets (5, 62), and NK cell depletion by anti-asialo GM1 or anti-NK1.1 antibodies results in parasitemia increases (10, 48, 62). Human NK cells also seem to respond to T. cruzi, inasmuch as stimulation of peripheral blood mononuclear cells from chagasic patients with parasite antigen boosts their NK cell cytotoxic activity (8). Accumulation of NK cells in the liver could result from cell recruitment mediated by locally released chemokines (49). Alternatively, it could be a consequence of cytokine-induced proliferation (40), a possibility supported by the high proportion of large cells within the liver NK cell population of acutely infected mice. In this respect, IL-12 produced by T. cruzi-infected macrophages (2) and released in acutely infected mice (62) could participate in the activation and proliferation of liver NK cells. This is indirectly supported by data showing that accumulation of NK cells is not observed in the spleen of T. cruzi-infected IL-12p35KO mice (39). Later on, at day 14 p.i., when the NK cell population shows no further increase, a noticeable increase in T-cell numbers occurs in the liver. The increase of T cells affects CD4+ and CD8+ cells and, more intensively, CD4− CD8− CD3+ cells, a population mainly composed of γδ T cells poorly represented in noninfected mice. Interestingly, very similar numbers of liver infiltrating T cells were observed in the four mouse strains, even though lower numbers of CD3+ CD4− CD8− cells (mostly γδ T cells) were present in C57BL/6 mice. By producing huge amounts of IFN-γ, all three liver T-cell populations guarantee a strong and diverse source of IFN-γ when its production by NK cells has already declined.
Besides IFN-γ-producing NK and T cells, other leukocyte populations were increased in the livers of acutely infected mice. Thus, macrophages and PMN cells were present in the four mouse strains from day 7 onward. These cells might contribute to T. cruzi control through phagocytosis of extracellular parasites. B cells were observed at day 14 p.i. in the livers of infected mice except in those of the C3H/HePAS strain, which also showed reduced B cell numbers in the spleen. This is an interesting matter that deserves further investigation, inasmuch as similar anti-parasite antibody titers were found in T. cruzi-infected C3H/He and C57BL/6 mice (45).
NK T cells participate in protection against diverse parasites (3, 21), by producing IFN-γ and by establishing a bridge between the innate and acquired immune responses. Since a large fraction of the CD4+, CD8+, and CD4− CD8− CD3+ cells in the livers of acutely infected mice were positive for the PanNK marker, the α chain of the VLA-2 integrin expressed by NK T cells (6), it was important to determine if NK T cells were included in the PanNK+ CD3+ cell population. Although classic NK T cells have CD4+ or CD4− CD8− phenotypes (32), PanNK+ CD8+ cells could still be nonclassic CD8+ NK T cells (22), a liver population increased during Plasmodium yoelii infection (35). However, our study suggests that during acute T. cruzi infection, most liver PanNK+ CD3+ cells are not NK T cells, considering that in infected C57BL/6 mice very few of them coexpress NK1.1, a more reliable NK T cell marker than PanNK, which is shared by a fraction of conventional T cells with effector functions (29, 56). This conclusion is reinforced by the fact that PanNK+ CD4+ and PanNK+ CD8+ cells, which express the TCRαβ, do not show a preferential usage of Vβ8, a TCR chain frequently used by classic NK T cells (32). Furthermore, follow-up of NK1.1+ CD3+ cells in acutely infected C57BL/6 mice revealed a progressive decrease in their frequencies among liver lymphocytes. A persistent reduction in the percentage of this cell population was also observed by Duthie et al. (19) in C57BL/6 mice infected with T. cruzi of the CL strain. These findings, however, do not rule out the participation of NK T cells in the acute liver response to T. cruzi, since the low production of IFN-γ by this population could be important for activating NK cells (31) and/or for T-cell polarization. Thus, in relation to the role of classic NK T cells in protection against acute T. cruzi infection (18, 19, 38, 44), our data indicate that this population does not show major expansions in the liver, nor does it directly contribute to protection with a strong IFN-γ production.
γδ T cells can be found in different tissues, where they are involved in innate immune responses, operating through recognition of pathogen-derived molecules (14) or of autologous proteins overexpressed as a consequence of infection, cell disregulation, or tumor transformation (13). In contrast to classic NK T cells, liver γδ T cells expressing a CD4− CD8− phenotype showed the most notable increase during acute T. cruzi infection. Except for C57BL/6 mice, this cell population attained values of the same magnitude or even higher than those of CD4+ and CD8+ T cells. In addition, a large fraction of γδ T cells in acutely infected C57BL/6 mice coexpressed PanNK and NK1.1, which could indicate the presence of a recently defined rare population of nonclassic NK T cells (22). Indeed, the most remarkable observation was that γδ T cells spontaneously produce IFN-γ, a fact compatible with a proinflammatory protective effect against T. cruzi. This observation extends previous studies showing that, at the beginning of infection by different viruses and bacteria, γδ T cells are frequently proinflammatory but, later on, when parasite proliferation is under control, they mediate regulatory functions (11). The fact that T. cruzi infection in γδ T-cell-deficient mice results in milder inflammatory heart lesions and lower mortality compared to control mice (50) reinforces the proinflammatory role of this population.
The present study suggests that a broad array of lymphocyte populations, such as NK, γδ T, CD4+ T, and CD8+ T cells, may provide the IFN-γ required for liver protection against acute T. cruzi infection. Moreover, a clear polarization to the type 1 functional profile is observed in T cells that infiltrate the liver of acutely infected mice, as evidenced by the boost in IFN-γ production upon in vitro restimulation with anti-CD3/anti-CD28 antibodies. Remarkably, IFN-γ production by liver CD4+, CD8+, and γδ T cells was found to attain similar levels in C57BL/6 and C3H/HePAS mice, two strains that show opposite behavior in terms of the ability to control the systemic proliferation of the parasite. This observation is of great relevance because it could explain why amastigote nests are rarely found in the livers of both resistant and susceptible mice. Considering the crucial role of IFN-γ in protection against T. cruzi infection, the fact that different liver populations respond to acute infection by secreting large amounts of IFN-γ could be very important to ensure the destruction of intrahepatic parasites, transforming the liver at a site where the effector arm of the immune response operates with great efficiency.
Acknowledgments
Financial support was provided by grants FAPESP, numbers 02/03133-7 and 00/13756-6, and CNPq, numbers 301291/85-3 and 305049/2004-6.
We acknowledge Rogério Silva do Nascimento and Bernardo Paulo Albe for technical support.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Alcantara, A., and Z. Brener. 1978. The in vitro interaction of Trypanosoma cruzi bloodstream forms and mouse peritoneal macrophages. Acta Trop. 35:209-219. [PubMed] [Google Scholar]
- 2.Aliberti, J. C., M. A. Cardoso, G. A. Martins, R. T. Gazzinelli, L. Q. Vieira, and J. S. Silva. 1996. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect. Immun. 64:1961-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amprey, J. L., J. S. Im, S. J. Turco, H. W. Murray, P. A. Illarionov, G. S. Besra, S. A. Porcelli, and G. F. Spath. 2004. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J. Exp. Med. 200:895-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade, Z. A., and E. A. Lopes. 1963. A histochemical study of experimental Chagas' disease. Rev. Inst. Med. Trop. Sao Paulo 19:236-242. [PubMed] [Google Scholar]
- 5.Antunez, M. I., and R. L. Cardoni. 2000. IL-12 and IFN-gamma production, and NK cell activity, in acute and chronic experimental Trypanosoma cruzi infections. Immunol. Lett. 71:103-109. [DOI] [PubMed] [Google Scholar]
- 6.Arase, H., T. Saito, J. H. Phillips, and L. L. Lanier. 2001. Cutting edge: the mouse NK cell-associated antigen recognized by DX5 monoclonal antibody is CD49b (alpha 2 integrin, very late antigen-2). J. Immunol. 167:1141-1144. [DOI] [PubMed] [Google Scholar]
- 7.Azogue, E., and C. Darras. 1995. Congenital Chagas in Bolivia: comparative study of the effectiveness and cost of diagnostic methods. Rev. Soc. Bras. Med. Trop. 28:39-43. [DOI] [PubMed] [Google Scholar]
- 8.Brodskyn, C. I., A. Barral, M. A. Bulhoes, T. Souto, W. C. Machado, and M. Barral-Netto. 1996. Cytotoxicity in patients with different clinical forms of Chagas' disease. Clin. Exp. Immunol. 105:450-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckner, F. S., A. J. Wilson, and W. C. Van Voorhis. 1999. Detection of live Trypanosoma cruzi in tissues of infected mice by using histochemical stain for β-galactosidase. Infect. Immun. 67:403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardillo, F., J. C. Voltarelli, S. G. Reed, and J. S. Silva. 1996. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect. Immun. 64:128-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carding, S. R., and P. J. Egan. 2002. Gammadelta T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2:336-345. [DOI] [PubMed] [Google Scholar]
- 12.Chagas, C. 1916. Tripanosomíase Americana: forma aguda da moléstia. Mem. Inst. Oswaldo Cruz 1:159-218. [Google Scholar]
- 13.Chien, Y. H., R. Jores, and M. P. Crowley. 1996. Recognition by gamma/delta T cells. Annu. Rev. Immunol. 14:511-532. [DOI] [PubMed] [Google Scholar]
- 14.Constant, P., F. Davodeau, M. A. Peyrat, Y. Poquet, G. Puzo, M. Bonneville, and J. J. Fournie. 1994. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science 264:267-270. [DOI] [PubMed] [Google Scholar]
- 15.Crispe, I. N. 1996. Isolation of mouse intrahepatic lymphocytes. Curr. Protoc. Immunol. 3:22-28. [DOI] [PubMed] [Google Scholar]
- 16.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 17.Doherty, D. G., and C. O'Farrelly. 2000. Innate and adaptive lymphoid cells in the human liver. Immunol. Rev. 174:5-20. [DOI] [PubMed] [Google Scholar]
- 18.Duthie, M. S., M. Kahn, M. White, R. P. Kapur, and S. J. Kahn. 2005. Critical proinflammatory and anti-inflammatory functions of different subsets of CD1d-restricted natural killer T cells during Trypanosoma cruzi infection. Infect. Immun. 73:181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duthie, M. S., M. Wleklinski-Lee, S. Smith, T. Nakayama, M. Taniguchi, and S. J. Kahn. 2002. During Trypanosoma cruzi infection CD1d-restricted NK T cells limit parasitemia and augment the antibody response to a glycophosphoinositol-modified surface protein. Infect. Immun. 70:36-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehlers, S., and E. Richter. 2001. Differential requirement for interferon-gamma to restrict the growth of or eliminate some recently identified species of nontuberculous mycobacteria in vivo. Clin. Exp. Immunol. 124:229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emoto, M., Y. Emoto, I. B. Buchwalow, and S. H. Kaufmann. 1999. Induction of IFN-gamma-producing CD4+ natural killer T cells by Mycobacterium bovis bacillus Calmette Guerin. Eur. J. Immunol. 29:650-659. [DOI] [PubMed] [Google Scholar]
- 22.Emoto, M., and S. H. Kaufmann. 2003. Liver NKT cells: an account of heterogeneity. Trends Immunol. 24:364-369. [DOI] [PubMed] [Google Scholar]
- 23.Emoto, M., M. Miyamoto, Y. Emoto, J. Zerrahn, and S. H. Kaufmann. 2001. A critical role of T-cell receptor gamma/delta cells in antibacterial protection in mice early in life. Hepatology 33:887-893. [DOI] [PubMed] [Google Scholar]
- 24.Gazzinelli, R. T., I. P. Oswald, S. Hieny, S. L. James, and A. Sher. 1992. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur. J. Immunol. 22:2501-2506. [DOI] [PubMed] [Google Scholar]
- 25.Gomes, J. A., L. M. Bahia-Oliveira, M. O. Rocha, O. A. Martins-Filho, G. Gazzinelli, and R. Correa-Oliveira. 2003. Evidence that development of severe cardiomyopathy in human Chagas' disease is due to a Th1-specific immune response. Infect. Immun. 71:1185-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graefe, S. E., T. Jacobs, I. Gaworski, U. Klauenberg, C. Steeg, and B. Fleischer. 2003. Interleukin-12 but not interleukin-18 is required for immunity to Trypanosoma cruzi in mice. Microbes Infect. 5:833-839. [DOI] [PubMed] [Google Scholar]
- 27.Grisotto, M. G., M. R. D'Imperio Lima, C. R. Marinho, C. E. Tadokoro, I. A. Abrahamsohn, and J. M. Alvarez. 2001. Most parasite-specific CD8+ cells in Trypanosoma cruzi-infected chronic mice are down-regulated for T-cell receptor-alphabeta and CD8 molecules. Immunology 102:209-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamano, S., K. Himeno, Y. Miyazaki, K. Ishii, A. Yamanaka, A. Takeda, M. Zhang, H. Hisaeda, T. W. Mak, A. Yoshimura, and H. Yoshida. 2003. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity 19:657-667. [DOI] [PubMed] [Google Scholar]
- 29.Kambayashi, T., E. Assarsson, B. J. Chambers, and H. G. Ljunggren. 2001. Expression of the DX5 antigen on CD8+ T cells is associated with activation and subsequent cell death or memory during influenza virus infection. Eur. J. Immunol. 31:1523-1530. [DOI] [PubMed] [Google Scholar]
- 30.Klotz, F. W., L. F. Scheller, M. C. Seguin, N. Kumar, M. A. Marletta, S. J. Green, and A. F. Azad. 1995. Co-localization of inducible-nitric oxide synthase and Plasmodium berghei in hepatocytes from rats immunized with irradiated sporozoites. J. Immunol. 154:3391-3395. [PubMed] [Google Scholar]
- 31.Kronenberg, M. 2005. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 23:877-900. [DOI] [PubMed] [Google Scholar]
- 32.Lantz, O., and A. Bendelac. 1994. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J. Exp. Med. 180:1097-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lima, E. C., I. Garcia, M. H. Vicentelli, P. Vassalli, and P. Minoprio. 1997. Evidence for a protective role of tumor necrosis factor in the acute phase of Trypanosoma cruzi infection in mice. Infect. Immun. 65:457-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin, Y., T. J. Roberts, V. Sriram, S. Cho, and R. R. Brutkiewicz. 2003. Myeloid marker expression on antiviral CD8+ T cells following an acute virus infection. Eur. J. Immunol. 33:2736-2743. [DOI] [PubMed] [Google Scholar]
- 35.Mannoor, M. K., A. Weerasinghe, R. C. Halder, S. Reza, M. Morshed, A. Ariyasinghe, H. Watanabe, H. Sekikawa, and T. Abo. 2001. Resistance to malarial infection is achieved by the cooperation of NK1.1(+) and NK1.1(−) subsets of intermediate TCR cells which are constituents of innate immunity. Cell Immunol. 211:96-104. [DOI] [PubMed] [Google Scholar]
- 36.Marinho, C. R., D. Z. Bucci, M. L. Dagli, K. R. Bastos, M. G. Grisotto, L. R. Sardinha, C. R. Baptista, C. P. Goncalves, M. R. Lima, and J. M. Alvarez. 2004. Pathology affects different organs in two mouse strains chronically infected by a Trypanosoma cruzi clone: a model for genetic studies of Chagas' disease. Infect. Immun. 72:2350-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martins, L. P., R. E. Castanho, J. A. da Rosa, L. C. da Silva, C. A. de Godoy, and M. Rosa Rde. 2003. Biological and histopathological characterization together with nucleic acids analysis of a Trypanosoma cruzi strain from Marilia, Sao Paulo State. Rev. Soc. Bras. Med. Trop. 36:35-39. (In Portuguese.) [DOI] [PubMed] [Google Scholar]
- 38.Miyahira, Y., M. Katae, K. Takeda, H. Yagita, K. Okumura, S. Kobayashi, T. Takeuchi, T. Kamiyama, Y. Fukuchi, and T. Aoki. 2003. Activation of natural killer T cells by α-galactosylceramide impairs DNA vaccine-induced protective immunity against Trypanosoma cruzi. Infect. Immun. 71:1234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller, U., G. Kohler, H. Mossmann, G. A. Schaub, G. Alber, J. P. Di Santo, F. Brombacher, and C. Holscher. 2001. IL-12-independent IFN-gamma production by T cells in experimental Chagas' disease is mediated by IL-18. J. Immunol. 167:3346-3353. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen, K. B., T. P. Salazar-Mather, M. Y. Dalod, J. B. Van Deusen, X. Q. Wei, F. Y. Liew, M. A. Caligiuri, J. E. Durbin, and C. A. Biron. 2002. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 169:4279-4287. [DOI] [PubMed] [Google Scholar]
- 41.Nogueira de Melo, A. C., M. N. Meirelles, R. Porrozzi, J. D. Costa, M. H. Branquinha, and A. B. Vermelho. 2004. Reduced activity of matrix metalloproteinase-9 in Trypanosoma cruzi-infected mouse embryo hepatocyte cell. Hepatol. Res. 28:49-56. [DOI] [PubMed] [Google Scholar]
- 42.Olivares Fontt, E., C. Heirman, K. Thielemans, and B. Vray. 1996. Granulocyte-macrophage colony-stimulating factor: involvement in control of Trypanosoma cruzi infection in mice. Infect. Immun. 64:3429-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ota, T., K. Takeda, H. Akiba, Y. Hayakawa, K. Ogasawara, Y. Ikarashi, S. Miyake, H. Wakasugi, T. Yamamura, M. Kronenberg, D. H. Raulet, K. Kinoshita, H. Yagita, M. J. Smyth, and K. Okumura. 2005. IFN-gamma-mediated negative feedback regulation of NKT-cell function by CD94/NKG2. Blood 106:184-192. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Procopio, D. O., I. C. Almeida, A. C. Torrecilhas, J. E. Cardoso, L. Teyton, L. R. Travassos, A. Bendelac, and R. T. Gazzinelli. 2002. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins from Trypanosoma cruzi bind to CD1d but do not elicit dominant innate or adaptive immune responses via the CD1d/NKT cell pathway. J. Immunol. 169:3926-3933. [DOI] [PubMed] [Google Scholar]
- 45.Rowland, E. C., K. S. Mikhail, and T. S. McCormick. 1992. Isotype determination of anti-Trypanosoma cruzi antibody in murine Chagas' disease. J. Parasitol. 78:557-561. [PubMed] [Google Scholar]
- 46.Russo, M., P. Minoprio, A. Coutinho, and M. Hontebeyrie-Joskowicz. 1988. Depletion of L3T4+ T lymphocytes during acute Trypanosoma cruzi infection abolish macrophage and B lymphocyte activation but not tissue inflammatory reaction. Mem. Inst. Oswaldo Cruz 83:527-538. [DOI] [PubMed] [Google Scholar]
- 47.Russo, M., N. Starobinas, M. C. Marcondes, P. Minoprio, and M. Honteyberie-Joskowicz. 1996. The influence of T cell subsets on Trypanosoma cruzi multiplication in different organs. Immunol. Lett. 49:163-168. [DOI] [PubMed] [Google Scholar]
- 48.Sakai, T., H. Hisaeda, Y. Nakano, H. Ishikawa, Y. Maekawa, K. Ishii, Y. Nitta, J. Miyazaki, and K. Himeno. 2000. Gene gun-mediated delivery of an interleukin-12 expression plasmid protects against infections with the intracellular protozoan parasites Leishmania major and Trypanosoma cruzi in mice. Immunology 99:615-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salazar-Mather, T. P., J. S. Orange, and C. A. Biron. 1998. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein 1alpha (MIP-1alpha)-dependent pathways. J. Exp. Med. 187:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santos Lima, E. C., and P. Minoprio. 1996. Chagas' disease is attenuated in mice lacking gamma delta T cells. Infect. Immun. 64:215-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scott, M. T., and L. Moyes. 1982. 75Se-methionine labelled Trypanosoma cruzi blood trypomastigotes: opsonization by chronic infection serum facilitates killing in spleen and liver. Clin. Exp. Immunol. 48:754-757. [PMC free article] [PubMed] [Google Scholar]
- 52.Seki, S., Y. Habu, T. Kawamura, K. Takeda, H. Dobashi, T. Ohkawa, and H. Hiraide. 2000. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol. Rev. 174:35-46. [DOI] [PubMed] [Google Scholar]
- 53.Shikanai-Yasuda, M. A., C. B. Marcondes, L. A. Guedes, G. S. Siqueira, A. A. Barone, J. C. Dias, V. Amato Neto, J. E. Tolezano, B. A. Peres, E. R. Arruda Junior, et al. 1991. Possible oral transmission of acute Chagas' disease in Brazil. Rev. Inst. Med. Trop. Sao Paulo 33:351-357. [DOI] [PubMed] [Google Scholar]
- 54.Snapper, C. M., and W. E. Paul. 1987. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236:944-947. [DOI] [PubMed] [Google Scholar]
- 55.Starobinas, N., M. Russo, P. Minoprio, and M. Hontebeyrie-Joskowicz. 1991. Is TNF alpha involved in early susceptibility of Trypanosoma cruzi-infected C3H/He mice? Res. Immunol. 142:117-122. [DOI] [PubMed] [Google Scholar]
- 56.Taniguchi, M., and T. Nakayama. 2000. Recognition and function of Vα14 NKT cells. Semin. Immunol. 12:543-550. [DOI] [PubMed] [Google Scholar]
- 57.Tarleton, R. L. 1990. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J. Immunol. 144:717-724. [PubMed] [Google Scholar]
- 58.Toledo, M. J., M. de Lana, C. M. Carneiro, M. T. Bahia, G. L. Machado-Coelho, V. M. Veloso, C. Barnabe, M. Tibayrenc, and W. L. Tafuri. 2002. Impact of Trypanosoma cruzi clonal evolution on its biological properties in mice. Exp. Parasitol. 100:161-172. [DOI] [PubMed] [Google Scholar]
- 59.Torrico, F., H. Heremans, M. T. Rivera, E. Van Marck, A. Billiau, and Y. Carlier. 1991. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J. Immunol. 146:3626-3632. [PubMed] [Google Scholar]
- 60.Trischmann, T. M. 1983. Non-antibody-mediated control of parasitemia in acute experimental Chagas' disease. J. Immunol. 130:1953-1957. [PubMed] [Google Scholar]
- 61.Umekita, L. F., H. A. Takehara, and I. Mota. 1988. Role of the antibody Fc in the immune clearance of Trypanosoma cruzi. Immunol. Lett. 17: 85-89. [DOI] [PubMed] [Google Scholar]
- 62.Une, C., J. Andersson, M. L. Eloranta, D. Sunnemark, R. A. Harris, and A. Orn. 2000. Enhancement of natural killer (NK) cell cytotoxicity and induction of NK cell-derived interferon-gamma (IFN-gamma) display different kinetics during experimental infection with Trypanosoma cruzi. Clin. Exp. Immunol. 121:499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wirth, J. J., F. Kierszenbaum, G. Sonnenfeld, and A. Zlotnik. 1985. Enhancing effects of gamma interferon on phagocytic cell association with and killing of Trypanosoma cruzi. Infect. Immun. 49:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang, R., Q. Liu, J. L. Grosfeld, and M. D. Pescovitz. 1994. Intestinal venous drainage through the liver is a prerequisite for oral tolerance induction. J. Pediatr. Surg. 29:1145-1148. [DOI] [PubMed] [Google Scholar]