Abstract
Borrelia burgdorferi strains exhibit various degrees of infectivity and pathogenicity in mammals, which may be due to their relative ability to evade initial host immunity. Innate immune cells recognize B. burgdorferi by Toll-like receptors (TLRs) that use the intracellular molecule MyD88 to mediate effector functions. To determine whether impaired TLR signaling enhances Ixodes scapularis acquisition of B. burgdorferi, we fed nymphs on wild-type (WT) and MyD88−/− mice previously infected with two clinical isolates of B. burgdorferi, BL206, a high-virulence strain, and B348, an attenuated strain. Seventy-three percent of the nymphs that fed on BL206-infected WT mice and 40% of the nymphs that fed on B348-infected WT mice acquired B. burgdorferi, whereas 100% of the nymphs that fed on MyD88−/− mice became infected, irrespective of B. burgdorferi strain. Ticks that acquired infection after feeding on MyD88−/− mice harbored more spirochetes than those that fed on WT mice, as assessed by quantitative PCR for B. burgdorferi DNA. Vector transmission of BL206 and B348 was also enhanced when MyD88−/− mice were the blood meal hosts, with the mean pathogen burden at the skin inoculation site significantly higher than levels in WT mice. These results show that the absence of MyD88 facilitates passage of both low- and high-infectivity B. burgdorferi strains between the tick vector and the mammal and enhances the infectivity of a low-infectivity B. burgdorferi strain.
Lyme disease due to infection with the spirochete Borrelia burgdorferi is the most common vector-borne disease in the United States (4, 19). The infection can be acquired when humans serve as incidental blood meal hosts for Ixodes scapularis or Ixodes pacificus ticks, the vectors for B. burgdorferi, and manifests first with localized skin infection and a characteristic skin rash called erythema migrans (EM) in 70 to 80% of cases. In untreated infection, spirochetes can disseminate widely to infect any organ system, with disease primarily found in the skin, heart, joints, and nervous system.
The severity of Lyme disease is related to infection duration before treatment (19), host genetic factors (2, 20), and the infectivity and pathogenicity of B. burgdorferi strains (23, 24, 28). Recently, restriction fragment length polymorphism analysis of the 16S-23S rRNA spacer has allowed the classification of B. burgdorferi sensu stricto isolated from Lyme disease patients into three rRNA gene spacer restriction fragment length polymorphism genotypes (RSTs) that correlate with disease severity (11-13). RST1 isolates are more likely to be found in patients with signs of disseminated infection, including multiple EM lesions and positive blood cultures, whereas RST3 isolates are associated with localized disease (28). In the mouse model of Lyme borreliosis, RST1 isolates exhibit earlier dissemination, achieve higher pathogen loads, and cause more severe disease compared to similar infection with RST3 isolates (23, 24). RST3 isolates can be further subdivided into RST3A and RST3B strains, which differ in infectivity and pathogenicity (24). In a previous study, RST3A strains could not be cultured from mice after intradermal inoculation, and only one of three strains examined showed signs of limited dissemination by PCR detection of spirochete DNA in tissues (23, 24). In contrast, RST3B strains could generally be recovered from mice by culture and disseminated to cause pathology, although pathogen burden and disease severity were attenuated in comparison to RST1 strains. The mechanisms that underlie differences in infectivity and pathogenicity among B. burgdorferi strains remain undefined but may be related in part to the ability of spirochetes to evade mammalian immune defenses.
Toll-like receptors (TLRs) comprise a family of pattern recognition molecules on innate immune cells that respond to pathogen-associated molecular motifs (8). Several TLRs participate in the immune recognition of B. burgdorferi, including TLR2, with synergy from TLR1, and TLR9, which interact with bacterial lipoprotein and CpG DNA, respectively. These TLRs utilize the common intracellular adaptor molecule myeloid differentiation antigen 88 (MyD88) to mediate effector functions. MyD88-deficient (MyD88−/−) mice in which B. burgdorferi infection has been introduced by syringe inoculation of cultured organisms have markedly elevated pathogen burdens in comparison to similarly infected wild-type (WT) mice, demonstrating the importance of TLR-mediated innate immunity in the initial containment of B. burgdorferi (5, 10). The ability to evade immune recognition by TLRs may be one reason that RST1 and RST3B strains of B. burgdorferi differ in their infectivity and pathogenicity.
The saliva from Ixodes ticks contains immunomodulatory factors that impair the host immune response and wound repair mechanisms to promote vector feeding (7, 15, 16, 21, 25). Transmission of B. burgdorferi to mammals is facilitated by tick saliva (25), and a salivary protein (Salp15) has been shown to enhance B. burgdorferi survival after experimental inoculation into mice (14). Conversely, local impairment of host immunity by tick saliva likely promotes vector acquisition of B. burgdorferi (18). Whether TLR-mediated immune responses influence B. burgdorferi survival in mammals after vector transmission or impede the passage of spirochetes between the tick vector and the blood meal host has not yet been evaluated. The study reported here was conducted to determine whether MyD88 deficiency altered I. scapularis tick feeding patterns or vector acquisition and transmission of B. burgdorferi RST1 and RST3B strains.
MATERIALS AND METHODS
Ticks.
All experiments were performed with I. scapularis ticks maintained in a laboratory-reared tick colony at the Yale University School of Medicine (6). To introduce infection into ticks, larvae were fed on mice inoculated 21 days earlier with the indicated strain of cultured spirochetes. Infected larvae were allowed to molt, and only groups of nymphs for which infection rates were at least 80%, as assessed by PCR of the B. burgdorferi flaB gene, were utilized for transmission experiments.
Mice.
C57BL/6J × 129/SvJ (B6129F2) MyD88−/− mice were originally obtained from Ruslan Medzhitov, Yale University School of Medicine (17), and maintained as a colony in specific-pathogen-free housing with autoclaved food, water, and bedding according to Yale institutional animal care and use guidelines. In addition, the broad-spectrum antibiotic sulfamethoxazole-trimethoprim (Sulfatrim) (0.25 mg/ml) was added to drinking water to further reduce opportunistic infection. Age- and sex-matched control B6129F2 mice were purchased from the Jackson Laboratories, Bar Harbor, Maine, and maintained under similar conditions. Sulfamethoxazole-trimethoprim, which has no effect on B. burgdorferi infection or disease, was provided to both WT and MyD88−/− mice throughout all experiments. Mice were killed by carbon dioxide asphyxiation. Animal use complied with all relevant federal guidelines and Yale institutional policies.
Spirochetes.
The high-infectivity B. burgdorferi RST1 strain BL206, originally isolated from the blood of a patient from Westchester County, New York, and the low-infectivity RST3B strain B348, isolated from a patient with EM, were used in these experiments (6). Frozen aliquots of low-passage spirochetes were thawed, expanded to log phase in Barbour-Stoenner-Kelly (BSK) II medium at 33°C (1), and then enumerated by dark-field microscopy using a Petroff-Hausser counting chamber. Mice were infected with the B. burgdorferi strains either by intradermal inoculation into the interscapular region with 104 spirochetes in 100 μl BSK II medium or by serving as blood meal hosts for infected nymphs.
Tick feeding.
Mice were mildly anesthetized with ketamine (100 mg/kg of body weight) and xylazine (10 mg/kg) prior to tick infestation. Larvae (∼100 larvae/mouse) or nymphs (3 to 20 nymphs/mouse) were placed on the head fur or in the ear canals, respectively, after which mice were placed in individual cages. Ticks were allowed to feed and detach naturally into a water bath from which they were collected on a daily basis for up to 1 week. Collected ticks were rinsed in water and allowed to air dry. In experiments that assessed vector feeding patterns, ticks were weighed daily using a Mettler balance in preweighed tubes. In some experiments, collected ticks were placed in glass tubes sealed with a fine nylon mesh and housed in 22°C environmental chambers for later analysis or until they molted.
Measurement of B. burgdorferi DNA.
DNA was extracted from engorged ticks 8 days after retrieval or from molted ticks using the Isoquick DNA isolation kit (ORCA Research) according to the manufacturer's protocol and was then resuspended in 20 μl of double-distilled H2O. Quantitative PCR of the B. burgdorferi recA or flaB gene or tick β-actin was performed using an ICycler (Bio-Rad, Hercules, CA) and the following primers and probes: for flaB (41G), 5′-GCTCCTTCCTGTTGAACACCC-3′ (forward), 5′-CTTTTCTCTGGTGAGGGAGCTC-3′ (reverse), and 5′-6-carboxyfluorescein-CTTGAACCGGTGCAGCCTGAGCA-6-carboxytetramethylrhodamine-3′ (probe); for recA, 5′-GTGGATCTATTGTATTAGATGAGGCTCTCG-3′ (forward) and 5′-GCCAAAGTTCTGCAACATTAACACCTAAAG-3′ (reverse); for tick β-actin, 5′-ATCAGGTAGTCGGTCAGG-3′ (forward) and 5′-GGTATCGTGCTCGACTC-3′ (reverse). Reactions were performed in a total volume of 50 μl containing a final concentration of 0.4 μM each primer and 0.2 μM deoxynucleoside triphosphates. For recA and tick β-actin PCR, SYBR green (Stratagene) was added to the reaction mixture to detect double-stranded DNA products, according to the manufacturer's protocol (Stratagene). DNA was also isolated from mouse urinary bladders and the ear skin (site of nymph feeding) using the DNeasy tissue kit (QIAGEN, Valencia, CA) and then resuspended in 50 μl of double-distilled H2O. B. burgdorferi DNA was quantitated by real-time PCR using recA primers with results normalized to mouse β-actin as described previously (10).
Culture.
At the time of mouse sacrifice, 2 drops of blood were cultured in BSK II medium as previously described (3). At 14 days, cultures were assessed for viable spirochetes by dark-field microscopy.
Antibody detection.
B. burgdorferi-specific immunoglobulin G (IgG) in the sera of mice infected by tick transmission was measured by enzyme-linked immunosorbent assay (ELISA). Ninety-six-well microtiter plates (ICN Biomedicals, Aurora, OH) were coated with BL206 or B348 sonicates (3 μg/well) and then blocked with phosphate-buffered saline containing 3% bovine serum albumin. Serum samples were added to the wells initially at a dilution of 1:200 and then serially diluted twofold in phosphate-buffered saline. After incubation for 2 h at room temperature, plates were washed three times in phosphate-buffered saline containing 0.1% Tween 20. Bound antibodies were labeled with alkaline phosphatase-conjugated goat anti-mouse IgG and then detected with the Vector Elite visualization kit (Vector Laboratories, Burlingame, CA), according to the manufacturer's protocol.
B. burgdorferi-specific antibodies were also detected by immunoblot analysis using BL206 lysates as previously described (10).
RESULTS
MyD88 deficiency does not alter Ixodes tick feeding patterns or rate of engorgement.
The saliva of I. scapularis contains potent anti-inflammatory and antihemostatic factors that facilitate vector feeding, the duration of which varies from 3 to 5 days for maximal engorgement (15). In order to determine whether impairment of MyD88-dependent immune responses would enhance the rate of blood meal ingestion, we examined the kinetics of larval and nymphal I. scapularis feeding (Table 1). The majority of nymphs fed to repletion within 4 days of placement, and mean weights were not statistically different. There was a trend for larvae to feed faster on WT mice, with most detaching by day 3, but the mean weight of these ticks was slightly less than the weight of larvae that fed on MyD88−/− mice. By the end of 4 days, equivalent numbers of larvae had fed on WT and MyD88−/− mice, and there was no difference in the mean weights.
TABLE 1.
Duration of I. scapularis feeding and detachment weighta
| Days after placement | WT blood meal host
|
MyD88−/− blood meal host
|
||
|---|---|---|---|---|
| No. of nymphs or larvae retrieved | Mean wt (mg/nymph or mg/larva) | No. of nymphs or larvae retrieved | Mean wt (mg/nymph or mg/larva) | |
| Nymph | ||||
| 0-2 | 0 | ND | 0 | ND |
| 3 | 11 | 3.48 | 7 | 3.16 |
| 4 | 61 | 4.24 | 81 | 4.35 |
| 5 | 4 | 5.10 | 10 | 5.10 |
| Avg mean wt ± SD | 4.27 ± 0.81 | 4.20 ± 0.98 | ||
| Larvae | ||||
| 0-2 | 0 | ND | 0 | ND |
| 3 | 518 | 0.45 | 364 | 0.62 |
| 4 | 196 | 0.55 | 399 | 0.52 |
| Avg mean wt ± SD | 0.50 ± 0.07 | 0.58 ± 0.06 | ||
ND, not determined.
MyD88 deficiency enhances spirochete acquisition by feeding ticks.
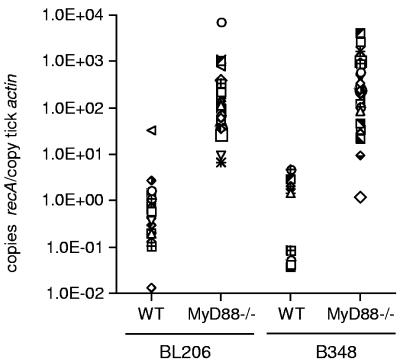
Having established that the feeding pattern was not altered when ticks fed on MyD88−/− mice, we next sought to determine whether the absence of MyD88-mediated immune responses influenced the ability of ticks to acquire B. burgdorferi from infected mice. Nymphal ticks were fed on groups of three WT or MyD88−/− mice previously infected with BL206 or B348 spirochetes by syringe inoculation of cultured organisms. As expected, MyD88−/− mice harbored significantly more spirochetes within tissues than infected WT mice (Table 2). Analysis of a randomly selected sample of 15 ticks retrieved from each group revealed that when MyD88−/− mice were the blood meal hosts, ticks were more likely to acquire infection with either BL206 or B348 than when ticks fed on WT mice (Table 2). Moreover, ticks that became infected with B348 after feeding on MyD88−/− mice had significantly more spirochetes than those that acquired spirochetes after feeding on WT mice, as assessed by quantitative PCR of B. burgdorferi DNA (P < 0.0001; Mann-Whitney test) (Fig. 1). A similar trend was observed with BL206, with ticks acquiring more spirochetes after feeding on MyD88−/− mice (P < 0.0001; Mann-Whitney test) (Fig. 1).
TABLE 2.
Nymphs acquire spirochetes more readily after feeding on B. burgdorferi-infected MyD88−/− mice
| Blood meal host | Mean pathogen burden in mouse urinary bladders ± SEMa | % of feeding nymphs that acquired B. burgdorferib |
|---|---|---|
| BL206-infected WT | 1.5 × 10−4 ± 0.2 × 10−4 | 73 |
| BL206-infected MyD88−/− | 9.3 ± 8.7 | 100 |
| B348-infected WT | 2.9 × 10−6 ± 2.9 × 10−6 | 40 |
| B348-infected MyD88−/− | 3.2 ± 2.5 | 100 |
Mean pathogen burden was determined by quantitative PCR and expressed as the ratio of nanograms of flaB to nanograms of mouse β-actin ± standard error of the mean.
Percent calculated from number that tested positive out of 15 ticks analyzed per mouse group.
FIG. 1.
Nymphs that acquire spirochetes from B. burgdorferi-infected MyD88−/− mice have higher pathogen loads. Quantitative PCR for the B. burgdorferi recA gene was performed on a randomly selected sample of nymphs that tested positive by flaB PCR from each group. Each data point represents results from one tick. Results, normalized to tick actin, show a statistical difference between pathogen loads of ticks that fed on MyD88−/− versus controls, irrespective of spirochete strain (P < 0.0001; Mann-Whitney test).
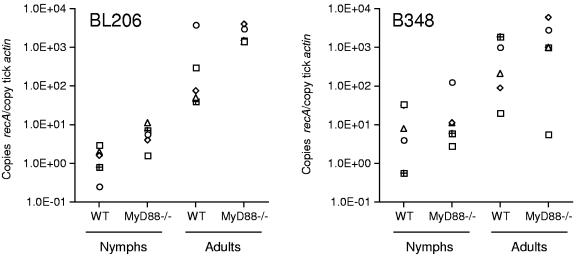
In a second experiment, larvae and nymphs that fed on BL206- and B348-infected mice were housed in environmental chambers until they molted into nymphs and adult ticks, respectively. The infection rate among molted ticks was similar to that observed previously in nymphs shortly after feeding, with enhanced acquisition of both BL206 and B348 in ticks that fed on MyD88−/− mice (data not shown). Quantitation of B. burgdorferi DNA in BL206-infected ticks revealed that the increased pathogen burden was retained at the larva-to-nymph stage (P = 0.0317; Mann-Whitney test) of development but not from nymphs to adults (P = 0.0952; Mann-Whitney test) (Fig. 2). This was not the case with B348-infected ticks, where, after molting to nymphs or to adults, differences noted at the acquisition stage did not quite achieve statistical significance (P = 0.8413 and P = 0.3290, respectively; Mann-Whitney test) (Fig. 2).
FIG. 2.
Pathogen burden in ticks after molting. Larvae and nymphal ticks were housed in environmental chambers until they molted. DNA was isolated and pooled from a representative sample of molted nymphs, and B. burgdorferi DNA was quantified as described in Materials and Methods. Each data point represents the result of DNA pooled from 10 nymphs retrieved from a single mouse. One molted adult tick representative of ticks retrieved from each mouse was similarly analyzed; each data point represents results of ticks that fed on individual mice. Differences between groups achieved significance only for nymphs that had molted from larvae fed on BL206-infected mice (P = 0.0317; Mann-Whitney test).
MyD88 deficiency results in high pathogen loads and enhanced bacteremia after vector transmission of B. burgdorferi strains BL206 and B348.
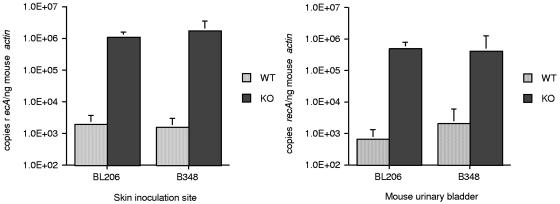
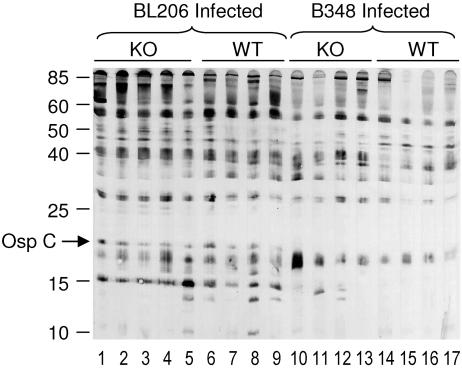
To assess whether MyD88 deficiency enhanced survival of the attenuated strain after vector transmission, the outcomes from B348- and BL206-infected nymphal feeding on MyD88−/− and WT mice were compared. Pathogen burdens at the skin inoculation site and urinary bladder were determined in mice 21 days after initial tick placement (Fig. 3). For both BL206 and B348, MyD88 deficiency resulted in an ∼1,000-fold increase in spirochete DNA in tissues, even in the urinary bladder, a site representative of disseminated infection. Blood cultures were generally positive in MyD88−/− mice but not in WT mice (Table 3), suggesting that the elevated levels of spirochete DNA correlated with an increased number of viable spirochetes. B. burgdorferi-specific IgG titers were similar in both the WT and MyD88−/− mice, irrespective of the infecting strain, but accurate detection of IgG titers required the use of the autologous B. burgdorferi strain as the antigen for the ELISA. B348 IgG titers were substantially lower when BL206 was used as the source of antigen for the ELISA in comparison to B348 antigen. Immunoblot analysis revealed that this was due to lower reactivity to several B. burgdorferi proteins, particularly those in the lower-molecular-weight range, such as OspC (Fig. 4).
FIG. 3.
Pathogen burden in skin and urinary bladders after vector transmission of BL206 and B348. DNA was isolated from mouse tissues 21 days after tick infestation. Differences between WT and MyD88−/− (KO) values achieved significance for all groups (P = 0.0159 for all skin and BL206-infected urinary bladder and P = 0.0317 for B348-infected urinary bladder; Mann-Whitney test).
TABLE 3.
IgG endpoint titers are similar in vector-borne infection of WT and MyD88−/− mice, but accurate detection is B. burgdorferi strain specifice
| Mouse group | No. of positive blood cultures/total no. | Endpoint IgG ELISA titer ± SEM using:
|
|
|---|---|---|---|
| BL206 antigen | B348 antigen | ||
| BL206-infected WT | 0/4 | 22,500 ± 3,200 | 19,200 ± 3,695 |
| BL206-infected MyD88−/− | 5/5a | 37,120 ± 22,933b | 15,360 ± 5,724 |
| B348-infected WT | 0/4 | 2,000 ± 400 | 12,800 ± 5,226 |
| B348-infected MyD88−/− | 4/5c | 3,520 ± 1,176d | 16,000 ± 6,072 |
These results were significantly different from WT values (P = 0.0079; Fisher's exact test).
No significant difference from WT values (P = 0.6201; Mann-Whitney test).
These results were significantly different from WT values (P = 0.0476; Fisher's exact test).
No significant difference from WT values (P = 0.6129; Mann-Whitney test).
All skin, heart, and joint cultures were culture positive (data not shown).
FIG. 4.
Immunoblot of BL206 lysates using sera from BL206- and B348-infected mice. IgG reactivities of 1:100 dilutions of sera from MyD88−/− (KO) (lanes 1 to 5) and WT (lanes 6 to 9) mice infected with BL206 were compared with reactivities in sera from B348-infected MyD88−/− (lanes 10 to 13) and WT (lanes 14 to 17) mice. The location of OspC was determined using a polyclonal rabbit anti-OspC (B. burgdorferi strain N40) antiserum and coincided with the 20-kDa molecular mass marker (data not shown).
DISCUSSION
B. burgdorferi genotypes correlate with various dissemination patterns within humans (28) and exhibit differences in infectivity and pathogenicity after inoculation into mice (23, 24), but the mechanisms for these differences have not yet been defined. A previous study in which RST1, RST3A, and RST3B strains were used to infect mice demonstrated their equivalent sensitivity to killing by mouse serum and suggested that differences in dissemination and disease were due to RST strain-specific resistance to innate immune mechanisms (24). In particular, RST3A strain B356 could not be cultivated from the skin inoculation site 24 h after injection into either C3H/He mice or C3H/He mice with severe combined immunodeficiency, implicating innate immune mechanisms in its destruction. However, RST3A strains could not be cultured from TLR2−/− mice, which lack one of several TLRs that contribute to the innate immune response to B. burgdorferi components (22). We attempted to include B356 in this study but could not establish infection in MyD88−/− mice with this organism. Similar to the findings with C3H SCID mice, we were unable to detect B356 DNA in the skin inoculation site, urinary bladder, or heart 14 days after inoculation into MyD88−/− mice. Moreover, we were unable to culture B356 within 24 h of inoculation into MyD88−/− mice (data not shown). This contrasts with the results we obtained with RST3B isolate B348, which proliferated and disseminated in MyD88−/− mice inoculated with cultured spirochetes to a degree equivalent to that of the more infectious BL206 strain. With the caveat that only single representatives of each genotype were evaluated, these results suggest that RST3B isolates are more susceptible to MyD88-dependent innate immune mechanisms than RST1 isolates, although both genotypes are clearly affected. RST3A strains may have other properties unrelated to their ability to withstand immune defenses that retard their infectivity and dissemination in mice.
In order to assess the effects of MyD88 deficiency on the transmission of B. burgdorferi between the tick and the mouse, it was necessary to first establish the feeding patterns of Ixodes ticks on these mice. I. scapularis saliva contains a number of bioactive agents that promote vector feeding. In addition to antihemostatic, antihistaminic, and fibrinolytic activities (16), several studies have demonstrated that I. scapularis saliva and/or individual salivary components can impair innate immunity (9, 25). Ixodes ricinus salivary gland extracts can suppress polyinosinic poly(C) [poly(I:C)] and lipopolysaccharide-mediated cytokine production by splenocytes in vitro, suggesting an inhibitory effect on some TLR-mediated responses, since poly(I:C) and lipopolysaccharide engage TLR3 and TLR4, respectively. The transcriptome of I. pacificus contains genes encoding metalloproteases and disintegrins (7); the latter group could impair immune cell migration. Our study is the first study to compare I. scapularis feeding patterns on WT and MyD88−/− mice and suggests that if MyD88-dependent immunity comprises part of the host defense against tick feeding, salivary components have a profound ability to neutralize those responses. Neither the duration of larva and nymph feeding nor their weights after detachment were altered when MyD88-deficient mice served as blood meal hosts. Although unlikely, it remains possible that the ability of ticks to feed repeatedly on mice could be impacted by the absence of MyD88, particularly if tick salivary components harness MyD88-dependent responses in a way that tolerizes the host to the tick.
In contrast to its lack of impact on vector feeding patterns, MyD88 deficiency significantly enhanced I. scapularis acquisition of B. burgdorferi and promoted survival of B. burgdorferi within the mouse. It is likely that the increased pathogen burden seen in B. burgdorferi-infected MyD88−/− mice enhanced transmission of B. burgdorferi to I. scapularis, as engorged ticks that fed on MyD88−/− mice harbored more spirochete DNA than those that fed on WT mice. Moreover, spirochetes are more readily cultured from the blood of MyD88−/− mice, suggesting that enhanced bacteremia as well as tissue pathogen burden contributed to the increased infection rate of feeding ticks.
Interestingly, although a lower percentage of ticks acquired infection after feeding on B348-infected WT mice in comparison to BL206-infected WT mice, the pathogen burden among infected ticks was equivalent. A previous study that evaluated the transmission efficiency of BL206 and B348 from infected Peromyscus leucopus mice to uninfected larvae showed a similar reduced rate of transmission for B348 in comparison to BL206, but the relative spirochete load among infected ticks was not assessed (6). P. leucopus mice appear to be particularly resistant to infection with B348 when inoculated with cultured organisms, as it was necessary to infect these outbred mice using B348-infected ticks. Although not directly measured in that study (6), a lower pathogen burden within P. leucopus mice infected with B348 in comparison to BL206 may explain the reduced rate of transmission to feeding ticks. Our results extend these findings by showing that even though BL206 achieves a higher pathogen burden than B348 in B6129 mice inoculated with cultured organisms, this difference is not reflected in the pathogen burden of ticks that successfully acquire infection after feeding. There may be a threshold level that must be exceeded in order for spirochetes to establish infection within feeding ticks. Our results suggest that the pathogen burden within infected ticks is only significantly raised when that threshold is greatly exceeded, as is the case when ticks feed on B. burgdorferi-infected MyD88−/− mice. After molting, ticks that fed on MyD88−/− mice showed a trend toward retaining the elevated pathogen burden in comparison to those that fed on WT mice, although differences only remained significant for BL206-infected larvae molting to nymphs.
The infectivity of vector-borne B348 and BL206 was also enhanced in the absence of MyD88. Both strains of spirochetes achieved higher pathogen loads in the inoculation sites and the urinary bladders of MyD88−/− mice than in WT mice, and only MyD88−/− mice had bacteremia detected by culture at the time of mouse sacrifice. Although the pathogen burden appeared equivalent in B348- and BL206-infected WT mice, this may be due to the use of five nymphs per mouse to transmit B348 in comparison to three BL206-infected nymphs per mouse. We did not assess whether the absence of MyD88 altered the pathogenicity of BL206 or B348, because our previous study with MyD88−/− mice infected with RST3 isolate N40 did not reveal differences in disease severity despite the marked alteration in pathogen burden (10). These findings underscore the importance of MyD88-mediated immunity in the control of B. burgdorferi infection in mice and show that B348 can replicate and disseminate as efficiently as BL206 when this immunity is impaired.
B. burgdorferi sensu stricto exhibits a high degree of genetic diversity, although genetic homogeneity of the 16S-23S rRNA gene spacer and ospC can be seen among RST1 isolates (24). In the case of ospC, B348 is significantly divergent from RST1 isolate BL206, but differences in the ospC sequence do not explain the variation in infectivity and pathogenicity between RST1 and RST3B strains (24). We observed in this study, however, that the pathogen-specific IgG titers were influenced by the antigen source used to detect them. IgG titers were significantly lower in B348-infected mice assessed using BL206 sonicates as the antigen, and much of this reduction appeared to be due to a lower reactivity to BL206 OspC and other lower-molecular-weight proteins. Selective reduction in OspC reactivity was similarly observed in sera of B348-infected C3H mice by use of commercially prepared immunoblot strips (24). This is not surprising, given the divergence in the ospC sequences. Moreover, decreased reactivity does not appear to be due to the level of OspC expression on cultured spirochetes, the source of lysate for immunoblots, as the use of syngeneic sera revealed prominent reactivity to OspC from both BL206 and B348 strains. We did not directly assess the level of OspC expression by host-adapted spirochetes, which could influence the infectivity of B. burgdorferi. OspC is known to exhibit a high degree of molecular and immunologic polymorphism among B. burgdorferi sensu lato strains, especially among European strains, and contributes to the variability of serologic diagnostic assays (26, 27). These observations may have bearing on the lower sensitivity of ELISA for detecting B. burgdorferi antibodies among North American patients with early Lyme disease, in which reactivity to OspC is considered a diagnostic criterion, and may be especially applicable to those individuals infected with RST3B strains. When the syngeneic strain of spirochete was used to detect antibodies, no difference in immunogenicity between BL206 and B348 was found.
In summary, our results show that the transmission dynamics of B. burgdorferi between mice and I. scapularis ticks is enhanced in the absence of MyD88, particularly for the low-infectivity RST3B isolate B348. They further implicate MyD88-dependent immune mechanisms in the reduced infectivity of B348 in comparison to RST1 isolate BL206 but not in the lower infectivity of RST3A isolate B356. It is possible that variations in MyD88-dependent immunity among reservoir hosts may, in part, determine the optimum reservoir for different B. burgdorferi genotypes. Additional studies are necessary to define the interaction between B. burgdorferi genotypes and MyD88-dependent immunity, which might explain the role of B. burgdorferi genotypic variation in infectivity and disease.
Acknowledgments
This work was supported by Cooperative Agreement U01 CI000159 from the Centers for Disease Control and Prevention (L.K.B.); Cooperative Agreement 58-0790-2-072 from the U.S. Department of Agriculture, Agricultural Research Service (D.F.); the G. Harold and Leila Y. Mathers Charitable Foundation (D.F.); and NIH grant AR41511 (I.S.).
We thank Michele Papero, Jialing Mao, and Ming Li for excellent technical assistance.
Editor: D. L. Burns
REFERENCES
- 1.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521-525. [PMC free article] [PubMed] [Google Scholar]
- 2.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 3.Barthold, S. W., M. S. de Souza, J. L. Janotka, A. L. Smith, and D. H. Persing. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 143:419-420. [PMC free article] [PubMed] [Google Scholar]
- 4.Bockenstedt, L. K. Lyme disease. In J. Imboden, D. Hellmann, and J. Stone (ed.), Current diagnosis and treatment in rheumatology, 2nd ed., in press. McGraw-Hill Co., New York, N.Y.
- 5.Bolz, D. D., R. S. Sundsbak, Y. Ma, S. Akira, C. J. Kirschning, J. F. Zachary, J. H. Weis, and J. J. Weis. 2004. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J. Immunol. 173:2003-2010. [DOI] [PubMed] [Google Scholar]
- 6.Derdakova, M., V. Dudioak, B. Brei, J. S. Brownstein, I. Schwartz, and D. Fish. 2004. Interaction and transmission of two Borrelia burgdorferi sensu stricto strains in a tick-rodent maintenance system. Appl. Environ. Microbiol. 70:6783-6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franchischetti, I. M., V. M. Pham, B. J. Mans, J. F. Anderson, T. N. Mather, R. S. Lane, and J. M. Ribeiro. 2005. The transcriptome of the salivary glands of the female western black-legged tick Ixodes pacificus (Acari: Ixodidae). Insect Biochem. Mol. Biol. 35:1142-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawai, T., and S. Akira. 2005. Pathogen recognition with Toll-like receptors. Curr. Opin. Immunol. 17:338-344. [DOI] [PubMed] [Google Scholar]
- 9.Kopecky, J., and M. Kuthejlova. 1998. Suppressive effect of Ixodes ricinus salivary gland extract on mechanisms of natural immunity in vitro. Parasite Immunol. 20:169-174. [PubMed] [Google Scholar]
- 10.Liu, N., R. R. Montgomery, S. W. Barthold, and L. K. Bockenstedt. 2004. Myeloid differentiation antigen 88 deficiency impairs phagocytosis but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect. Immun. 72:3195-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liveris, D., A. Gazumyan, and I. Schwartz. 1995. Molecular typing of Borrelia burgdorferi sensu lato by PCR-restriction fragment length polymorphism analysis. J. Clin. Microbiol. 33:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liveris, D., S. Varde, R. Iyer, S. Koenig, S. Bittker, D. Cooper, D. McKenna, J. Nowakowski, R. B. Nadelman, G. P. Wormser, and I. Schwartz. 1999. Genetic diversity of Borrelia burgdorferi in Lyme disease patients as determined by culture versus direct PCR with clinical specimens. J. Clin. Microbiol. 37:565-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liveris, D., G. P. Wormser, J. Nowakowski, R. Nadelman, S. Bittker, D. Cooper, S. Varde, F. H. Moy, G. Forseter, C. S. Pavia, and I. Schwartz. 1996. Molecular typing of Borrelia burgdorferi from Lyme disease patients by PCR-restriction fragment length polymorphism analysis. J. Clin. Microbiol. 34:1306-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramamoorthi, N., S. Narasimhan, U. Pal, F. Bao, X. F. Yang, D. Fish, J. Anguita, M. V. Norgard, F. S. Kantor, J. F. Anderson, R. A. Koski, and E. Fikrig. 2005. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436:573-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribeiro, J. M. C., and I. M. B. Franchischetti. 2003. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu. Rev. Entomol. 48:73-88. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro, J. M. C., G. T. Makoul, J. Levine, D. R. Robinson, and A. R. Spielman. 1985. Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick, Ixodes dammini. J. Exp. Med. 161:332-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnare, M., G. M. Barton, A. C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2:947-950. [DOI] [PubMed] [Google Scholar]
- 18.Shih, C.-M., and L.-P. Liu. 1996. Accelerated infectivity of tick-transmitted Lyme disease spirochetes to vector ticks. J. Clin. Microbiol. 34:2297-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steere, A. C., J. Coburn, and L. Glickstein. 2004. The emergence of Lyme disease. J. Clin. Investig. 113:1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steere, A. C., B. Falk, E. E. Drouin, L. A. Baxter-Lowe, J. Hammer, and G. T. Nepom. 2003. Binding of outer surface protein A and human lymphocyte function-associated antigen 1 peptides to HLA-DR molecules associated with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum. 48:534-540. [DOI] [PubMed] [Google Scholar]
- 21.Valenzuela, J. G., I. M. Franchischetti, V. M. Pham, M. K. Garfield, T. N. Mather, and J. M. Ribeiro. 2002. Exploring the sialome of the tick Ixodes scapularis. J. Exp. Biol. 205:2843-2864. [DOI] [PubMed] [Google Scholar]
- 22.Wang, G., Y. Ma, A. Buyuk, S. McClain, J. J. Weis, and I. Schwartz. 2004. Impaired host defense to infection and Toll-like receptor 2-independent killing of Borrelia burgdorferi clinical isolates in TLR2-deficient C3H/HeJ mice. FEMS Microbiol. Lett. 231:219-225. [DOI] [PubMed] [Google Scholar]
- 23.Wang, G., C. Ojaimi, R. Iyer, V. Saksenberg, S. A. McClain, G. P. Wormser, and I. Schwartz. 2001. Impact of genotypic variation of Borrelia burgdorferi sensu stricto on kinetics of dissemination and severity of disease in C3H/HeJ mice. Infect. Immun. 69:4303-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang, G., C. Ojaimi, H. Wu, V. Saksenberg, R. Iyer, D. Liveris, S. A. McClain, G. P. Wormser, and I. Schwartz. 2002. Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J. Infect. Dis. 186:782-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wikel, S. K. 1999. Tick modulation of host immunity: an important factor in pathogen transmission. Int. J. Parasitol. 29:851-859. [DOI] [PubMed] [Google Scholar]
- 26.Wilske, B., A. G. Barbour, S. Bergstron, N. Burman, B. I. Restropo, P. A. Rosa, T. Schwan, E. Soutschek, and R. Wallich. 1992. Antigenic variation and strain heterogeneity in Borrelia spp. Res. Microbiol. 143:583-596. [DOI] [PubMed] [Google Scholar]
- 27.Wilske, B., V. Preac-Mursic, S. Jauris, A. Hofmann, I. Pradel, E. Soutschek, E. Schwab, G. Will, and G. Wanner. 1993. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect. Immun. 61:2182-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wormser, G. P., D. Liveris, J. Nowakowski, R. B. Nadelman, L. F. Cavaliere, D. McKenna, D. Holmgren, and I. Schwartz. 1999. Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J. Infect. Dis. 180:720-725. [DOI] [PubMed] [Google Scholar]