Abstract
Longitudinal investigations of an adult male population of Kenyan car washers who have heavy and quantifiable occupational exposure to Schistosoma mansoni cercariae revealed that some individuals develop resistance to reinfection while others remain highly susceptible. We sought to characterize immune correlates associated with host protection in this population. Previous studies have demonstrated an association of peripheral eosinophilia with resistance to reinfection with schistosomes. Thus, we investigated the relationship between the percentage of circulating eosinophils and the effect of human immunodeficiency virus type 1 (HIV-1) coinfection on the susceptibility of the car washers to reinfection with schistosomes. Elevated percentages of circulating eosinophils were associated with resistance to reinfection by S. mansoni in HIV-1-seronegative persons. In the HIV-1-seropositive cohort, low CD4+-T-cell counts were associated with a less intense eosinophilia. Moreover, eosinophils from the car washers expressed high levels of FcεRI β chain, a molecule important in immunoglobulin E (IgE)-mediated immunity. Levels of FcεRI β chain expression correlated with serum levels of total and antigen-specific IgE for HIV-1-negative car washers, but this was not the case for individuals coinfected with HIV-1. Overall, these data further implicate eosinophils as having a potential role in development of protective immunity against schistosomes and suggest that changes associated with HIV-1 coinfection increase susceptibility to reinfection.
Schistosomes are parasitic trematodes responsible for causing disease in over 200 million people worldwide (14). A better understanding of the immunologic components involved in the development of protection against infection is needed to lay the foundation for the advancement of efficacious vaccines (49). A common finding in many studies is that a high concentration of serum parasite-specific immunoglobulin E (IgE) is related to resistance against schistosomes (6, 12, 13, 27, 39). However, whether IgE is directly involved in protective mechanisms in vivo has not been elucidated, as IgE exerts its effector function through FcεRI-expressing cells. Several studies have demonstrated an association between eosinophilia and host protection against schistosomes (2, 18, 45), although whether eosinophils express the high-affinity IgE receptor remains under debate (1, 3, 4, 17, 21, 23, 26, 40, 42, 43). Purified human eosinophils are cytotoxic to schistosomula in vitro in the presence of parasite-specific IgE, with eosinophils from helminth-infected individuals having greater potency (11, 16, 23, 50). These observations suggest that IgE and eosinophils are likely to have an important role in host protection against schistosomes.
Our laboratory is conducting a longitudinal study on Schistosoma mansoni infections in a cohort of adult male Kenyan car washers who are occupationally exposed to infective cercariae in the waters along the shores of Lake Victoria (22). Over 200 men in this group have been actively monitored for water contact and evaluated for schistosome and human immunodeficiency virus type 1 (HIV-1) infection from 1995 to the present (0 to 10 years). About one-third of these individuals are seropositive for HIV-1. A statistical model using data from 96 men who were comprehensively tracked for 1 to 5 years demonstrated that infection with schistosomes and subsequent praziquantel treatment decreased the risk for reinfection (i.e., increased time to reinfection) for some individuals, suggesting an augmentation of resistance resulting from cycles of drug treatment and reinfection (22). Conversely, an HIV-1-mediated reduction in CD4+ T cells was a risk factor for reinfection (22). These observations suggest that in an adult population in which water contact is quantifiable, a delay in reinfection may be related to an active immune-mediated mechanism involving CD4+ T cells. We have, based on the work mentioned above, evaluated the contribution of eosinophils to resistance against S. mansoni reinfection in this population.
We report that development of peripheral eosinophilia correlates with resistance to reinfection in HIV-1-negative car washers. Furthermore, an HIV-related reduction in CD4+ T cells was associated with reduced peripheral blood eosinophil percentages, posing a possible explanation for their increased susceptibility to reinfection. These data, as well as the demonstration that FcεRI β is expressed on eosinophils of schistosomiasis patients, are consistent with the hypothesis that eosinophils may play an important role in host protection against human schistosomiasis (6, 16).
MATERIALS AND METHODS
Study participants.
This study was performed in western Kenya, near Kisumu, in an area along the shores of Lake Victoria where infestation by S. mansoni-infected Biomphalaria sudanica snails has been confirmed (unpublished data). Study participants, of ages 18 to 55 (median, 24.5), included occupationally exposed car washers or fishermen, who had been followed for 0 to 10 years (Table 1) . An on-site member of the car wash consortium recorded the number of cars each individual washed daily. These records, which the consortium uses as the basis for determining each man's pay, were also utilized to calculate level of exposure for our study. Water contact data for fishermen were not available, but multiple daily contacts are usual for these men.
TABLE 1.
Medians and 95% confidence intervals for HIV-negative and -positive schistosomiasis patients included in this study
| Patient group | No. of patients | Median value (95% CI)a
|
||
|---|---|---|---|---|
| EPG | CD4 count | CD8 count | ||
| HIV negative | 43 | 12 (12-362) | 752 (696-926)* | 525 (477-618)* |
| HIV positive | 24 | 18 (3-176) | 478 (399-604) | 882 (779-1232) |
CI, confidence interval; EPG, number of S. Mansoni eggs per gram of feces.
P < 0.001 compared to HIV-positive patients.
The study was approved by the institutional review boards of the Centers for Disease Control and Prevention and the University of Georgia, the Scientific Steering Committee of the Kenya Medical Research Institute, and the National Ethics Review Board of Kenya. Upon giving informed consent, study participants were screened for S. mansoni eggs and for other helminth ova by using the modified Kato Katz technique (Helm Tec R Kato/Katz kit; Pesquisas E Desenvolmento Limitada, Brazil). Infected individuals were treated with praziquantel (40 mg/kg) as well as albendazole (400 mg) if nematode infections were detected. A person was considered to be reinfected with S. mansoni if he was found to have a positive egg count following successful treatment (drug treatment and three consecutive egg counts of zero at 6 weeks after treatment).
For determination of absolute CD4+-T-cell counts, whole blood collected in EDTA tubes was tested using FACSCount instrument and reagents according to the manufacturer's specifications (BD Biosciences Immunocytometry Systems, San Jose, CA). HIV-1 screening was performed on patients' plasma samples by serostrip (Saliva Diagnostic Systems, Vancouver, WA). Qualified personnel offered study participants confidential HIV screening and pre- and posttest counseling in their native language. Eight volunteers were also included as helminth-negative controls.
Calculation of IoS/R.
Each study participant had a different history of exposure and reinfection and had been monitored for different lengths of time. Therefore, a mathematical formula was created to normalize these factors to generate an index of susceptibility/resistance (IoS/R), representative of each participant's relative susceptibility: (number of times reinfected × 100)/(amount of time followed [weeks] × mean number of cars washed per week) = IoS/R. The higher the IoS/R value, the greater the individual's susceptibility; the lower the index value, the greater the resistance to reinfection.
Serum immunoglobulin profiles.
A 5-μg/ml soluble worm antigen preparation (SWAP) (9) or 2.5 μg/ml of anti-human IgE was coated onto plates in carbonate buffer overnight at 4°C. Plates were washed with phosphate-buffered saline (PBS)-0.05% Tween 20 (Invitrogen, Carlsbad, CA) and blocked with PBS-1% bovine serum albumin (Sigma, St. Louis, MO) for 1 h at room temperature. After a washing, serum was added at a 1:10 dilution for the detection of SWAP-specific IgE and 1:25 for the detection of total IgE in blocking buffer. Serum was incubated overnight at 4°C. Plates were washed and horseradish peroxidase-conjugated anti-human IgE (Zymed, San Francisco, CA) was used at 1:500. Plates were incubated at room temperature for 1 h. After a washing, TMB (Kirkegaard & Perry Laboratories, Gaithersburg, MD) was added to plates. Reactions were stopped with 1 M H2SO4 and read at 450 nm on an enzyme-linked immunosorbent assay (ELISA) plate reader (Molecular Devices, Sunnyvale, CA).
Cell surface staining and FACS analysis.
Four milliliters of whole blood was collected by venipuncture into EDTA tubes (BD Vacutainer Systems, Franklin Lakes, NJ) for fluorescence-activated cell sorter (FACS) analysis and absolute CD4+-T-cell counts. For flow-cytometric analysis, all antibodies were purchased from BD Pharmingen (San Diego, CA) except the anti-FcεRI β chain antibody (Upstate Biotechnology, Lake Placid, NY). To evaluate the percentages of eosinophils and their levels of activation, the following antibodies and reagents were used: CD11b/CD18 (Cy-Chrome [cy-c]), anti-CD23 (phycoerythrin [PE]), anti-CD25 (PE), anti-CD69 (fluorescein isothiocyanate [FITC]), anti-CD125 (PE), biotinylated anti-CD131w (common chain β for interleukin 3 [IL-3], IL-5, GM-CSF receptors) plus streptavidin FITC, and anti-FcεRI β chain plus anti-rabbit FITC (Sigma).
Anti-CD14 (FITC), anti-CD4 (cy-c), anti-CCR5 (PE), and anti-CXCR4 were utilized to identify monocytes. Anti-Mac-1 (cy-c), biotinylated anti-CD131w, and anti-CD125 (PE) were used to distinguish neutrophils. Anti-CD3 (FITC), anti-CD4 (PE), anti-CD8 (cy-c), and anti-CD19 (FITC) were used to identify lymphocytes. Isotype controls included mouse (BD Pharmingen) and rabbit IgG (Sigma).
For each stain, 100 μl of whole blood was incubated with antibodies for 30 min at 4°C in the dark. Red blood cells were then lysed with 2 ml of 1 × FACS lysing solution (BD Pharmingen) for 30 min at room temperature in the dark. Cells were washed with 2 ml of 0.2% bovine serum albumin-PBS and fixed with 2% paraformaldehyde (Electron Microscopy Sciences, Washington, PA). The remaining whole blood was centrifuged; the plasma collected and stored at −20°C for the detection of antibodies or used fresh for rapid HIV-1 screening.
Peripheral blood cells obtained from unexposed and uninfected subjects were stained with antibodies to FcεRIβ, CD11b/CD18, and CD125 as described above. Data were collected on a FACSCalibur (BD Biosciences) using CellQuest software and analyzed with WinMDI software (Joseph Trotter, The Scripps Research Institute, Palo Alto, CA). A Z1 single-threshold analyzer/Coulter Counter (Beckman Coulter, Fullerton, CA) was used according to the protocol to generate cell differentials for some subjects.
Cell culture.
Peripheral blood was obtained by venipuncture into heparin-coated vacutainer tubes (BD Vacutainer Systems). Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque gradient (Atlanta Biologicals, Lawrenceville, GA). PBMCs were cultured at 2 × 106 cells/ml in 48-well plates in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 1 mM penicillin-streptomycin, and 2 mM l-glutamine (Invitrogen). Cells were stimulated with medium alone, phytohemagglutinin (Sigma), soluble schistosome egg antigen, SWAP, Escherichia coli lipopolysaccharide (Sigma), schistosomula soluble protein, r-schistosomal paramyosin (a generous gift from John Andersen, Laboratory of Parasitic Diseases, NIAID/NIH), or Brugia pahangi antigens for 48 h. At 48 h, supernatants were collected for measurement of secreted cytokines.
Measurement of secreted cytokines.
Cytokines were measured by standard capture ELISA on cell-free culture supernatants. Plates were coated with anticytokine antibodies at 5 μg/ml (IFN-γ and IL-4) or at 2 μg/ml (IL-5, IL-10, IL-13, and tumor necrosis factor alpha [TNF-α]) (R&D Systems, Minneapolis, MN). Biotinylated mouse anti-human cytokine antibodies were diluted in PBS at 0.5 μg/ml (IFN-γ, IL-5, and IL-13), at 2 μg/ml (IL-10 and TNF-α), or at 2.5 μg/ml (IL-4), all obtained from Endogen (Rockford, IL). Streptavidin-horseradish peroxidase conjugate was used at 0.5 μg/ml in PBS (Sigma). Cytokine concentrations were calculated by using standard curves created with known amounts of the recombinant purified cytokines. Standard values ranged between 81.25 and 6,000 pg/ml for IFN-γ, 62.5 and 4,000 pg/ml for IL-13 and TNF-α, 31.25 and 2,000 pg/ml for IL-5, and 10.15 to 750 pg/ml for IL-4 and IL-10.
Statistical analyses.
Statistical analyses were performed using the Instat2 statistical package (GraphPad Software) and Microsoft Excel. Nonparametric comparisons of the distribution of group ranks were made with the Mann-Whitney U test. Spearman's rank correlation test was used to test for nonzero nonparametric correlation coefficients. Sample sizes differ for different tests due to the unavailability of certain patient samples.
RESULTS
Eosinophilia in schistosome-infected subjects.
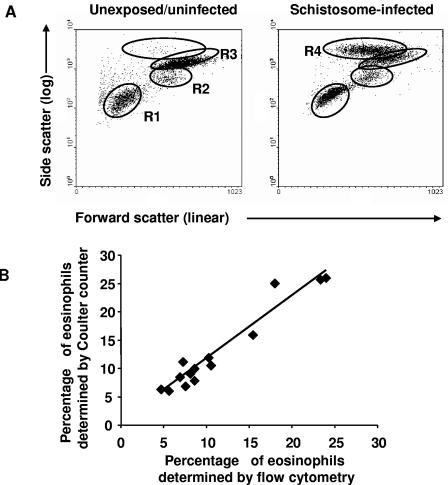
To evaluate eosinophilia in schistosomiasis patients and uninfected controls, cells from whole blood were electronically gated using forward and side scatter (linear forward scatter/log side scatter) as previously described (32). In individuals who did not have schistosomiasis, three distinct cell populations were evident (Fig. 1A). Fluorescence staining revealed that region 1 contained mostly lymphocytes, region 2 contained CD14+CD4+CXCR4+/−CCR5+/− cells (monocytes) (33, 34) and CD117+ CD4+/−CCR5+/−CXCR4+/−CD125−FcεRI β chainlow cells (mast cell precursors) (28), and region 3 contained neutrophils, which were CD11b/CD18 (Mac-1)high, CD131w+, and CD125+/− (19, 54). In the schistosomiasis patients, a fourth cell population was also prominent. The cells in region 4 were CD125(IL-5R)+, Mac-1+ CD131w+, and CD23 (low-affinity FcεRII)low (10, 26), consistent with characteristics of eosinophils. To confirm that the cells in region 4 were eosinophils, blood from a subset of patients was also analyzed on a Coulter Counter to generate cellular differentials. Eosinophil percentages estimated by flow cytometry plotted against Coulter Counter-assessed percentages demonstrated a strong correlation (r = 0.890, P < 0.0001) between the two systems (Fig. 1B).
FIG. 1.
Eosinophilia in schistosome-infected car washers. (A) Whole blood was stained with fluorescently conjugated antibodies, followed by lysis of red blood cells and fixation of remaining leukocytes. Cells were electronically separated using forward and side scatter. Four distinct regions labeled R1 through R4 were analyzed. Region 1 contained mainly CD3+ and CD19+ lymphocytes, region 2 contained CD14+CD4+CXCR4+CCR5+ cells (monocytes) and CD117+CD4+/−CXCR4+/−CCR5+/−CD125−FcεRI β chainlow cells (mast cell precursors), region 3 contained CD11b/CD18 (Mac-1)high, CD131w+, CD125+/− cells (neutrophils), and region 4 contained CD125+Mac-1+CD131w+CD23low cells (eosinophils). (B) Eosinophil percentages generated by Coulter Counter were plotted again eosinophil percentages estimated by flow cytometry in the region 4 gate (r = 0.890, n = 14, P < 0.0001).
CD4+-T-cell counts and antigen-specific-IL-5 production correlate with percentages of peripheral blood eosinophils.
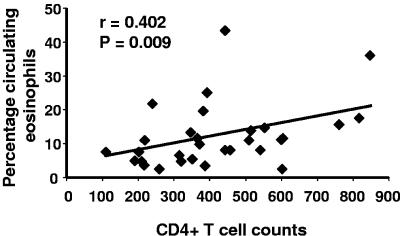
An HIV-1-related decline in CD4+ T cells is a risk factor for reinfection with schistosomes in this population (22). To determine whether alteration in eosinophilia may in part be related to this observation, we plotted percentages of eosinophils against CD4+-T-cell counts. In HIV-positive car washers, there was a positive correlation between CD4+-T-cell counts and percentages of eosinophils (r = 0.402, n = 20, P = 0.009) (Fig. 2), but there was no correlation between CD4+-T-cell counts and eosinophil percentages in HIV-negative subjects (r = 0.032, n = 43, P = 0.841) (data not shown). CD4+-T-cell counts were not related to percentages of neutrophils in either group (data not shown).
FIG. 2.
CD4+-T-cell counts correlate with percentages of eosinophils in HIV-1-seropositive subjects. CD4+-T-cell counts generated by FACSCount were plotted against the corresponding percentage of peripheral blood eosinophils of HIV-1-seropositive individuals (n = 20).
Because IL-5 is an eosinophil growth and differentiation factor (56), we evaluated whether schistosome antigen-specific-cytokine production was correlated with eosinophilia. We found that IL-5 produced by SWAP-exposed PBMCs correlated significantly with percent eosinophils (r = 0.415, n = 33, P = 0.016). As HIV-1-infected individuals have fewer CD4+ T cells than persons without viral coinfection, we tested whether there was a difference in the amounts of SWAP-induced IL-5 on a per-CD4+-T-cell basis between HIV-positive and HIV-negative car washers. Although the median IL-5 production per CD4+ T cell by HIV-negative car washers' PBMCs was more than sevenfold higher than that from HIV-positive subjects (0.0731 pg/ml compared to 0.0095 pg/ml), with the current number of participants and high degree of variation of these data, this difference was not statistically significant. There were no other correlations between eosinophilia and PBMC antigen-specific-cytokine production for any other cytokines or antigens tested (data not shown).
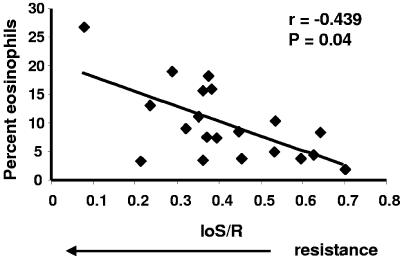
Percentages of peripheral blood eosinophils demonstrate an inverse relationship with IoS/R.
To evaluate whether eosinophils were associated with resistance to reinfection by S. mansoni in this population, we analyzed the patients' eosinophil percentages against their IoS/R values. In HIV-negative subjects there was a positive correlation between resistance to reinfection (lower IoS/R values) and proportions of circulating eosinophils (r = −0.439, n = 20, P = 0.040) (Fig. 3). There was no significant relationship between eosinophilia and resistance in HIV-positive subjects (r = −0.010, n = 21, P = 0.958) (data not shown). For comparison, we also evaluated IoS/Rs in relationship to neutrophil percentages, but there were no statistically significant correlations observed between these parameters in either group of car washers (data not shown).
FIG. 3.
A high percentage of circulating eosinophils correlates with a resistant phenotype. Percentages of eosinophils plotted against IoS/R values (n = 20) are shown. A low IoS/R value indicates a resistant phenotype.
Eosinophils express FcεRI β chain.
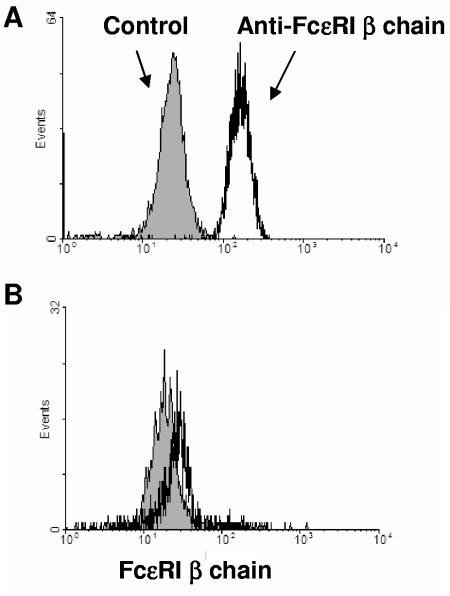
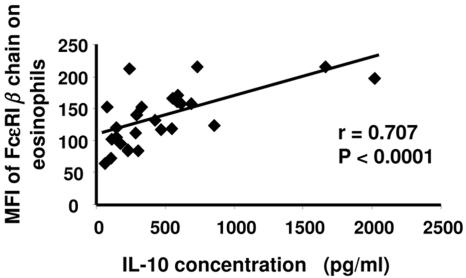
Because IgE levels have been associated with resistance to schistosomiasis (6, 12, 13, 27, 39), IgE receptor expression on eosinophils was evaluated. Antibodies specific for the β chain of FcεRI were used to assess levels of the high-affinity IgE receptor by flow cytometry. While most similar studies have evaluated FcεRI α chain expression, IgE must be stripped to effectively detect the α chain, and we wished to avoid in vitro manipulation of the cells. Eosinophils from all schistosome-exposed subjects had high-level expression (median fluorescence intensity [MFI]) of the FcεRI β chain (Fig. 4A). In contrast, nonexposed subjects had virtually undetectable fluorescence on what few eosinophils they had in the peripheral blood (Fig. 4B). FcεRI β MFIs of HIV-negative (134.8 ± 43.4; n = 20) and HIV-positive (154.6 ± 53.0; n = 11) (P > 0.05) patients were similar, and FcεRI β chain expression did not significantly correlate with IoS/R in either population, although the sample sizes available for analysis were small. However, because FcεRI β chain is not usually detected on eosinophils (1, 25, 40, 42, 43), we sought to determine the possible mechanisms of its upregulation in schistosomiasis. We analyzed levels of antigen-specific-cytokine production in relation to the MFIs of FcεRI β chain on eosinophils and found that SWAP-specific IL-10 production (r = 0.707, n = 27, P < 0.0001), but not IL-5, TNF-α, IL-4, IFN-γ, or IL-13 production (or other antigen-stimulated PBMCs) (data not shown), strongly correlated with FcεRI β chain expression in all car washers (Fig. 5). The low-affinity IgE receptor (CD23) was also assessed on eosinophils. CD23 expression was minimal and did not correlate with IoS/R or cytokine production (data not shown). Similarly, there were no differences observed between expression of CD23 in HIV-negative and HIV-positive subjects (data not shown).
FIG. 4.
Eosinophils from S. mansoni-infected individuals express FcεRI β chain. Whole blood cells were stained with antibody specific for FcεRI β chain or isotype control and processed by flow cytometry. Shown is the gated eosinophil population of cells from a representative schistosome-infected car washer (A) and cells from a representative control individual without schistosome infection (B). The histogram of the isotype control antibody is shown in gray.
FIG. 5.
SWAP-induced IL-10 production by PBMCs correlates with the level of FcεRI β chain on eosinophils. PBMCs were cultured in the presence of 10 μg of SWAP for 48 h and supernatants were assayed for IL-10 by standard ELISA. IL-10 concentrations were plotted against the corresponding MFI of FcεRI β chain on eosinophils (n = 27).
Surface expression of FcεRI β chain correlates with serum IgE levels.
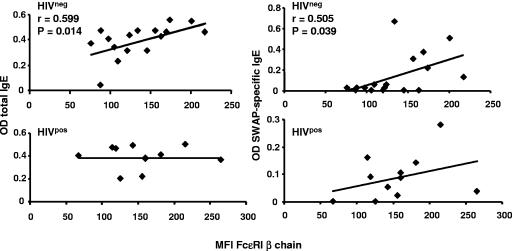
Surface FcεRI is regulated in part by IgE and concentrations of serum IgE correlate with levels of FcεRI on mast cells and basophils (1, 29, 40, 41, 43, 55). We sought to determine if a similar relationship to IgE existed with FcεRI expression on eosinophils and whether the relationship was maintained in HIV-positive individuals. The level of FcεRI β chain on eosinophils strongly correlates with levels of both total serum IgE (r = 0.599, n = 16, P = 0.014) and SWAP-specific IgE (r = 0.505, n = 16, P = 0.039) in HIV-negative individuals but not in HIV-positive patients (Fig. 6).
FIG. 6.
Serum IgE concentrations correlate with levels of FcεRI β chain on eosinophils. Total serum IgE or SWAP-specific IgE levels were determined by standard ELISA. OD values were plotted against the corresponding MFI of FcεRI β chain on eosinophils. FcεRI β chain MFI correlated with total IgE levels (n = 16) and SWAP-specific IgE (n = 16) for HIV-1-seronegative car washers but not for HIV-1-seropositive subjects (n = 11, P > 0.05).
Mac-1 expression on eosinophils is associated with susceptibility.
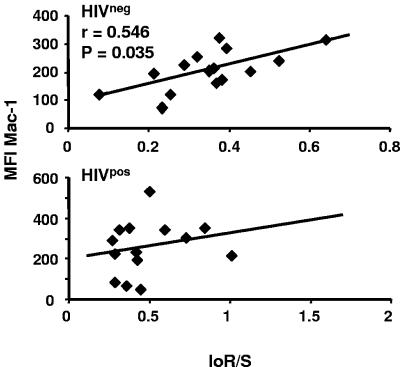
Activated eosinophils demonstrate increased larvicide activity in vitro compared to nonactivated cells (11, 16, 24). Thus, molecules associated with eosinophil activation, including CD11b/CD18 (Mac-1) (46), CD25 (32), and CD69 (32), were evaluated. A higher MFI of Mac-1 (P = 0.02) expression was found on eosinophils isolated from HIV-positive study participants (260.1 ± 126.3; n = 17) than was found on those from HIV-negative subjects (185.4 ± 124.5; n = 26). MFIs of Mac-1 plotted against IoS/R unexpectedly demonstrated that increased expression on eosinophils was associated with susceptibility to reinfection (higher IoS/R values) in HIV-negative subjects (r = 0.546, n = 15, P = 0.035), but no relationship existed in the HIV-positive group (r = 0.282, n = 14, P = 0.228) (Fig. 7). Interestingly, levels of Mac-1 did not correlate with the MFIs of CD25 or CD69, the other markers of eosinophils activation that we evaluated (P > 0.05; data not shown). Also in contrast to Mac-1, there were no differences in CD25 and CD69 expression between HIV-positive and HIV-negative groups, nor were there associations with IoS/R (data not shown). The MFI of FcεRI β chain correlated with the levels of CD25 (r = 0.755, n = 26, P < 0.0001) and CD69 (r = 0.589, n = 26, P = 0.001) but not Mac-1 (P > 0.05). No differences in expression of Mac-1 on neutrophils of the HIV-negative and HIV-positive cohorts were found, nor were there any associations between these activation markers on neutrophils and IoS/R values (data not shown).
FIG. 7.
Eosinophil expression of Mac-1 is associated with a susceptible phenotype. The MFIs of Mac-1 on gated eosinophils were plotted against IoS/R. Mac-1 expression on eosinophils correlated with a susceptible phenotype in the HIV-1-seronegative car washers (n = 15) but not in the HIV-1-seropositive individuals (n = 14, P > 0.05).
DISCUSSION
Our laboratory is investigating the mechanisms of host protection against schistosomiasis and the effect of HIV-1 coinfection in a population of Kenyan car washers who are occupationally exposed to infective cercariae of S. mansoni in a setting where their water contact can be well documented. This ongoing study has revealed individuals who become more resistant or who remain highly susceptible to reinfection with schistosomes after repeated treatments with praziquantel (22). By analyzing immune responses in resistant and susceptible individuals, we hope to identify correlates of protective immunity.
We found that a high percentage of circulating eosinophils is associated with a resistant phenotype in HIV-1-seronegative individuals, which corroborates findings from several previous studies (2, 18, 45). Eosinophil activation generally occurs in inflamed tissues (52), but circulating eosinophils from helminth-infected individuals exhibit an activated phenotype, suggesting that parasite infection may up regulate eosinophil function (32, 47). This is reflected in data showing that eosinophils isolated from schistosomiasis patients have increased IgE-mediated larvicidal activity in vitro compared to eosinophils from noninfected individuals (16).
A relatively consistent observation reported by many investigators, in spite of the assortment of parameters used to define resistance to schistosomiasis and the differences in the demographics of the many study populations, is that an elevated concentration of serum parasite-specific IgE is related to resistance against schistosomes (6, 12, 13, 27, 39). Thus, cells bearing IgE receptors may be important in protective mechanisms. Expression of CD23, the low-affinity IgE receptor, was minimal on the car washers' eosinophils, consistent with an activated phenotype (32), and there was no relationship of CD23 levels with resistance or susceptibility. Eosinophils isolated from allergic subjects have minimal expression of the higher-affinity IgE receptor, FcεRI (1, 25, 40, 42, 43). In contrast, we found that eosinophils from most members of our study group expressed the FcεRI β chain. This particular finding is significant because there are two isoforms of FcεRI. The tetrameric form of FcεRI consists of an α chain, two disulfide-linked γ chains, and a β chain and is expressed on mast cells and basophils (25). The trimeric form, found on monocytes, Langerhans cells, and dendritic cells, contains the α chain and 2 γ chains but lacks the β chain (25). The α chain binds IgE, whereas the β and γ chains are involved in cell signaling through immunoreceptor tyrosine-based kinase motifs in their cytoplasmic tails. Phosphorylation of the β chain amplifies signals triggered by IgE binding five- to sevenfold (25). Thus, it appears that infection with schistosomes may induce expression of tetrameric FcεRI and subsequently influence eosinophil effector function differently than in atopy. IL-10 production by SWAP-stimulated PBMCs strongly correlated with the level of corresponding FcεRI β chain on eosinophils, suggesting that parasite-induced IL-10 production, or other responses that we did not measure, may upregulate tetrameric FcεRI expression.
FcεRI on eosinophils was associated with CD25 and CD69, suggesting an association with cellular activation. Moreover, levels of FcεRI β chain significantly correlated with IgE concentrations in plasma of HIV-1 seronegative car washers, indicating that the regulation of the high-affinity IgE receptor on eosinophils is similar in some respects to that noted for basophils and mast cells (1, 29, 40, 41, 43, 55). The relationship between the levels of FcεRI β on eosinophils with IoS/R was not apparent, although our ability to detect anything but a very strong relationship was hindered by the sample size. Nevertheless, the expression of this molecule indicates a possible role for IgE-mediated immunity by activated eosinophils in schistosomiasis.
Expression of CD69 or CD25 was not related to an individual's IoS/R but increased Mac-1 expression was significantly associated with a history of susceptibility to reinfection. These data suggest that Mac-1 may interfere with protective mechanisms or that its upregulation is a result of undefined events that contribute to susceptibility. The absence of a relationship between Mac-1 and CD25, CD69, or FcεRI β chain levels on eosinophils does not fit with the conventional description of cellular activation (46, 51). Thus, high levels of Mac-1 may indicate an alternative activation state. Eosinophils from HIV-1-coinfected schistosomiasis patients also demonstrated significantly higher Mac-1 expression, emphasizing its ambiguous role in host susceptibility. Paradoxically, adherence of eosinophils to IgE-coated schistosomula is inhibited by anti-Mac-1 antibodies, which suggests that Mac-1 has a role in antibody-dependent cellular-mediated killing of worms (5). In addition, Mac-1-dependent cellular adhesion promotes degranulation and superoxide production by eosinophils, which are important events in causing damage to worms (20). High levels of Mac-1 on eosinophils at the site of worm attrition might therefore be protective, and its role on circulating eosinophils and association with a susceptible phenotype in this study group is of unknown significance.
In addition to the data presented in this report, our laboratory has analyzed cytokine production by PBMCs isolated from the car washers. Mwinzi et al. found that PBMCs isolated from HIV-1-coinfected individuals secrete decreased levels of Th2 cytokines compared to cells isolated from HIV-1-negative schistosomiasis patients (35). Interestingly, in other settings, HIV-1-infected patients with reduced CD4+-T-cell counts develop an allergic phenotype characterized by an elevated serum level of IgE and eosinophilia (8, 30, 38, 44, 48). The etiology of the allergic response is unknown (7, 15), but recent evidence implicates HIV-1 gp120 in inducing IL-4 and IL-13 release from FcεRI+ cells, such as basophils, by binding to the VH3 region of bound IgE (31, 37). Thus, putative intact immune responses, such as FcεRI expression, noted in the HIV-1-coinfected subjects in this study may result from the complexities of HIV-1 infection rather than an appropriate host reaction to a parasitic infection. Because HIV-1 can directly infect and kill eosinophils (53), HIV-1-coinfected schistosomiasis patients may have alterations in their normal eosinophil functions. The decreased ability to develop eosinophilia as CD4+ T cells decline, coupled with possible cellular dysregulation, such as increased Mac-1 expression (36) may impede the capacity of the HIV-1-positive car washers for resisting reinfection with schistosomes as effectively as their HIV-1-negative counterparts.
In conclusion, we have confirmed a correlation between peripheral blood eosinophilia and host resistance against reinfection with schistosomes and extended it into a setting of known occupational exposures. Furthermore, we have also broadened our knowledge regarding eosinophil cell surface receptors, their activation, and associated immune responses in this setting.
Acknowledgments
This work was published with the permission of the Director of the Kenya Medical Research Institute. We would like to thank Karen Wozniak for critical review of the manuscript, Kennedy Matuda for stool evaluation, and especially all the study participants.
L.M.G.-L. was supported by the American Society for Microbiology/National Center for Infectious Disease Postdoctoral Fellowship (2000-2002) and NIH grant T32 AI52070 (2003-2005) and C.B.C.-S. by Fondation pour la Recherche Médicale. This work was supported in part by NIH AI053695.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Borkowski, T. A., M. H. Jouvin, S. Y. Lin, and J. P. Kinet. 2001. Minimal requirements for IgE-mediated regulation of surface FcεRI. J. Immunol. 167:1290-1296. [DOI] [PubMed] [Google Scholar]
- 2.Butterworth, A. E., M. Capron, J. S. Cordingley, P. R. Dalton, D. W. Dunne, H. C. Kariuki, G. Kimani, D. Koech, M. Mugambi, J. H. Ouma, M. A. Prentice, B. A. Richardson, T. K. Arap Siongok, R. F. Sturrock, and D. W. Taylor. 1985. Immunity after treatment of human schistosomiasis mansoni. II. Identification of resistant individuals, and analysis of their immune responses. Trans. R. Soc. Trop. Med. Hyg. 79:393-408. [DOI] [PubMed] [Google Scholar]
- 3.Capron, M., H. L. Spiegelberg, L. Prin, H. Bennich, A. E. Butterworth, R. J. Pierce, M. A. Ouaissi, and A. Capron. 1984. Role of IgE receptors in effector function of human eosinophils. J. Immunol. 132:462-468. [PubMed] [Google Scholar]
- 4.Capron, M., J. P. Kusnierz, L. Prin, H. L. Spiegelberg, G. Ovlaque, P. Gosset, A. B. Tonnel, and A. Capron. 1985. Cytophilic IgE on human blood and tissue eosinophils: detection by flow microfluorometry. J. Immunol. 134:3013-3018. [PubMed] [Google Scholar]
- 5.Capron, M., M. D. Kazatchkine, E. Fischer, M. Joseph, A. E. Butterworth, J. P. Kusnierz, L. Prin, J. P. Papin, and A. Capron. 1987. Functional role of the α-chain complement receptor type 3 in human eosinophil-dependent antibody-mediated cytotoxicity against schistosomes. J. Immunol. 139:2059-2065. [PubMed] [Google Scholar]
- 6.Capron, M., and A. Capron. 1994. Immunoglobulin E and effector cells in schistosomiasis. Science 264:1876-1877. [DOI] [PubMed] [Google Scholar]
- 7.Clerici, M., and G. Shearer. 1994. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol. Today 15:575-581. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, A. J., and R. T. Steigbigel. 1996. Eosinophilia in patients infected with human immunodeficiency virus. J. Infect. Dis. 174:615-618. [DOI] [PubMed] [Google Scholar]
- 9.Colley, D. G., C. W. Todd, F. A. Lewis, and R. W. Goodgame. 1979. Immune responses during human schistosomiasis mansoni. VI. In vitro nonspecific suppression of phytohemagglutinin responsiveness induced by exposure to certain schistosomal preparation. J. Immunol. 122:1447-1453. [PubMed] [Google Scholar]
- 10.Conesa, A., P. Tassinari, H. Rivera, J. B. De Sanctis, N. Bianco, and O. Aldrey. 2002. Hypodense eosinophils: characterization of surface molecule expression. Allergy Asthma Proc. 23:117-124. [PubMed] [Google Scholar]
- 11.David, J. R., M. A. Vadas, A. E. Butterworth, P. A. de Brito, E. M. Carvalho, R. A. David, J. C. Bina, and Z. A. Andrade. 1980. Enhanced helminthotoxic capacity of eosinophils from patients with eosinophilia. N. Engl. J. Med. 303:1147-1152. [DOI] [PubMed] [Google Scholar]
- 12.Demeure, C. E., P. Rihet, L. Abel, M. Ouattara, A. Bourgois, and A. J. Dessein. 1993. Resistance to Schistosoma mansoni in humans: influence of IgE/IgG4 balance and IgG2 in immunity to reinfection after chemotherapy. J. Infect. Dis. 168:1000-1008. [DOI] [PubMed] [Google Scholar]
- 13.Dunne, D. W., A. E. Butterworth, A. J. Fulford, J. H. Ouma, and R. F. Sturrock. 1992. Human IgE responses to Schistosoma mansoni and resistance to reinfection. Mem. Inst. Oswaldo Cruz 87(Suppl. 4):99-103. [DOI] [PubMed] [Google Scholar]
- 14.Engels, D., L. Chitsulo, A. Montresor, and L. Savioli. 2002. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 82:139-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galhardo, M. C., M. Perez, M. G. Morgado, S. Almeida, L. M. Azevedo, I. Georg, H. Ferreira, and E. N. Sarno. 2000. Search for evidence of a Th2 profile in HIV+ patients. Int. J. Dermatol. 39:109-115. [DOI] [PubMed] [Google Scholar]
- 16.Gounni, A. S., B. Lamkhioued, K. Ochiai, Y. Tanaka, E. Delaporte, A. Capron, J. P. Kinet, and M. Capron. 1994. High-affinity IgE receptor on eosinophils is involved in defense against parasites. Nature 367:183-186. [DOI] [PubMed] [Google Scholar]
- 17.Gounni, A. S., B. Lamkioued, E. Delaporte, A. Dubost, J. P. Kinet, A. Capron, and M. Capron. 1994. The high affinity IgE receptor on eosinophils: from allergy to parasites or from parasites to allergy? J. Allergy Clin. Immunol. 94:1214-1216. [DOI] [PubMed] [Google Scholar]
- 18.Hagan, P., U. J. Blumenthal, M. Chaudri, B. M. Greenwood, R. J. Hayes, J. Hodgson, C. Kelly, M. Knight, A. J. G. Simpson, S. R. Smithers, and H. A. Wilkins. 1987. Resistance to reinfection with Schistosoma haematobium in Gambian children: analysis of their immune responses. Trans. R. Soc. Trop. Med. Hyg. 81:938-946. [DOI] [PubMed] [Google Scholar]
- 19.Hickstein, D. D., J. Ozols, S. A. Williams, J. U. Baenziger, R. M. Locksley, and G. J. Roth. 1987. Isolation and characterization of the receptor on human neutrophils that mediates cellular adherence. J. Biol. Chem. 262:5576-5580. [PubMed] [Google Scholar]
- 20.Horie, S., and H. Kita. 1994. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human recombinant granulocytes-macrophage colony-stimulating factor and platelet activating factor. J. Immunol. 152:5457-5467. [PubMed] [Google Scholar]
- 21.Iikura, M., M. Yamaguchi, K. Hirai, M. Miyamasu, H. Yamada, T. Nakajima, T. Fujisawa, C. Ra, Y. Morita, and K. Yamamoto. 2001. Regulation of surface FcεRI expression on human eosinophils by IL-4 and IgE. Int. Arch. Allergy Immunol. 124:470-477. [DOI] [PubMed] [Google Scholar]
- 22.Karanja, D. M. S., A. W. Hightower, D. G. Colley, P. N. M. Mwinzi, K. Galil, J. Andove, and W. E. Secor. 2002. Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and effect of HIV-1 co-infection on susceptibility to schistosomiasis: a longitudinal study. Lancet 360:592-596. [DOI] [PubMed] [Google Scholar]
- 23.Kayaba, H., D. Dombrowicz, G. Woerly, J. P. Papin, S. Loiseau, and M. Capron. 2001. Human eosinophils and human high affinity IgE receptor transgenic mouse eosinophils express low levels of high affinity IgE receptor but release IL-10 upon receptor activation. J. Immunol. 167:995-1003. [DOI] [PubMed] [Google Scholar]
- 24.Kimani, G., G. M. Mkoji, J. R. Rashid, J. M. Mbugua, D. Koech, T. Kamau, and B. Mungai. 1993. Enhancement of eosinophil-mediated cytotoxicity to schistosomula of Schistosoma mansoni by autologous mononuclear cells from patients. Parasite Immunol. 15:251-260. [DOI] [PubMed] [Google Scholar]
- 25.Kinet, J. P. 1999. The high-affinity IgE receptor (FcεRI): from physiology to pathology. Annu. Rev. Immunol. 17:931-936. [DOI] [PubMed] [Google Scholar]
- 26.Kita, H., M. Kaneko, K. R. Bartemes, D. A. Weiler, A. W. Schimming, C. E. Reed, and G. J. Gleich. 1999. Does IgE bind to and activate eosinophils from patients with allergy? J. Immunol. 162:6901-6911. [PubMed] [Google Scholar]
- 27.Li, Y., A. C. Sleigh, A. G. Ross, Y. Li, X. Zhang, G. M. Williams, X. Yu, M. Tanner, and D. P. McManus. 2001. Human susceptibility to Schistosoma japonicum in China correlates with antibody isotypes to native antigens. Trans. R. Soc. Trop. Med. Hyg. 95:441-448. [DOI] [PubMed] [Google Scholar]
- 28.Li, Y., L. Li, R. Wadley, S. W. Reddel, J. C. Qi, C. Archis, A. Collins, E. Clark, M. Cooley, S. Kouts, H. M. Naif, M. Alali, A. Cunningham, G. W. Wong, R. L. Stevens, and S. A. Krilis. 2001. Mast cells/basophils in the peripheral blood of allergic individuals who are HIV-1 susceptible due to their surface expression of CD4 and the chemokine receptors CCR3, CCR5, and CXCR4. Blood 97:3484-3490. [DOI] [PubMed] [Google Scholar]
- 29.Malveaux, F. J., M. C. Conroy, N. F. Adkinson, Jr., and L. M. Lichtenstein. 1978. IgE receptors on human basophils. Relationship to serum IgE concentration. J. Clin. Investig. 62:176-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marone, G., G. Florio, M. Triggiani, A. Petraroli, and A. de Paulis. 2000. Mechanisms of IgE elevation in HIV-1 infection. Crit. Rev. Immunol. 20:477-496. [PubMed] [Google Scholar]
- 31.Marone, G., G. Florio, A. Petraroli, and A. de Paulis. 2001. Dysregulation of the IgE/FcεRI network in HIV-1 infection. J. Allergy Clin. Immunol. 107:22-30. [DOI] [PubMed] [Google Scholar]
- 32.Mawhorter, S. D., D. A. Stephany, E. A. Ottesen, and T. B. Nutman. 1996. Identification of surface molecules associated with physiologic activation of eosinophils. Application of whole-blood flow cytometry to eosinophils. J. Immunol. 156:4851-4858. [PubMed] [Google Scholar]
- 33.Melendez-Guerrero, L. M., J. K. Nicholson, and J. S. McDougal. 1990. In vitro infection of monocytes with HIVBa-L. Effect on cell surface expression of CD4, CD14, HLA-DR, and HLA-DQ. AIDS Res. Hum. Retrovir. 6:731-741. [DOI] [PubMed] [Google Scholar]
- 34.Moore, J. P., A. Trkola, and T. Dragic. 1997. Co-receptors for HIV-1 entry. Curr. Opin. Immunol. 9:551-562. [DOI] [PubMed] [Google Scholar]
- 35.Mwinzi, P. N. M., D. M. S. Karanja, D. G. Colley, A. S. S. Orago, and W. E. Secor. 2001. Cellular immune response of schistosomiasis patients are altered by human immunodeficiency virus type 1 coinfection. J. Infect. Dis. 184:488-496. [DOI] [PubMed] [Google Scholar]
- 36.Palmer, S., and A. S. Hamblin. 1993. Increased CD11/CD18 expression on the peripheral blood leukocytes of patients with HIV disease: relationship to disease severity. Clin. Exp. Immunol. 93:344-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patella, V., G. Florio, A. Petraroli, and G. Marone. 2000. HIV-1 gp120 induces IL-4 and IL-13 release from human FcεRI+ cells through interaction with the VH3 region of IgE. J. Immunol. 164:589-595. [DOI] [PubMed] [Google Scholar]
- 38.Rancinan, C., P. Morlat, G. Chene, S. Guez, A. Baquey, J. Beylot, and R. Salamon. 1998. IgE serum level: a prognostic marker for AIDS in HIV-infected adults? J. Allergy Clin. Immunol. 102:329-330. [DOI] [PubMed] [Google Scholar]
- 39.Rihet, P., C. E. Demeure, A. Bourgois, A. Prata, and A. J. Dessein. 1991. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur. J. Immunol. 21:2679-2686. [DOI] [PubMed] [Google Scholar]
- 40.Saini, S. S., A. D. Klion, S. M. Holland, R. G. Hamilton, B. S. Bochner, and D. W. MacGlashan, Jr. 2000. The relationship between serum IgE and surface levels of FcεR on human leukocytes in various diseases: correlation of expression with FcεRI on basophils but not on monocytes or eosinophils. J. Allergy Clin. Immunol. 106:514-520. [DOI] [PubMed] [Google Scholar]
- 41.Saini, S. S., J. J. Richardson, C. Wofsy, S. Lavens-Philips, B. S. Bochner, and D. W. MacGlashan, Jr. 2001. Expression and modulation of FcεRIα and FcεRIβ in human blood basophils. J. Allergy Clin. Immunol. 107:832-841. [DOI] [PubMed] [Google Scholar]
- 42.Seminario, M. C., S. S. Saini, D. W. MacGlashan, Jr., and B. S. Bochner. 1999. Intracellular expression and release of FcεRIα by human eosinophils. J. Immunol. 162:6893-6900. [PubMed] [Google Scholar]
- 43.Sihra, B. S., O. M. Kon, J. A. Grant, and A. B. Kay. 1997. Expression of high-affinity IgE receptors (FcεRI) on peripheral blood basophils, monocytes, and eosinophils in atopic and non-atopic subjects: Relationship to total serum IgE concentrations. J. Allergy Clin. Immunol. 99:699-706. [DOI] [PubMed] [Google Scholar]
- 44.Skiest, D. J., and P. Keiser. 1997. Clinical significance of eosinophilia in HIV-infected individuals. Am. J. Med. 102:449-453. [DOI] [PubMed] [Google Scholar]
- 45.Sturrock, R. F., R. Kimani, B. J. Cottrell, A. E. Butterworth, H. M. Seitz, T. K. Siongok, and V. Houba. 1983. Observations on possible immunity to reinfection among Kenyan school children after treatment for Schistosoma mansoni. Trans. R. Soc. Trop. Med. Hyg. 77:363-371. [DOI] [PubMed] [Google Scholar]
- 46.Thorne, K. J., B. A. Richardson, G. Mazza, and A. E. Butterworth. 1990. A new method for measuring eosinophil activating factors based on increased expression of CR3 α chain (CD11b) on the surface of activated eosinophils. J. Immunol. Methods 133:47-54. [DOI] [PubMed] [Google Scholar]
- 47.Thorne, K. J., and G. Mazza. 1991. Eosinophilia, activated eosinophils and human schistosomiasis. J. Cell Sci. 98:265-270. [DOI] [PubMed] [Google Scholar]
- 48.Tietz, A., L. Sponagel, P. Erb, H. Butcher, M. Battagay, and W. Zimmerli. 1997. Eosinophilia in patients infected with the human immunodeficiency virus. Eur. J. Clin. Microbiol. Infect. Dis. 16:675-677. [DOI] [PubMed] [Google Scholar]
- 49.Todd, C. W., and D. G. Colley. 2002. Practical and ethical issues in the development of a vaccine against schistosomiasis mansoni. Am. J. Trop. Med. Hyg. 66:348-358. [DOI] [PubMed] [Google Scholar]
- 50.Vadas, M. A., J. R. David, A. E. Butterworth, V. Houba, R. F. Sturrock, L. David, R. Herson, T. A. Siongok, and R. Kimani. 1980. Functional studies on purified eosinophils and neutrophils from patients with Schistosoma mansoni infections. Clin. Exp. Immunol. 39:683-694. [PMC free article] [PubMed] [Google Scholar]
- 51.Walker, C., S. Rihs, R. K. Braun, S. Betz, and P. L. Bruijnzeel. 1993. Increased expression of CD11b and functional changes in eosinophils after migration across endothelial cell monolayers. J. Immunol. 150:4061-4071. [PubMed] [Google Scholar]
- 52.Weller, P. F. 1994. Eosinophils: structure and functions. Curr. Opin. Immunol. 6:85-90. [DOI] [PubMed] [Google Scholar]
- 53.Weller, P. F., W. L. Marshall, D. R. Lucey, T. H. Rand, A. M. Dvorak, and R. W. Finberg. 1995. Infection, apoptosis, and killing of mature human eosinophils by human immunodeficiency virus-1. Am. J. Respir. Cell Mol. Biol. 13:610-620. [DOI] [PubMed] [Google Scholar]
- 54.Yamada, T., Q. Sun, K. Zeibecoglou, J. Bungre, J. North, A. B. Kay, A. F. Lopez, and D. S. Robinson. 1998. IL-3, IL-5, granulocyte-macrophage colony-stimulating factor receptor alpha-subunit, and common beta-subunit expression by peripheral leukocytes and blood dendritic cells. J. Allergy Clin. Immunol. 101:677-682. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi, M., K. Sayama, K. Yano, C. S. Lantz, N. Noben-Trauth, C. Ra, J. J. Costa, and S. J. Galli. 1999. IgE enhances Fcε receptor I expression and IgE-dependent release of histamine and lipid mediators from human umbilical cord blood-derived mast cells: synergistic effect of IL-4 and IgE on human mast cell Fcε receptor I expression and mediator release. J. Immunol. 162:5455-5465. [PubMed] [Google Scholar]
- 56.Yamaguchi, Y., Y. Hayashi, Y. Sugama, Y. Miura, T. Kasahara, S. Kitamura, M. Torisu, S. Mita, A. Tominaga, and K. Takatsu. 1988. Highly purified murine interleukin 5 (IL-5) stimulates eosinophil function and prolongs in vitro survival. IL-5 as an eosinophil chemotactic factor. J. Exp. Med. 167:1737-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]