Abstract
Oral immunization of healthy adults with 107 CFU BCG Moreau Rio de Janeiro was well tolerated and significantly boosted gamma interferon responses to purified protein derivative, Ag85, and MPB70 from previous childhood intradermal BCG immunization. Oral BCG offers the possibility of a needle-free tuberculosis vaccine and of boosting the protective immunity from intradermal tuberculosis vaccines.
DNA, recombinant viruses (13), subunit proteins, and genetically modified mycobacteria (2, 4, 5, 15) are being developed as vaccines against adult pulmonary tuberculosis, but down-selection of candidates will be slow, expensive, and contentious. A vaccine based on an adaptation of existing technology that could enter the market rapidly is therefore attractive. As a booster vaccine for infants, or immunocompetent adults or adolescents, to extend the protection afforded by neonatal BCG (19) will almost certainly be required, a “BCG-plus” strategy is gaining attention. M. bovis naturally spreads via the gastrointestinal tract, and BCG was developed by Calmette and Guérin as an oral vaccine. In Brazil, Assis demonstrated that repeated oral doses of BCG Moreau were highly effective in preventing tuberculosis (1), and Brazil routinely employed single-dose oral immunization with 100 mg of BCG Moreau up to the mid-1970s. We used the oral vaccine, still licensed for children and adults in Brazil, prepared from a World Health Organization-defined seed lot designated Mycobacterium bovis BCG substrain Moreau Rio de Janeiro, cultured in a proprietary Sauton medium, suspended in 5 ml 1.5% sodium glutamate solution, and presented as a single 100-mg dose that we found to contain between 3.4 × 107 and 7.3 × 107 CFU viable bacilli. BCG Moreau substrains retain the RD2 sequence deleted from BCG Pasteur but have a unique RD16 deletion and characteristically do not produce cervical adenitis, otitis media, or retropharyngeal abscess after oral delivery (12), even after repeated high doses during cancer chemotherapy (18).
Subjects, immunization, and reactogenicity.
The phase 1 study protocol was approved by the Wandsworth Local Research Ethics Committee (reference 00.60.03) and the UK MCA/MHRA (MF8000/10030). All subjects provided written informed consent. Fifteen minutes after receiving 100 ml of 2% sodium bicarbonate buffer, 29 healthy volunteers (12 male, 17 female; mean age, 25), all of whom had received intradermal BCG in childhood or adolescence, received a single oral 100-mg dose of BCG, mixed with bicarbonate buffer. The vaccine was extremely well tolerated with no vaccine-related serious adverse events. Eight subjects (28%) reported mild to moderate, transient odynophagia, 9 (29%) reported headache, and 6 (21%) reported gastrointestinal symptoms related to bicarbonate buffer (which we used to abolish gastric acid but which the manufacturer's data sheet does not routinely recommend, and its omission could increase palatability and eliminate the requirement for clean water to dispense). Clinical examination, including ear, nose, and throat inspection, and C-reactive protein levels of subjects reporting odynophagia after immunization were normal.
Immune responses induced by immunization.
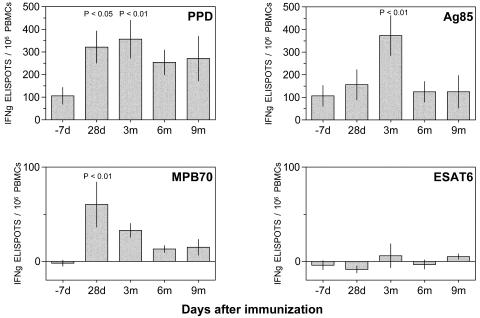
Antigen-specific peripheral blood mononuclear cells (PBMCs) secreting gamma interferon (IFN-γ) were detected by ELISPOT based on that reported by Lalvani et al. (10), in which unfractionated PBMCs are stimulated for 18 h with M. tuberculosis antigens (purified protein derivative [PPD], Statens Serum Institute, Copenhagen, Denmark; MPB70 recombinant protein, Lionex, Braunschweig, Germany; ESAT-6 recombinant protein, antigen 85 complex, gift from NIH, NIAID, Tuberculosis Research Materials and Vaccine Testing contract no. NO1 AI-75320). The background activity of IFN-γ-secreting cells in control wells was constant (data not shown). As all subjects had previously received intradermal BCG, PBMCs secreting IFN-γ in response to PPD were detectable prior to immunization (Fig. 1), and boosting of this baseline frequency was observed from day 7 after a single oral dose of liquid vaccine, sustained throughout the 9 months of follow-up, and significantly raised from baseline at months 1 and 3 in a strict per-protocol analysis. MPB70 accounts for as much as 10% of BCG Moreau culture filtrate but is expressed to a much lower level in BCG Glaxo (14), which would have been the strain subjects had previously received. Consequently, IFN-γ-secreting cells responding to MPB70 were not detectable before immunization but appeared following oral immunization, peaking at day 28 (P < 0.01 from day 0), followed by a gradual decline to a plateau, which was still raised from baseline at 9 months. The ability of oral BCG to stimulate an immune response to this internal secreted antigen provides encouragement for development of recombinant BCG (rBCG) vaccines expressing heterologous antigens. Ag85 is a highly immunogenic major secretion product of many mycobacterial strains (20) and is being exploited in subunit and recombinant live tuberculosis vaccine candidates. PBMCs secreting IFN-γ in response to Ag85 were detectable prior to immunization, and there were increases to peak mean numbers of 460 per 106 on day 7 after immunization for the whole group and 373 per 106 at 3 months when only per-protocol subjects were analyzed (Fig. 1). This is similar to that obtained recently with modified vaccinia Ankara-Ag85 as a single-antigen delivery system to boost prior intradermal BCG immunization (peak 783 per 106), although in that study the numbers fell sharply after day 7 (13). ESAT-6 is coded in the RD1 gene segment, which has been deleted from BCG, and an immune response to this antigen has been used to differentiate exposure to M. tuberculosis from that to BCG (10). Although sporadic low-level background activity could be detected, there was no discernible increase in the frequency of IFN-γ-secreting cells after immunization (Fig. 1), indicating that exposure to other mycobacteria was not the cause of the immune responses we observed.
FIG. 1.
Frequency of PBMCs secreting IFN-γ in response to mycobacterial antigens at primary protocol time points after oral immunization with BCG. Values are frequency of IFN-γ-secreting cells per 106 PBMCs (corrected for background activity in wells without antigen) for 13 subjects with complete data sets at primary protocol time points after oral immunization. Columns are group means. Error bars are standard errors of the means. Differences from day 0 were analyzed using GraphPad Prism by repeated-measures one-way analysis of variance and corrected by Dunnett's test for multiple comparisons.
Effect of oral BCG immunization on skin tuberculin sensitivity.
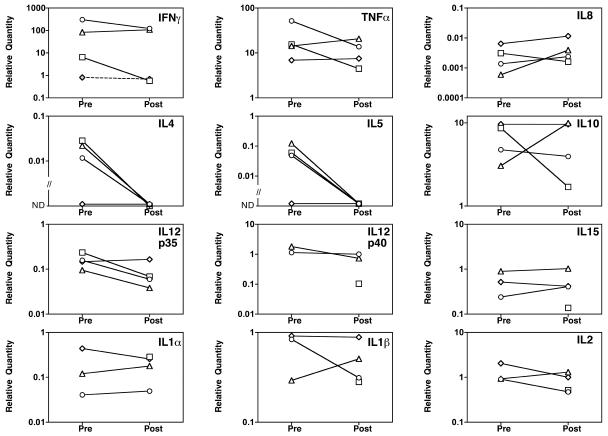
A strong skin delayed-type hypersensitivity (DTH) response to tuberculin has been linked to protection against tuberculosis. Intriguingly, studies with repeated high doses of lyophilized BCG preparations given orally confirmed early observations that when the first exposure to BCG is via the oral route, it infrequently primes for a skin response to tuberculin (8) and may even suppress induction of tuberculin response by subsequent intradermal BCG boost. We investigated whether oral BCG boosting modulated skin tuberculin reactions of subjects that were present from previous parenteral immunization, by taking 3-mm skin biopsies from the area of inflammation induced 7 days after standard Heaf testing in four subjects, before and 1 month after oral BCG immunization. Relative quantities of cytokine mRNA expression was determined after cDNA conversion using Superscript II, with a TaqMan cytokine gene expression plate according to the manufacturer's instructions, from RNA extracted by silica/ceramic bead ribolysis and chloroform-sodium acetate precipitation, and cleaned with a QIAGEN kit (79254). Before immunization, as might be expected, there was a high expression of TH1-type cytokine mRNA at the site of the skin tuberculin response and only low or undetectable RNA expression of interleukin 4 (IL-4) and IL-5 (Fig. 2). After oral BCG immunization, the expression of IL-4 and IL-5 became undetectable in all subjects, while proinflammatory and TH1 cytokines remained unchanged. There was also no significant change in the Heaf grade after oral BCG boosting (data not shown). These very preliminary data do not suggest that oral boosting of previous parenteral immunization abrogates skin tuberculin reactivity or causes a switch from TH1 to TH2 type responses to intradermal tuberculin challenge.
FIG. 2.
Relative quantity of cytokine mRNA in skin biopsies of Heaf tests before and after oral liquid BCG vaccine. Values indicate relative amounts of cytokine RNA extracted from skin biopsies taken 7 days after a standard tuberculin Heaf test, either before oral BCG immunization or 1 month after. Subject V039, diamonds; subject V040, circles; subject V041, squares; subject V043, triangles. ND, not detected. All cytokine mRNAs were assessed relative to a standard control RNA except V039 IFN-γ, which used a different control RNA (indicated by dotted line). No preimmunization results on V041 for IL-1α and -β, IL-2, IL-12p40, and IL-15 were available due to insufficient sample. The y axes are plotted on a logarithmic scale.
Intradermal BCG immunization requires technical skill, and although intradermal reimmunization appears to be safe (11, 17), it does not confer additional protection (6, 7, 9, 16), possibly as a result of prompt removal of viable bacilli by anti-BCG skin DTH responses. Bulk culture of BCG is inexpensive, and eliminating lyophilization further reduces costs, while oil-based or other diluent systems that prolong thermostability while retaining efficacy of oral BCG (3) or freezing may prolong the shelf life of liquid vaccines. The 8-decade safety profile of oral BCG Moreau Rio de Janeiro may offer an expedited route through the regulatory process, opening the possibility of using oral BCG with the 6-, 10-, and 14-week immunizations in the current EPI (Expanded Program on Immunization) childhood schedule to boost neonatal intradermal BCG vaccine or as a needle-free oral booster for adolescents and adults where live vaccines are not contraindicated by prevalence of HIV or other immune deficiency. Whether needle-free immunization with oral BCG could ultimately supplant intradermal BCG, and whether attenuated forms of rBCG will be suitable for oral use in immunodeficient adults, will require further trials of efficacy and safety.
Acknowledgments
This work was supported by Wellcome Trust Programme grant 043139 and the Commission of the European Union Integrated Project ‘MUVAPRED,’ contract number LSHP-CT-2003-503240.
Editor: J. L. Flynn
REFERENCES
- 1.Assis, A. 1950. Novas perspectivas de Calmettizaçâo. Hospital (RioJ) 37:337-353. [Google Scholar]
- 2.Brennan, M. J., F. M. Collins, and S. L. Morris. 1999. Propelling novel vaccines directed against tuberculosis through the regulatory process. Tuber. Lung Dis. 79:145-151. [DOI] [PubMed] [Google Scholar]
- 3.Buddle, B. M., F. E. Aldwell, M. A. Skinner, G. W. de Lisle, M. Denis, H. M. Vordermeier, R. G. Hewinson, and D. N. Wedlock. 2005. Effect of oral vaccination of cattle with lipid-formulated BCG on immune responses and protection against bovine tuberculosis. Vaccine 23:3581-3589. [DOI] [PubMed] [Google Scholar]
- 4.Dietrich, G., J. F. Viret, and J. Hess. 2003. Mycobacterium bovis BCG-based vaccines against tuberculosis: novel developments. Vaccine 21:667-670. [DOI] [PubMed] [Google Scholar]
- 5.Doherty, T. M., and P. Andersen. 2002. Tuberculosis vaccine development. Curr. Opin. Pulm. Med. 8:183-187. [DOI] [PubMed] [Google Scholar]
- 6.Dourado, I., M. H. Rios, S. M. Pereira, S. S. Cunha, M. Y. Ichihara, J. C. Goes, L. C. Rodrigues, A. L. Bierrenbach, and M. L. Barreto. 2003. Rates of adverse reactions to first and second doses of BCG vaccination: results of a large community trial in Brazilian schoolchildren. Int. J. Tuberc. Lung Dis. 7:399-402. [PubMed] [Google Scholar]
- 7.Ferreira, A. A., F. Ferreira Mde, E. A. Macedo, I. Cunha, S. L. Santos, A. R. Reis, M. G. Fortunato, A. A. Siquinelli, A. B. Figueiredo, I. Menezes, and W. D. Moreno. 2002. BCG revaccination in school children: evolution of the lesion at the vaccination site between 48 hours and 10 weeks. J. Pediatr. (Rio J.). 78:289-294. [DOI] [PubMed] [Google Scholar]
- 8.Hoft, D. F., E. B. Kemp, M. Marinaro, O. Cruz, H. Kiyono, J. R. McGhee, J. T. Belisle, T. W. Milligan, J. P. Miller, and R. B. Belshe. 1999. A double-blind, placebo-controlled study of Mycobacterium-specific human immune responses induced by intradermal bacille Calmette-Guerin vaccination. J. Lab. Clin. Med. 134:244-252. [DOI] [PubMed] [Google Scholar]
- 9.Karonga Prevention Trial Group. 1996. Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet 348:17-24. [PubMed] [Google Scholar]
- 10.Lalvani, A., A. A. Pathan, H. Durkan, K. A. Wilkinson, A. Whelan, J. J. Deeks, W. H. Reece, M. Latif, G. Pasvol, and A. V. Hill. 2001. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet 357:2017-2021. [DOI] [PubMed] [Google Scholar]
- 11.Leung, C. C., C. M. Tam, S. L. Chan, M. Chan-Yeung, C. K. Chan, and K. C. Chang. 2001. Efficacy of the BCG revaccination programme in a cohort given BCG vaccination at birth in Hong Kong. Int. J. Tuberc. Lung Dis. 5:717-723. [PubMed] [Google Scholar]
- 12.Lotte, A., O. Wasz-Hockert, N. Poisson, N. Dumitrescu, M. Verron, and E. Couvet. 1984. BCG complications. Estimates of the risks among vaccinated subjects and statistical analysis of their main characteristics. Adv. Tuberc. Res. 21:107-193. [PubMed] [Google Scholar]
- 13.McShane, H., A. A. Pathan, C. R. Sander, S. M. Keating, S. C. Gilbert, K. Huygen, H. A. Fletcher, and A. V. Hill. 2004. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 10:1240-1244. [DOI] [PubMed] [Google Scholar]
- 14.Miura, K., S. Nagai, M. Kinomoto, S. Haga, and T. Tokunaga. 1983. Comparative studies with various substrains of Mycobacterium bovis BCG on the production of an antigenic protein, MPB70. Infect. Immun. 39:540-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohara, N., and T. Yamada. 2001. Recombinant BCG vaccines. Vaccine 19:4089-4098. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues, L. C., S. M. Pereira, S. S. Cunha, B. Genser, M. Y. Ichihara, S. C. de Brito, M. A. Hijjar, I. Dourado, A. A. Cruz, C. Sant'Anna, A. L. Bierrenbach, and M. L. Barreto. 2005. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet 366:1290-1295. [DOI] [PubMed] [Google Scholar]
- 17.Tala-Heikkila, M. M., J. E. Tuominen, and E. O. Tala. 1998. Bacillus Calmette-Guerin revaccination questionable with low tuberculosis incidence. Am. J. Respir. Crit. Care Med. 157:1324-1327. [DOI] [PubMed] [Google Scholar]
- 18.Varella, A. D., D. C. Bandiera, A. R. de Amorim, Sr., L. A. Calvis, I. O. Santos, N. Escaleira, and F. Gentil. 1981. Treatment of disseminated malignant melanoma with high-dose oral BCG. Cancer 48:1353-1362. [DOI] [PubMed] [Google Scholar]
- 19.Wang, L., M. O. Turner, R. K. Elwood, M. Schulzer, and J. M. FitzGerald. 2002. A meta-analysis of the effect of Bacille Calmette Guerin vaccination on tuberculin skin test measurements. Thorax 57:804-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiker, H. G., and M. Harboe. 1992. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 56:648-661. [DOI] [PMC free article] [PubMed] [Google Scholar]