Abstract
The kinetics of effector CD8+-T-cell responses to specific Trypanosoma cruzi epitopes was investigated after challenge. Our results suggest that the delayed kinetics differs from that observed in other microbial infections and facilitates the establishment of the disease in naïve mice. In contrast, in vaccinated mice, the swift CD8+-T-cell response helps host survival after challenge.
Major histocompatibility complex class Ia-restricted CD8+ T cells are critical for the survival of naïve and vaccinated mice infected with the human protozoan parasite Trypanosoma cruzi (1, 5, 9, 11, 15, 16, 18). The antiparasitic mechanisms mediated by these cells are multiple, including cytokine secretion and possibly direct cytotoxicity against infected cells (6, 12, 13). In spite of their importance for host resistance, limited information is available regarding the kinetics of effector CD8+ T cells following parasite challenge. Here, we describe studies aimed at characterizing the kinetics of effector CD8+-T-cell responses specific to epitopes present in the trans-sialidase (TS) or the amastigote surface protein 2 (ASP-2), prime candidates for vaccine development against Chagas' disease (1, 4, 5, 18).
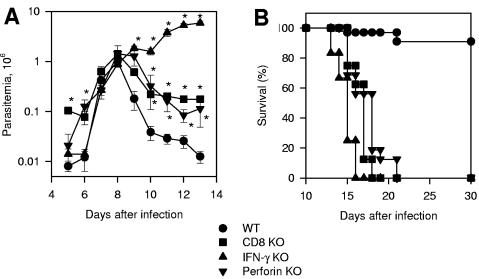
After infection with trypomastigotes of the Y strain, we observed that the parasitemia of wild-type (WT) C57BL/6, CD8α knockout (KO), gamma interferon (IFN-γ) KO, or perforin KO mice was not significantly different on day 8 after challenge (peak parasitemia of WT mice). From days 9 to 13, WT mice quickly reduced their parasitemia, and only 9% of them died after challenge (Fig. 1A and B). In contrast, IFN-γ KO mice were unable to control the parasitemia, dying faster than the other mouse groups. CD8α KO or perforin KO mice were unable to control their parasitemia at the same rate as WT animals, dying between days 15 and 21 after challenge (Fig. 1A and B). We concluded that IFN-γ secreted by non-CD8+ cells is important until day 13. CD8+ cells and perforin are critical for survival after day 14.
FIG. 1.
Trypomastigote-induced parasitemia and mortality in WT, CD8α KO, IFN-γ KO, or perforin KO mice. Groups of mice were infected intraperitoneally with 104 bloodstream trypomastigotes of the Y strain of T. cruzi. (A) Parasitemia was followed daily from days 5 to 13 after challenge. The results represent the mean of five or six mice ± standard deviation. The asterisks denote values statistically higher than the values in control WT mice (P < 0.05; one-way analysis of variance). The results are representative of two independent experiments. (B) Kaplan-Meier curves for survival of each mouse group: (i) WT, n = 32; (ii) CD8α KO, n = 8; (iii) IFN-γ KO, n = 12; (iv) perforin KO, n = 16. The results were pooled from two different experiments. Statistical analysis revealed significant differences in the survival of WT mice compared to the other mouse groups (P < 0.0001 in all cases; log rank test). CD8α KO and perforin KO mice survived longer than IFN-γ KO (P < 0.01 in both cases).
Based on the observations that CD8+ cells, IFN-γ, and perforin are critical for mouse survival after challenge, we followed the kinetics of specific effector CD8+ T cells by using the ex vivo enzyme-linked immunospot (ELISPOT) assay for IFN-γ (4) and the in vivo cytotoxicity assay, which measures the elimination of peptide-coated target cells mediated by perforin (3). Target cells were coated with synthetic peptides representing the CD8 T-cell epitope IYNVGQVSI (TS) or VNHRFTLV (ASP-2) (Table 1). The results of the in vivo cytotoxicity assay were obtained by measuring the carboxyfluorescein diacetate succinimidyl diester-labeled cells in the spleens of recipient mice (3). Identical results were seen when we analyzed labeled cells in lymph nodes (data not shown).
TABLE 1.
Peptides used in the present study
| Peptide | Antigen | aaa | MHCb restriction | Form of the parasite | Reference |
|---|---|---|---|---|---|
| IYNVGQVSI | TS | 359-367 | H-2Kd | Trypomastigote | 14 |
| VNHRFTLV | ASP-2 | 553-560 | H-2Kb | Amastigote | 8 |
| TEWETGQI | ASP-2 | 320-327 | H-2Kk | Amastigote | 1 |
aa, amino acids.
MHC, major histocompatibility complex.
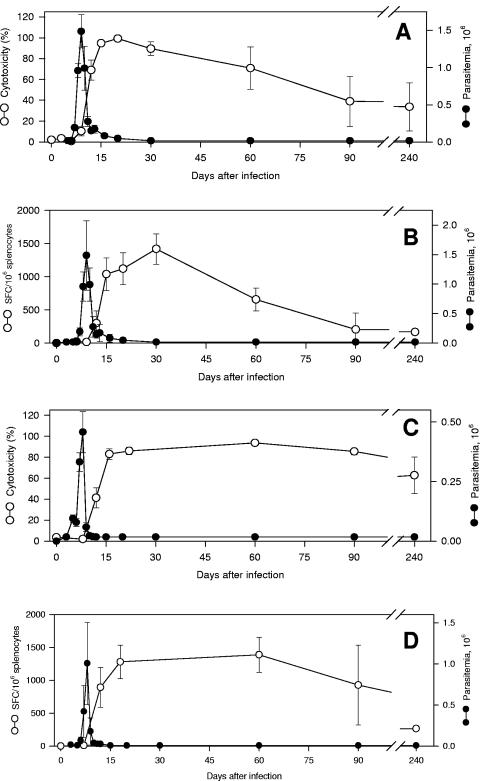
In BALB/c mice, the in vivo cytotoxicity and IFN-γ-secreting cells specific to the TS peptide IYNVGQVSI were first detected at the peak of parasitemia (day 9). Both T-cell activities rose quickly until the 15th day and were kept high until the 30th day after infection. During the subsequent period, they declined slowly but were still detectable by day 240 (Fig. 2A and B).
FIG.2.
Kinetics of peptide-specific cell-mediated immune responses in BALB/c or C57BL/6 mice after challenge with trypomastigotes of T. cruzi. BALB/c mice or C57BL/6 mice were challenged with 2.5 × 103 or 2.5 × 104 bloodstream trypomastigotes of T. cruzi, respectively. On the indicated days, the parasitemia was monitored in these animals (closed symbols in all panels). The results represent the mean of six mice ± standard deviation (SD). (A) The in vivo cytotoxic activity of BALB/c mice against target cells coated with peptide IYNVGQVSI was determined (open symbols). The results represent the mean of three to nine mice ± SD per group. (B) IFN-γ-producing spleen cells of BALB/c mice specific to the peptide IYNVGQVSI were estimated by the ELISPOT assay (4). The results represent the mean number of spot-forming cells (SFC) per 106 splenocytes ± SD (n = 4; open symbols). (C) The in vivo cytotoxic activity of C57BL/6 mice against target cells coated with peptide VNHRFTLV was determined. The results represent the mean of three or four mice ± SD per group (open symbols). (D) IFN-γ-producing spleen cells of C57BL/6 mice specific to the peptide VNHRFTLV were estimated by the ELISPOT assay. The results represent the mean number of SFC per 106 splenocytes ± SD (n = 4; open symbols). The results are representative of two or more independent experiments.
A similar picture emerged when we evaluated the kinetics of effector cells specific to the ASP-2 peptide VNHRFTLV. In C57BL/6 mice, both T-cell activities were not detectable at the peak of parasitemia (day 8). They rose quickly until the 16th day and were kept high until the 60th day after infection. During the subsequent period, both activities declined but were still detectable by day 240 (Fig. 2C and D).
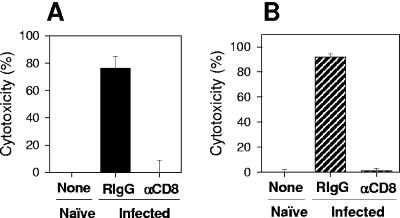
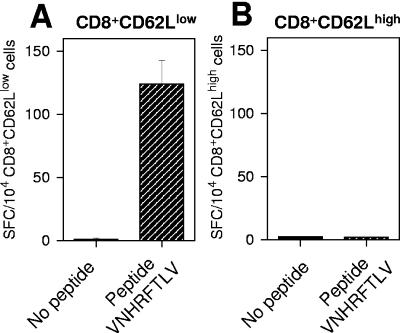
Treatment of T. cruzi-infected BALB/c or C57BL/6 mice with anti-CD8 monoclonal antibody resulted in a selective depletion of splenic CD8+ T cells in vivo (>98.6%) and a complete reversion of the in vivo cytotoxicity response to the peptide IYNVGQVSI or VNHRFTLV (Fig. 3A and B, respectively). The phenotypes of splenic IFN-γ-secreting cells were determined by sorting, with the aid of a FACS Vantage (Becton Dickinson), CD8+ cells costained with antibodies to CD62L (anti-CD8-Pe-CY5, clone 53-67, and anti-CD62L-FITC, clone Mel14; BD-Pharmingen) from C57BL/6 mice infected 80 days earlier. ELISPOT assays performed with these purified cells demonstrated that splenic IFN-γ-secreting cells were CD8+ CD62LLow (Fig. 4). Similar results had been described earlier for mice infected with T. cruzi (Brazil strain) for more than 150 days (10).
FIG.3.
Phenotypes of the cells mediating in vivo cytotoxic activity. BALB/c (A) or C57BL/6 (B) mice were challenged 30 days before the in vivo cytotoxic assay was performed with bloodstream trypomastigotes of T. cruzi as described in the legend to Fig. 1. Two days earlier, infected mice were treated with Rat immunoglobulin G (RIgG) or αCD8 monoclonal antibody (18). The in vivo cytotoxic activity against target cells coated with peptide IYNVGQVSI (A) or VNHRFTLV (B) was determined. The results represent the mean of three mice ± standard deviation and are representative of two independent experiments.
FIG. 4.
Phenotypes of IFN-γ-producing cells. C57BL/6 mice were challenged 80 days before the assay was performed with bloodstream trypomastigotes of T. cruzi as described in the legend to Fig. 1. CD8+ CD62LLow or CD8+ CD62LHigh cells were 97.96% or 94.41% pure, respectively (data not shown). An ELISPOT assay was used to estimate the number of IFN-γ-producing cells specific to the peptide VNHRFTLV in purified CD8+ CD62LLow (A) or CD8+ CD62LHigh (B) cells. The results represent the mean number of spot-forming cells (SFC) per 104 splenocytes ± standard deviation for triplicate cultures. The results are representative of two independent experiments.
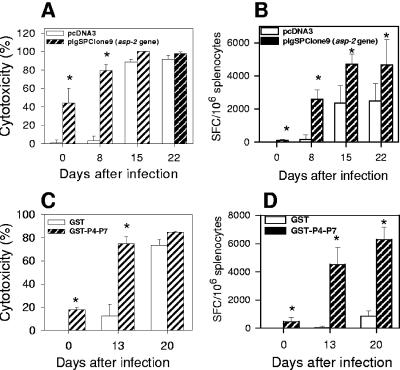
Finally, we compared the kinetics of effector CD8+-T-cell expansion following infection of immune or naïve animals. C57BL/6 or A/Sn mice were immunized with the plasmid pIgSPclone 9 (asp-2 gene) or with the recombinant protein gluthatione S-transferase (GST)-P4-P7, respectively (1, 4). After challenge, mice vaccinated with plasmid pIgSPclone9 (C57BL/6) or with the recombinant protein GST-P4-P7 (A/Sn) quickly developed in vivo specific cytotoxic cells against target cells coated with the peptide VNHRFTLV or TEWETGQI, respectively (Fig. 5A and C). Control mice injected with pcDNA3 or recombinant GST presented a delay in the generation of in vivo cytotoxicity.
FIG. 5.
Kinetics of the CD8+-T-cell-mediated immune responses after challenge in mice vaccinated with the asp-2 gene or a recombinant protein representing ASP-2 antigen. C57BL/6 mice were immunized intramuscularly with three doses of 100 μg plasmids pIgSPclone9 (asp-2 gene; hatched bars) or pcDNA3 (control; white bars). Two weeks after the last dose, the mice were challenged or not intraperitoneally (i.p.) with 104 bloodstream trypomastigotes. (A) On the indicated days, the in vivo cytotoxic activity against target cells coated with peptide VNHRFTLV was determined. The results represent the mean of three mice ± standard deviation (SD) per group. (B) On the indicated days, IFN-γ-producing spleen cells specific to the peptide VNHRFTLV were estimated by the ELISPOT assay. The results represent the mean number of spot-forming cells (SFC) specific to the peptide VNHRFTLV per 106 splenocytes ± SD (n = 4). A/Sn mice were immunized with three doses of the recombinant protein GST-P4-P7 (hatched bars) or GST (white bars) as described previously (1). Two weeks after the last dose, the mice were challenged or not i.p. with 250 bloodstream trypomastigotes. (C) On the indicated days, the in vivo cytotoxic activity against target cells coated with peptide TEWETGQI was determined. The results represent the mean of three mice ± SD per group. (D) On the indicated days, numbers of IFN-γ-producing spleen cells specific to the peptide TEWETGQI were estimated by the ELISPOT assay. The results represent the mean number of SFC specific to the peptide TEWETGQI per 106 splenocytes ± SD (n = 4). The asterisks denote values statistically higher than the values for control mice (P < 0.05; one-way analysis of variance). The results are representative of two independent experiments.
The enumeration of specific CD8+ T cells by ELISPOT showed a similar pattern of immune response. After challenge, mice vaccinated with the plasmid pIgSPclone9 or with the recombinant protein GST-P4-P7 displayed faster expansion and significantly higher numbers of IFN-γ-secreting cells (Fig. 5B and D).
An interesting observation we made in naïve mice was that the expansion of specific splenic CD8+ T cells occurred in the days following the peak parasitemia, between days 9 and 15 after challenge of BALB/c or C57BL/6 mice. The delayed kinetics of specific CD8+-T-cell expansion may be an important factor for the establishment of infection in naïve hosts. These results are in agreement with the data collected after the challenge of naïve KO mice. Mouse survival mediated by CD8+ T cells, perforin, and IFN-γ occurred mainly between days 14 and 17 after challenge. This timing correlated closely with the moment that specific effector CD8+ T cells of C57BL/6 mice reached their maximum activity (days 15 and 16).
Overall, the kinetics of T. cruzi-specific CD8+ cytotoxic T cells differs sharply from the observations made with mice infected with lymphocytic choriomeningitis virus, Listeria monocytogenes, and Plasmodium yoelli. In these cases, maximum CD8+-T-cell immune response was achieved between days 4 and 8 following challenge and rapidly declined after that period (2, 17, 19). Studies of mice infected with β-galactosidase-transgenic Toxoplasma gondii described much slower kinetics (7). The distinct kinetics can be explained by differences in the natures of these infections (acute versus chronic).
As opposed to naïve mice, vaccinated immune animals exhibited a significantly faster in vivo cytotoxic immune response. This swift immune response correlated with protective immunity, helping host survival after challenge.
Acknowledgments
We thank Fidel Zavala for reviewing the manuscript. We are in debt to Maria L. Juliano (UNIFESP-EPM) for providing one of the peptides.
This work was supported by grants from FAPESP, The Millennium Institute for Vaccine Development and Technology (CNPq-420067/2005-1), and FAPEMIG (EDT 24.000). R.T.G., P.M.P., and M.M.R. are recipients of fellowships from CNPq. F.T. and B.C.G.A. are recipients of fellowships from FAPESP.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Araujo, A. F., B. C. de Alencar, J. R. Vasconcelos, M. I. Hiyane, C. R. Marinho, M. L. Penido, S. B. Boscardin, D. F. Hoft, R. T. Gazzinelli, and M. M. Rodrigues. 2005. CD8+-T-cell-dependent control of Trypanosoma cruzi infection in a highly susceptible mouse strain after immunization with recombinant proteins based on amastigote surface protein 2. Infect. Immun. 73:6017-6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badovinac, V. P., A. R. Tvinnereim, and J. T. Harty. 2000. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science 290:1354-1358. [DOI] [PubMed] [Google Scholar]
- 3.Barber, D. L., E. J. Wherry, and R. Ahmed. 2003. Cutting edge: rapid in vivo killing by memory CD8 T cells. J. Immunol. 171:27-31. [DOI] [PubMed] [Google Scholar]
- 4.Boscardin, S. B., S. S. Kinoshita, A. E. Fujimura, and M. M. Rodrigues. 2003. Immunization with cDNA expressed by amastigotes of Trypanosoma cruzi elicits protective immune response against experimental infection. Infect. Immun. 71:2744-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg, N., and R. Tarleton. 2002. Genetic immunization elicits antigen-specific protective immune responses and decreases disease severity in Trypanosoma cruzi infection. Infect. Immun. 70:5547-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henriques-Pons, A., G. M. Oliveira, M. M. Paiva, A. F. Correa, M. M. Batista, R. C. Bisaggio, C. C. Liu, V. Cotta-De-Almeida, C. M. Coutinho, P. M. Persechini, and T. C. Araujo-Jorge. 2002. Evidence for a perforin-mediated mechanism controlling cardiac inflammation in Trypanosoma cruzi infection. Int. J. Exp. Pathol. 83:67-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwok, L. Y., S. Lutjen, S. Soltek, D. Soldati, D. Busch, M. Deckert, and D. Schluter. 2003. The induction and kinetics of antigen-specific CD8 T cells are defined by the stage specificity and compartmentalization of the antigen in murine toxoplasmosis. J. Immunol. 170:1949-1957. [DOI] [PubMed] [Google Scholar]
- 8.Low, H. P., M. A. Santos, B. Wizel, and R. L. Tarleton. 1998. Amastigote surface proteins of Trypanosoma cruzi are targets for CD8+ CTL. J. Immunol. 160:1817-1823. [PubMed] [Google Scholar]
- 9.Martin, D., and R. Tarleton. 2004. Generation, specificity, and function of CD8+ T cells in Trypanosoma cruzi infection. Immunol. Rev. 201:304-317. [DOI] [PubMed] [Google Scholar]
- 10.Martin, D. L., and R. L. Tarleton. 2005. Antigen-specific T cells maintain an effector memory phenotype during persistent Trypanosoma cruzi infection. J. Immunol. 174:1594-1601. [DOI] [PubMed] [Google Scholar]
- 11.Miyahira, Y., Y. Takashima, S. Kobayashi, Y. Matsumoto, T. Takeuchi, M. Ohyanagi-Hara, A. Yoshida, A. Ohwada, H. Akiba, H. Yagita, K. Okumura, and H. Ogawa. 2005. Immune responses against a single CD8+-T-Cell epitope induced by virus vector vaccination can successfully control Trypanosoma cruzi infection. Infect. Immun. 73:7356-7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller, U., V. Sobek, S. Balkow, C. Holscher, A. Mullbacher, C. Museteanu, H. Mossmann, and M. M. Simon. 2003. Concerted action of perforin and granzymes is critical for the elimination of Trypanosoma cruzi from mouse tissues, but prevention of early host death is in addition dependent on the FasL/Fas pathway. Eur. J. Immunol. 33:70-78. [DOI] [PubMed] [Google Scholar]
- 13.Nickell, S. P., and D. Sharma. 2000. Trypanosoma cruzi: roles for perforin-dependent and perforin-independent immune mechanisms in acute resistance. Exp. Parasitol. 94:207-216. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues, M. M., M. Ribeirao, V. Pereira-Chioccola, L. Renia, and F. Costa. 1999. Predominance of CD4 Th1 and CD8 Tc1 cells revealed by characterization of the cellular immune response generated by immunization with a DNA vaccine containing a Trypanosoma cruzi gene. Infect. Immun. 67:3855-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues, M. M., S. B. Boscardin, J. R. Vasconcelos, M. I. Hiyane, G. Salay, and I. S. Soares. 2003. Importance of CD8 T cell-mediated immune response during intracellular parasitic infections and its implications for the development of effective vaccines. Ann. Acad. Bras. Cienc. 75:443-468. [DOI] [PubMed] [Google Scholar]
- 16.Tarleton, R. L., B. H. Koller, A. Latour, and M. Postan. 1992. Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature 356:338-340. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji, M., and F. Zavala. 2003. T cells as mediators of protective immunity against liver stages of Plasmodium. Trends Parasitol. 19:88-93. [DOI] [PubMed] [Google Scholar]
- 18.Vasconcelos, J. R., M. I. Hiyane, C. R. F. Marinho, C. Claser, A. M. Vieira-Machado, R. T. Gazinelli, O. Bruña-Romero, J. M. Alvarez, S. B. Boscardin, and M. M. Rodrigues. 2004. Protective immunity against Trypanosoma cruzi infection in a highly susceptible mouse strain following vaccination with genes encoding the Amastigote Surface Protein-2 and trans-sialidase. Hum. Gene Ther. 15:878-886. [DOI] [PubMed] [Google Scholar]
- 19.Wherry, E. J., and R. Ahmed. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 78:5535-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]