Abstract
The innate immune system surveys the extra- and intracellular environment for the presence of microbes. Among the intracellular sensors is a protein known as Nod2, a cytosolic protein containing a leucine-rich repeat domain. Nod2 is believed to play a role in determining host responses to invasive bacteria. A key element in upregulating host defense involves activation of the NF-κB pathway. It has been suggested through indirect studies that NF-κB-inducing kinase, or NIK, may be involved in Nod2 signaling. Here we have used macrophages derived from primary explants of bone marrow from wild-type mice and mice that either bear a mutation in NIK, rendering it inactive, or are derived from NIK−/− mice, in which the NIK gene has been deleted. We show that NIK binds to Nod2 and mediates induction of specific changes induced by the specific Nod2 activator, muramyl dipeptide, and that the role of NIK occurs in settings where both the Nod2 and TLR4 pathways are activated by their respective agonists. Specifically, we have linked NIK to the induction of the B-cell chemoattractant known as BLC and suggest that this chemokine may play a role in processes initiated by Nod2 activation that lead to improved host defense.
The innate immune system detects the presence of microorganisms and some viruses using transmembrane and cytosolic receptors with specificities for distinct components of the infectious agents. Members of the Toll-like receptor (TLR) family are well recognized for this function (1-3). A second family known as the Nod/CATERPILLAR protein family includes at least two proteins, Nod1 (Card4) and Nod2 (Card15), that recognize substructures present in bacterial peptidoglycans (10, 12). Nod2 has been shown to be involved in genetically determined human diseases; mutations in Nod2 have been linked to susceptibility to Crohn's disease and to Blau's syndrome (10, 12). Crohn's disease and Blau's syndrome are chronic diseases with autoinflammatory and autoimmune components. Although Nod2 was initially thought to be a receptor for bacterial lipopolysaccharide (LPS), more recent studies have shown that Nod2-expressing cells respond to peptidoglycan-derived structures including muramyl dipeptides (MDP) (9, 13). Whether or not direct binding between MDP and Nod2 occurs has yet to be determined, despite the fact that there are extensive structure-function data for Nod2 responses to MDP (22).
Because of the well-characterized role of NF-κB activation in inflammation, we felt that a more in-depth analysis of MDP-induced NF-κB activation might enhance our understanding of how Nod2 regulates host responses to bacteria. There are two major pathways of NF-κB activation, known as the canonical (classical) and noncanonical (alternative) activation pathways. To date, studies of Nod2 signaling have been focused on the canonical NF-κB pathway, with essentially no direct studies of the role of the noncanonical pathway (4, 6). Functional characterization of Nod2 demonstrated a role for RIP2 (RICK) in Nod2-dependent NF-κB activation with the use of cells from RIP2-deficient mice (7, 14). In contrast Nod2 signaling is independent of adaptor proteins such as MyD88 (18) and Trif (R. J. Ulevitch et al., unpublished data) required for TLR signaling. The noncanonical NF-κB activation pathway involves NF-κB-inducing kinase (NIK), IκB kinase α (IKK1), and p100 leading to cleavage of p100 and formation of p52:RelB dimmers (4, 8, 23, 24). Here we have examined a possible role for NIK in Nod2-dependent signaling. Using bone marrow-derived macrophages from mice with a targeted deletion or mutation of NIK, we show that NIK participates in several Nod2-dependent cellular responses to MDP. Importantly, these data provide new information about the downstream signaling pathways of Nod2 and may help us to further understand the role of Nod2 in health and disease.
MATERIALS AND METHODS
Mice and reagents.
aly/+ (aly heterozygous) and aly/aly (aly homozygous) mice (17) were purchased from CLEA, Osaka, Japan. NIK−/− mice were generously provided by Tularik Inc. (South San Francisco, CA) (24); Nod2−/− mice were obtained from Koichi Kobayashi and Richard Flavell (Yale University School of Medicine, New Haven, Conn.) (15). All experimental protocols involving use of mice were reviewed and approved by the TSRI Institutional Animal Care and Use Committee.
MDP was purchased from Sigma; LPS isolated from Escherichia coli O111:B4 was purchased from List Biological Laboratories. CpG oligodeoxynucleotide 1826 (TCCATGACGTTCCTGACGTT) was ordered from Sigma-Genosys.
Preparation of bone marrow-derived macrophages (BMDM).
Femors and tibia were collected aseptically from euthanized mice, and after removal of muscle, the ends of the bones were cut off and the marrow was flushed out using 5-ml syringes with 25-gauge needles and RPMI 1640 medium containing 10% heat-inactivated (56°C for 30 min) HyClone defined fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cells were spun at 1,000 rpm for 10 min and resuspended in Dulbecco's modified Eagle medium (DMEM; Gibco) (with 4.5 g of d-glucose and 110 mg of sodium pyruvate per liter and with the addition of 30% L929 cell-conditioned medium, 10% heat-inactivated FBS, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin), and 30-ml aliquots were added to 150-mm-style non-tissue culture-treated dishes (Fisher 08-757-14K). The plates were placed in a tissue culture incubator in 10% CO2 at 37°C, and after 4 days 10 ml of additional medium was added. When cells reached confluence (typically after 5 to 7 days in culture), the medium was removed, and the cells were washed once and detached by a 15-min exposure to ice-cold Dulbecco's phosphate-buffered saline (PBS) without calcium or magnesium (Gibco). The detached cells were suspended by pipetting, centrifuged at 1,000 rpm for 5 min, resuspended in DMEM-L929 conditioned medium, and plated in tissue culture plates. Typically, the cell yield from one mouse was initially plated in three 150-mm plates, yielding 1 × 108 cells after 5 to 7 days in culture. The cells were observed to be >94% CD11b positive by fluorescence-activated cell sorting analysis. The BMDM were plated at 1 × 106 cells per ml in 24-well tissue culture plates 18 h prior to use in experiments. L929 conditioned medium was produced by plating L929 cells (ATCC CCL-1) in 150-cm2 tissue culture flasks at an initial density of 1 × 106 cells per ml in DMEM (high glucose) supplemented with 10% nonheated FBS, 2 mM l-glutamine, and penicillin/streptomycin as described above. After 5 to 7 days in culture, when the adherent cells were fully confluent, the culture supernatant was centrifuged at 2,000 rpm for 10 min, aliquoted in 50-ml tubes, and stored at −20°C.
RNA quantification.
Total RNA was isolated using Trizol reagent (Invitrogen Inc.), and 1 μg of RNA was reverse transcribed using Superscript II (Invitrogen Inc.). Real-time PCR was done in an Applied Biosystems HT7900 using a SYBR Green detection protocol. The following primers were used to amplify specific genes: B lymphocyte chemoattractant (BLC) 5′, CCCCAAAACTGAAGTTGTGATCT, and BLC 3′, CAGGCAGCTCTTCTCTTACTCACT; RANTES 5′, GCCCACGTCAAGGAGTATTTCTA, and RANTES 3′, ACACACTTGGCGGTTCCTTC; 18sRNA 5′, CCGCGGTTCTATTTTGTTGGT, and 18sRNA 3′, CTCTAGCGGCGCAATACGA; interleukin-1β (IL-1β) 5′, GCAACTGTTCCTGAACTCAACT, and IL-1β 3′, ATCTTTTGGGGTCCGTCAACT.
Immunoprecipitation and Western blot analysis.
For immunoprecipitation experiments, HEK 293 cells were transfected with Lipofectamine 2000 (Life Technologies, Inc.) and cultured for an additional 24 h. Cells were suspended in lysis buffer (225 mM NaCl, 50 mM Tris 7.4, 1% NP-40, supplemented with Protease Inhibitor Cocktail Set III [CalBiochem Inc.]) for 30 min at 4°C. Cell lysates were centrifuged at 14,000 rpm for 5 min, and supernatants were first preincubated with protein G-Sepharose beads for 2 h and spun at 13,000 rpm for 5 min. The supernatants were collected and incubated with anti-FLAG antibody (2 μg/ml; Santa Cruz Biotechnology) and protein G beads overnight at 4°C. The precipitated immunocomplex was washed five times with lysis buffer and separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE). Proteins were transferred to Immobilon-P (polyvinylidene difluoride) membranes (Millipore). The membrane was blocked with a solution containing PBS (PH 7.4), 0.5% Tween 20, and 5% nonfat milk. Incubation with anti-Myc mouse monoclonal antibody (clone 9E10, 1:2,000 dilution; Santa Cruz Biotechnology) was carried out at room temperature overnight. The blot was developed with ECL Plus reagent (Amersham Bioscience). For detection of NF-κB2 protein, cells were stimulated as indicated in the figure legends and lysed directly with SDS loading buffer. Cell lysates were separated on SDS-10% PAGE gels, and NF-κB proteins were detected with antibody which recognized both p100 and p52 (at a dilution of 1:2,000; anti-human p100 [Upstate Biotech] or anti-murine p100 [Santa Cruz Biotechnology]). Antitubulin antibody (Santa Cruz Biotechnology Inc., CA) was used as a gel loading control.
Preparation of nuclear extracts.
Cells (2 × 106) were washed with ice-cold PBS and resuspended in 0.4 ml of buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride). After 10 min, Nonidet P-40 was added to 0.6%. Nuclei were separated from the cytosol by centrifugation at 13,000 × g for 10 seconds and resuspended in 50 μl of buffer B (20 mM HEPES, pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 0.1 mM phenylmethylsulfonyl fluoride). After 30 min at 4°C, lysates were centrifuged at 13,000 × g for 30 s, and the supernatant containing nuclear proteins was collected.
Quantitation of Western blot band intensities.
Western blot films were scanned with a GS-800 Densitometer (Bio-Rad). The band intensity was presented as density (optical density [OD]/mm2) and normalized by the ratio of tubulin band density.
Measurement of cytokine production.
The concentrations of BLC, macrophage-derived chemokine (MDC), IL-6, and tumor necrosis factor α (TNF-α) in culture supernatants of BMDM were measured by enzyme-linked immunosorbent assay (ELISA) using reagents purchased from R&D, Inc. (Minneapolis, MN).
Helicobacter felis culture and infection.
H. felis (ATCC 49179) was grown under microaerobic conditions in a BBL Campy Pouch microaerophilic system (catalog no. 260656; Becton Dickinson) containing N2, H2, and CO2 (80:10:10) at 37°C on brucella broth agar plates supplemented with 5% sheep blood. The bacteria were harvested after 24 to 48 h of growth, washed, and resuspended in PBS. The OD at 600 nm (OD600) was determined, and the bacteria titer was calculated (1 × 108 CFU per OD unit). The presence of H. felis was confirmed by characteristic colony morphology, motility, and a urease test. BMDM were infected by bacteria for 24 h in the absence of antibiotics, and cell culture supernatants were assayed for specific cytokine secretion.
Statistical analysis.
A Student's t test (two-tailed) was used to evaluate the data. A P value of <0.05 was considered significant.
RESULTS
p52 production via Nod2.
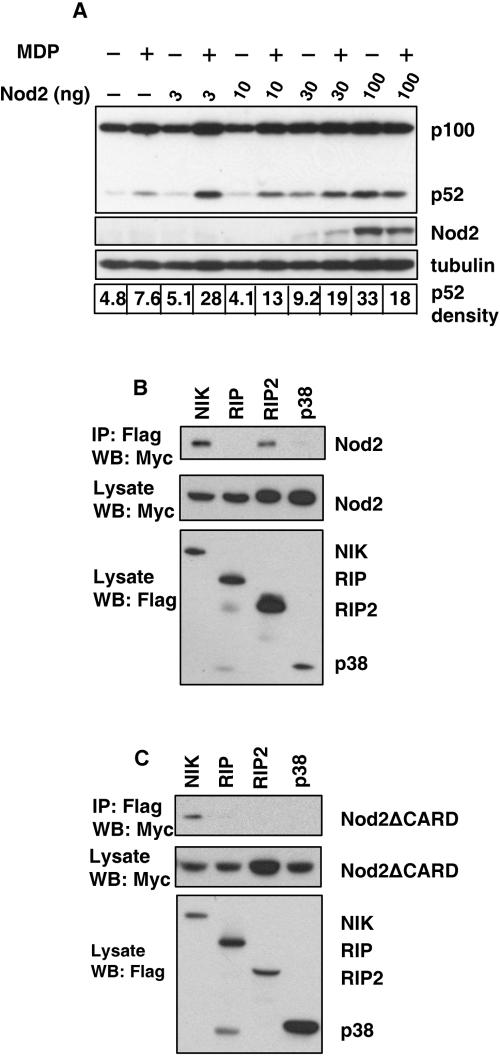
In order to determine if activation of the Nod2 pathway leads to events associated with the noncanonical NF-κB pathway, we focused our efforts on the role of the enzyme known as NIK. NIK is critical for the processing of p100 to p52 (4). Mammalian cells express five NF-κB proteins: NF-κB1 (the DNA-binding subunit p50 and its precursor p105), NF-κB2 (the DNA-binding subunit p52 and its precursor p100), RelA (p65), RelB, and c-Rel. NF-κB1 is critical for the canonical pathway, while processing of p100 activates noncanonical pathway. We first sought to determine whether MDP-induced cell activation leads to Nod2-dependent p52 production. To address this question, we transiently expressed p100 and Nod2 in HEK 293 cells and asked whether we could detect MDP- and Nod2-dependent p52 production. p52 accumulation was observed when MDP was added to Nod2-transfected cells, supporting a link between MDP, Nod2, and the NIK pathway (Fig. 1A). In studies not shown, we also noted that p52 was found in the nucleus of MDP-treated cells. Further, we observed constitutive production of p52 with the highest input of Nod2 cDNA, in keeping with ligand-independent activation of Nod2 when it is overexpressed (Fig. 1A). Several lines of evidence show that RIP2/RICK is important for Nod2 activation of the canonical NF-κB pathway and that it binds to Nod2 via CARD-CARD domain interactions (14, 18). RIP2 is upstream of the IKK1/IKK2 pathway, while NIK is upstream of IKK1 in the noncanonical pathway. We next asked if we could detect interactions of NIK and Nod2. We coexpressed FLAG-tagged constructs encoding RIP, RIP2, NIK, and p38 with Myc-tagged wild-type Nod2 or Nod2 containing deletions in the CARD domain (Nod2ΔCARD) in HEK 293 cells; cell lysates were immunoprecipitated with anti-FLAG, and the resulting immunoprecipitates were resolved by SDS-PAGE and visualized by Western blotting with anti-Myc antibody. We observed that both RIP2 and NIK, but not RIP or p38, interacted with wild-type Nod2 (Fig. 1B) and that NIK but not RIP2 interacted with NOD2ΔCARD (Fig. 1C). Thus, Nod2 appears to utilize CARD domain-dependent and -independent interactions to transduce signals to the canonical and noncanonical NF-κB pathways. In studies not shown, we also determined that NIK binds similarly to wild-type Nod2 or Nod2 containing various mutations (R702W, P268S, G908R, or the frameshift mutation L1007fsinsC), which are often found in Crohn's disease patients (12).
FIG. 1.
NIK interacts with Nod2. (A) MDP induces p100 processing in a Nod2-dependent manner. HEK 293 cells were transfected with 100 ng of p100 expression construct in combination with different amounts of Myc-tagged Nod2 expression plasmid. Cells were stimulated with 20 μg/ml MDP for 24 h, and p100 processing was detected with anti-NF-κB2 monoclonal antibody, which recognizes both p100 and p52. Myc-tagged Nod2 protein was detected by anti-Myc immunoblotting. The film was scanned, and the band intensity of p52 was calibrated as described in Materials and Methods. (B and C) Interaction of NIK with Nod2. HEK 293 cells were cotransfected with Myc-tagged Nod2 (B) or truncated Myc-tagged Nod2ΔCARD (C) and with FLAG-tagged NIK, RIP, RICK, and p38 expression plasmids. The cell lysates were immunoprecipitated with rabbit polyclonal anti-FLAG antibody overnight. The resulting immune complexes were fractionated by SDS-PAGE, transferred to membranes, and subsequently probed with monoclonal anti-Myc antibody. The lysates (10% of immunoprecipitation input) derived from each transfection were also loaded in gels as control and immunoblotted using anti-FLAG and anti-Myc antibodies.
Pathway requirements for p52 production.
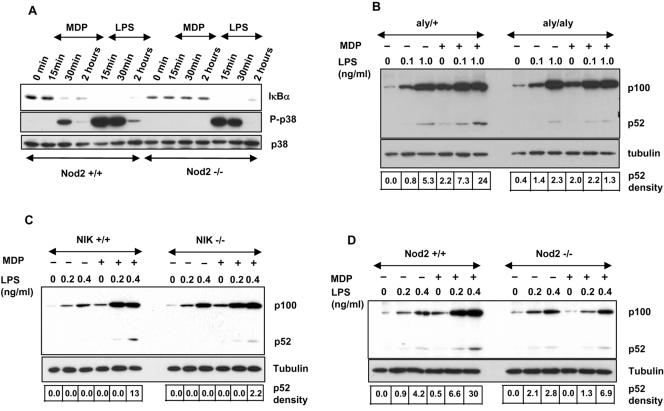
We next used BMDM from Nod2−/−, NIK−/−, and aly/aly strains of mice to further study the role of NIK in MDP signaling. The aly/aly strain has a point mutation in NIK that renders NIK inactive while aly/+ strain has a wild-type phenotype (23). It is now well established that Nod2 does not recognize LPS but, rather, is a receptor for structures found in bacterial peptidoglycans such as MDP. It is very likely that in physiological settings, a variety of TLR ligands might be present that influence Nod2 signaling via TLR activation pathways. In support of this concept are multiple studies showing synergies between TLR and Nod2 signaling. Here we have used LPS as a prototypic TLR4 activator and examined its effects in combination with MDP-induced Nod2 signaling. BMDM from Nod2−/− and Nod2+/+ mice were treated with MDP or LPS, and subsequent changes in IκBα degradation and p38 activation were measured as indicators of cell activation. MDP induced IκBα degradation and p38 activation in Nod2+/+ but not Nod2−/− cells, while the LPS responses were identical in Nod2−/− and Nod2+/+ BMDM (Fig. 2A). Thus, the absence of Nod2 does not reduce LPS responses but completely blunts MDP-induced cell activation. BMDM derived from aly/aly or NIK−/− cells that are defective in the NIK pathway due to a mutation in the former and gene deletion in the latter were used to further characterize endogenous Nod2 signaling pathways. Specifically, we determined how MDP, LPS, or the combination of the two influenced processing of p100 to p52 in wild-type or mutant cell lines. Addition of MDP alone produced barely detectable p52, but when increasing amounts of LPS were added to aly/+ cells, a synergistic production of p52 was observed (Fig. 2B). In addition we observed that MDP or LPS alone or when added together produced a strong increase in p100 production. When we compared aly/+ and aly/aly cells, we observed that the enhanced p52 production noted when MDP and LPS were added together was absent in the aly/aly cells homozygous for the NIK mutation. In contrast, the marked increase in p100 protein expression induced by MDP, LPS, or the combination of the two occurred to a similar extent when responses in aly/+ and aly/aly cells were compared. We also observed a comparable response pattern when MDP and LPS were added together to NIK+/+ or NIK−/− BMDM. NIK+/+ cells treated with LPS and MDP showed enhanced, synergistic production of p52 that was not observed with the NIK−/− BMDM (Fig. 2C). In contrast, increases in p100 expression were identical in NIK+/+ and NIK−/− BMDM. The synergy between LPS and MDP required the presence of Nod2 since we observed enhanced p52 production when MDP and LPS were added to Nod2+/+ cells but not when we performed the identical experiment using Nod2−/− BMDM (Fig. 2D). Thus, the totality of the data provided here support the contention that MDP signals via both the canonical and noncanonical NF-κB pathways and requires NIK for multiple and distinct responses in both transfected cell lines and in primary cell isolates and that synergies exist as shown here by the enhanced responses triggered by the combined effects of MDP and LPS.
FIG. 2.
Essential roles of Nod2 and NIK in mediating responses to MDP and LPS. (A) Nod2 mediates MDP-induced IκBα degradation and p38 phosphorylation. Nod2+/+ and Nod2−/− BMDM were stimulated with MDP (20 μg/ml) or LPS (10 ng/ml) for different times. Cell lysates were prepared, and IκBα degradation and p38 phosphorylation were detected by Western blotting using specific antibodies. NIK (B and C) and Nod2 (D) are required for synergistic production of p52 by MDP and LPS. BMDM from aly/+ and aly/aly mice lacking functional NIK (B) or BMDM from NIK+/+ and NIK−/− mice (C) and also BMDM from WT and Nod2−/− mice (D) were stimulated by various concentrations of LPS in the presence or absence of MDP (20 μg/ml) for 24 h, and processing of p100 was analyzed by Western blotting using anti-NF-κB2 antibody recognizing both p100 and p52. The films were scanned, and the band density for p52 was normalized to the tubulin constitutive control.
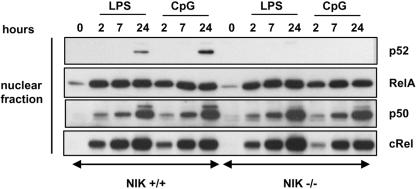
To determine if NIK is also required for p52 generation and subsequent translocation in cells activated by other TLR activators, BMDM were challenged with LPS (100 ng/ml) and CpG (0.2 mM), and nuclear fractions were subjected to immunoblot analyses. Both LPS and CpG induced p52 generation in wild-type but not NIK−/− cells. In contrast, translocation of other members of NF-κB family (RelA, p50, and cRel) were found in approximately equal amounts in wild-type and NIK−/− cells normally (Fig. 3).
FIG. 3.
NIK regulates LPS- and CpG-induced p52 nuclear translocation. BMDM were stimulated with LPS (100 ng/ml) and CpG (0.2 μM) for the indicated time points. Cells were lysed, and nuclear fractions were subjected to Western blot analysis with the indicated antibody.
NIK-dependent gene induction via the Nod2 pathway.
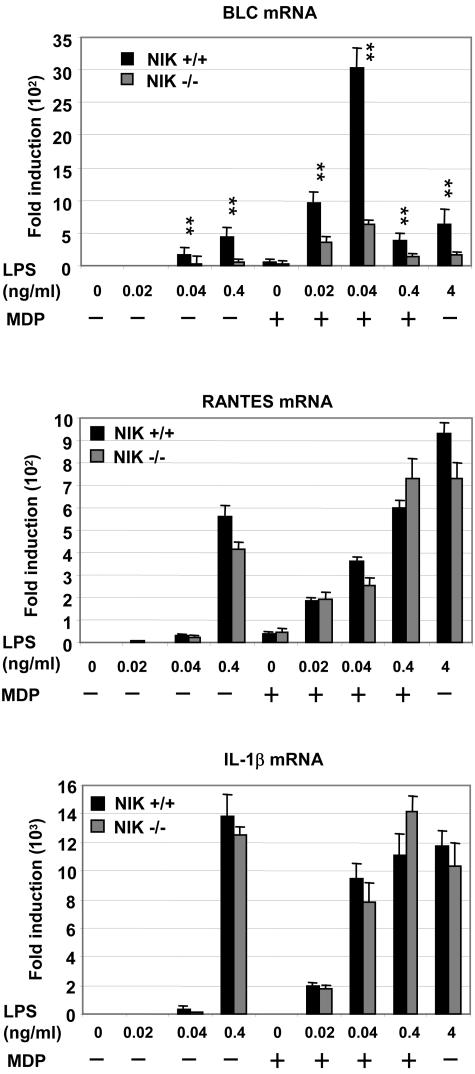
We next performed an expression analysis on total RNA from resting and activated BMDM obtained from wild-type and NIK−/− mice comparing the effects of stimulation with MDP, LPS, and MDP plus LPS to determine if we could identify one or more genes that are selectively upregulated by the combination of LPS and MDP compared to the effects of either ligand alone. Analysis of the arrays to be presented elsewhere (R. J. Ulevitch, Q. Pan, C. Fearns, et al., unpublished data) revealed that the gene encoding BLC (CXCL13) was strongly upregulated by the combined effects of LPS and MDP in wild-type but not NIK−/− cells. Based on these findings, we conducted experiments to quantify BLC, RANTES, and IL-1β mRNA expression by reverse transcription-PCR in BMDM from wild-type or NIK−/− mice following stimulation by LPS, MDP, or LPS plus MDP. Results shown in Fig. 4 demonstrate that BLC mRNA expression is markedly upregulated in wild-type but not NIK−/− BMDM when LPS and MDP are added simultaneously. In contrast, expression of RANTES and IL-1β mRNA was identical in the parental and NIK−/− BMDM.
FIG. 4.
Impaired BLC gene induction in NIK−/− cells. BMDM were stimulated for 24 h with LPS (0.02 to 0.4 ng/ml) and 20 μg/ml MDP. Gene induction (mRNA) was determined by real-time PCR. The relative expression of genes was normalized to the level of 18s RNA. Data are presented as means ± standard deviations of triplicate wells. **, P < 0.01 for cytokine concentrations from knockout mice compared with data from wild-type mice. Results are representative of two independent experiments.
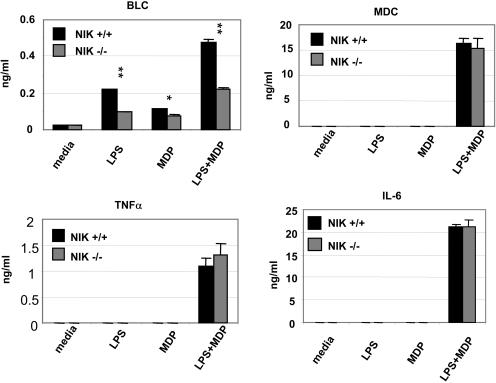
We also observed strong synergies between LPS and MDP in secretion of chemokines and proinflammatory cytokines. This was observed for release of BLC, MDC, TNF-α, and IL-6. A key role for the NIK pathway was only noted for BLC with a marked reduction in release when wild-type and NIK−/− BMDM were compared (Fig. 5). These data reflect the same pattern of mRNA expression when BLC induction was compared with RANTES and IL-1β. However, the levels of production of other cytokines (TNF-α, IL-6, and MDC) were similar in wild-type and NIK−/− cells.
FIG. 5.
Diminished BLC secretion in NIK−/− cells. BMDM were stimulated for 24 h (LPS, 0.04 ng/ml; MDP, 20 μg/ml) and the production of cytokine and chemokine were determined by specific ELISA. Data are presented as means ± standard deviations of triplicate wells. *, P < 0.05, and **, P < 0.01, for cytokine concentrations from knockout mice compared with data from wild-type mice. Results are representative of three independent experiments.
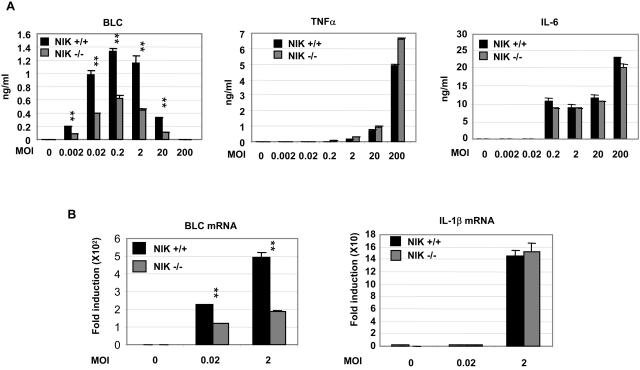
To further establish the functional significance of NIK in innate immunity, we infected BMDM with the gram-negative bacterium H. felis. H. felis is a mouse gastric pathogen, and infection of mouse by H. felis mimics many pathogenic changes commonly found in humans infected with Helicobacter pylori. Our data showed that H. felis activated murine macrophages (NIK+/+ and NIK−/−) and induced comparable levels of expression of TNF-α and IL-6 in NIK+/+ and NIK−/− macrophages (Fig. 6A). In contrast, the absence of NIK resulted in a decrease in BLC production that was seen both at the protein (Fig. 6A) and mRNA levels (Fig. 6B).
FIG. 6.
Helicobacter infection induced BLC gene activation and chemokine secretion in a NIK-dependent manner. (A) BMDM were infected with H. felis at the indicated multiplicity of infection (MOI) for 24 h, and induction of cytokines was detected with specific ELISA kits. Data are presented as means ± standard deviations of triplicate wells. Results are representative of three independent experiments. (B) BMDM were infected with H. felis at the indicated multiplicity of infection (MOI) for 24 h. Relative gene expression was detected by real-time PCR. Data are presented as means ± standard deviations of triplicate wells. **, P < 0.01 for cytokine concentrations from knockout mice compared with data from wild-type mice. Results are representative of three independent experiments.
DISCUSSION
It is our contention that a better understanding of the role of Nod2 in disease will derive from defining Nod2-dependent signaling pathways. Few studies have addressed questions about Nod2 function under physiological conditions where endogenous signal transduction pathways can be studied. Here we have provided several distinct experimental approaches that establish a role for NIK in Nod2-dependent signaling and that support the contention that Nod2 is linked to both the canonical and noncanonical NF-κB pathways. This contention is supported by studies that show NIK binding to Nod2, MDP-dependent processing of p100 to p52 requiring both Nod2 and NIK, and a requirement for NIK in Nod2-dependent induction of the chemokine encoded by the BLC gene. The requirement for NIK is not global since induction levels of multiple other genes including IL-1, TNF, IL-6, and other matrix metalloproteinase family members are identical in NIK-sufficient and NIK-deficient cells. We further showed that there is a striking synergy between LPS and MDP in activating the noncanonical NF-κB pathway and in gene expression and that in some cases this synergy requires NIK as well. Previous studies have used dominant-negative forms of NIK to probe Nod2-dependent signaling pathways (18). No previous studies have investigated a role for NIK in primary cell types such as BMDM or under conditions that reflect signaling via endogenous pathways.
The major role of the noncanonical NF-κB pathway in immunology has been assigned to adaptive immunity with a specific emphasis on its role in the development and maintenance of secondary lymphoid organs (4). Here we show that the role of NIK-dependent gene expression is not confined to these latter responses but include genes and pathways associated with innate immunity and inflammation. We have identified one specific gene encoding the chemokine known as BLC. Here we have linked BLC production to Nod2- and NIK-dependent pathways. BLC is usually expressed in secondary lymphoid tissue constitutively, and aberrant expression of BLC has also been associated with chronic inflammatory diseases, such as H. pylori gastritis (16) and rheumatoid arthritis (5, 20). These data suggested an important role for NIK in innate responses to bacteria infection and associated chronic disease as well.
The work described here provides a new framework to investigate the downstream events triggered by Nod2 activation and to further define the physiological role of Nod2 in health and disease. Whether the current studies provide the basis to understand recent results of Hollenbach et al. (11) will require further work in appropriate animal models. In their study, they examined the effects of p38 inhibitor SB203580. They showed the activation of the noncanonical NF-κB pathway in a dextran sulfate sodium-induced murine model of inflammatory bowel disease. Their studies also suggested that SB203580 could inhibit both the canonical and noncanonical NF-κB pathways leading to an improved clinical score in the bowel. Despite continued uncertainty about the pathogenesis of Crohn's disease, the results provided herein may well lead to a novel means to regulate key events linked to Nod2 signaling. This would include distinct modulation of the canonical and noncanonical NF-κB pathways as well as the basis for a better understanding of the influence of mutations in Nod2 or other genes linked to Crohn's disease susceptibility (19, 21).
Acknowledgments
This study was supported in part by the National Institutes of Health (grants AI15136 and GM28485 to R.J.U. and grant AI058047 to R.D.S.), Novartis SFP-1475 (R.J.U.), and The Eli and Edythe L. Broad Foundation (K.K.).
Editor: A. D. O'Brien
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 2.Beutler, B., K. Hoebe, X. Du, and R. J. Ulevitch. 2003. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J. Leukoc. Biol. 74:479-485. [DOI] [PubMed] [Google Scholar]
- 3.Beutler, B., and J. Hoffmann. 2004. Innate immunity. Curr. Opin. Immunol. 16:1-3. [DOI] [PubMed] [Google Scholar]
- 4.Bonizzi, G., and M. Karin. 2004. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25:280-288. [DOI] [PubMed] [Google Scholar]
- 5.Carlsen, H. S., E. S. Baekkevold, H. C. Morton, G. Haraldsen, and P. Brandtzaeg. 2004. Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood 104:3021-3027. [DOI] [PubMed] [Google Scholar]
- 6.Chen, C. M., Y. Gong, M. Zhang, and J. J. Chen. 2004. Reciprocal cross-talk between Nod2 and TAK1 signaling pathways. J. Biol. Chem. 279:25876-25882. [DOI] [PubMed] [Google Scholar]
- 7.Chin, A. I., P. W. Dempsey, K. Bruhn, J. F. Miller, Y. Xu, and G. Cheng. 2002. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature 416:190-194. [DOI] [PubMed] [Google Scholar]
- 8.Fagarasan, S., R. Shinkura, T. Kamata, F. Nogaki, K. Ikuta, K. Tashiro, and T. Honjo. 2000. Alymphoplasia (aly)-type nuclear factor κB-inducing kinase (NIK) causes defects in secondary lymphoid tissue chemokine receptor signaling and homing of peritoneal cells to the gut-associated lymphatic tissue system. J. Exp. Med. 191:1477-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girardin, S. E., I. G. Boneca, J. Viala, M. Chamaillard, A. Labigne, G. Thomas, D. J. Philpott, and P. J. Sansonetti. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278:8869-8872. [DOI] [PubMed] [Google Scholar]
- 10.Girardin, S. E., and D. J. Philpott. 2004. The role of peptidoglycan recognition in innate immunity. Eur. J. Immunol. 34:1777-1782. [DOI] [PubMed] [Google Scholar]
- 11.Hollenbach, E., M. Neumann, M. Vieth, A. Roessner, P. Malfertheiner, and M. Naumann. 2004. Inhibition of p38 MAP kinase- and RICK/NF-κB-signaling suppresses inflammatory bowel disease. FASEB J. 18:1550-1552. [DOI] [PubMed] [Google Scholar]
- 12.Inohara, N., and G. Nunez. 2003. NODs: intracellular proteins involved in inflammation and apoptosis. Nat. Rev. Immunol. 3:371-382. [DOI] [PubMed] [Google Scholar]
- 13.Inohara, N., Y. Ogura, A. Fontalba, O. Gutierrez, F. Pons, J. Crespo, K. Fukase, S. Inamura, S. Kusumoto, M. Hashimoto, S. J. Foster, A. P. Moran, J. L. Fernandez-Luna, and G. Nunez. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J. Biol. Chem. 278:5509-5512. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi, K. S., N. Inohara, L. D. Hernandez, J. E. Galan, G. Nunez, C. A. Janeway, R. Medzhitov, and R. A. Flavell. 2002. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416:194-199. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi, K. S., M. Chamaillard, Y. Ogura, O. Henegariu, N. Inohara, G. Nunez, and R. A. Flavell. 2005. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307:731-734. [DOI] [PubMed] [Google Scholar]
- 16.Mazzucchelli, L., A. Blaser, A. Kappeler, P. Scharli, J. A. Laissue, M. Baggiolini, and M. Uguccioni. 1999. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J. Clin. Investig. 104:1333-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyawaki, S., Y. Nakamura, H. Suzuka, M. Koba, R. Yasumizu, S. Ikehara, and Y. Shibata. 1994. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur. J. Immunol. 24:429-434. [DOI] [PubMed] [Google Scholar]
- 18.Ogura, Y., N. Inohara, A. Benito, F. F. Chen, S. Yamaoka, and G. Nunez. 2001. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-κB. J. Biol. Chem. 276:4812-4818. [DOI] [PubMed] [Google Scholar]
- 19.Peltekova, V. D., R. F. Wintle, L. A. Rubin, C. I. Amos, Q. Huang, X. Gu, B. Newman, M. Van Oene, D. Cescon, G. Greenberg, A. M. Griffiths, P. H. George-Hyslop, and K. A. Siminovitch. 2004. Functional variants of OCTN cation transporter genes are associated with Crohn's disease. Nat. Genet. 36:471-475. [DOI] [PubMed] [Google Scholar]
- 20.Shi, K., K. Hayashida, M. Kaneko, J. Hashimoto, T. Tomita, P. E. Lipsky, H. Yoshikawa, and T. Ochi. 2001. Lymphoid chemokine B cell-attracting chemokine-1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J. Immunol. 166:650-655. [DOI] [PubMed] [Google Scholar]
- 21.Stoll, M., B. Corneliussen, C. M. Costello, G. H. Waetzig, B. Mellgard, W. A. Koch, P. Rosenstiel, M. Albrecht, P. J. Croucher, D. Seegert, S. Nikolaus, J. Hampe, T. Lengauer, S. Pierrou, U. R. Foelsch, C. G. Mathew, M. Lagerstrom-Fermer, and S. Schreiber. 2004. Genetic variation in DLG5 is associated with inflammatory bowel disease. Nat. Genet. 36:476-480. [DOI] [PubMed] [Google Scholar]
- 22.Tanabe, T., M. Chamaillard, Y. Ogura, L. Zhu, S. Qiu, J. Masumoto, P. Ghosh, A. Moran, M. M. Predergast, G. Tromp, C. J. Williams, N. Inohara, and G. Nunez. 2004. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J. 23:1587-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada, T., T. Mitani, K. Yorita, D. Uchida, A. Matsushima, K. Iwamasa, S. Fujita, and M. Matsumoto. 2000. Abnormal immune function of hemopoietic cells from alymphoplasia (aly) mice, a natural strain with mutant NF-κB-inducing kinase. J. Immunol. 165:804-812. [DOI] [PubMed] [Google Scholar]
- 24.Yin, L., L. Wu, H. Wesche, C. D. Arthur, J. M. White, D. V. Goeddel, and R. D. Schreiber. 2001. Defective lymphotoxin-β receptor-induced NF-κB transcriptional activity in NIK-deficient mice. Science 291:2162-2165. [DOI] [PubMed] [Google Scholar]