Abstract
Endosymbiotic Wolbachia bacteria that infect the filarial nematode Onchocerca volvulus were previously found to have an essential role in the pathogenesis of river blindness. The current study demonstrates that corneal inflammation induced by Wolbachia or O. volvulus antigens containing Wolbachia is completely dependent on expression of myeloid differentiation factor 88.
The presence of intracytoplasmic bacteria in Onchocerca volvulus was first described in 1977 (13), and these bacteria were later identified as endosymbiotic Wolbachia (10). Proinflammatory responses in filarial-infected individuals and animals exposed to filaria have indicated that Wolbachia rather than the nematode is the cause of inflammation (3, 4, 12, 21). Wolbachia bacteria are essential for the pathogenesis of O. volvulus-induced keratitis, since (i) corneal inflammation was not induced by extracts derived from O. volvulus depleted of Wolbachia by doxycycline treatment of infected individuals and (ii) related filarial species containing Wolbachia induce keratitis but aposymbiotic species lacking Wolbachia do not (18). Both the systemic inflammatory response and the inflammatory response in the cornea were significantly reduced in C3H/HeJ mice (18, 21), which have a point mutation in the gene encoding Toll-like receptor 4 (TLR4) (16). Furthermore, the major surface protein of Wolbachia activates TLR2 and TLR4 (2). As TLR2 and TLR4 signal through the common myeloid differentiation factor 88 (MyD88) adaptor molecule, (19, 20, 22), we utilized MyD88−/− mice to examine the role of this intracellular signaling molecule in Wolbachia- and O.volvulus-induced keratitis.
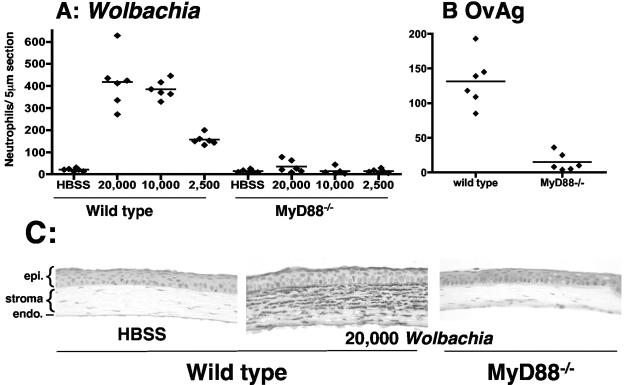
Although our previous study demonstrated that Wolbachia has an essential role in O. volvulus-induced keratitis, we did not determine whether Wolbachia could induce keratitis in the absence of filarial antigens. Since insect Wolbachia from the Aa23 mosquito cell line activates macrophages (21), we determined whether this Wolbachia also induces keratitis. Isolated insect Wolbachia and a soluble O. volvulus antigen extract containing Wolbachia (OvAg) were quantified by PCR for the single-copy wsp gene as described previously (5, 14). Four microliters was injected into the corneal stroma of MyD88−/− mice and wild-type littermates, and the mice were sacrificed 18 h later at the peak of neutrophil infiltration (6). Eyes were embedded in paraffin, and 5-μm sections of the corneal stroma were immunostained with NIMP-R14, which is specific for neutrophils (6). As shown in Fig. 1A, Wolbachia induced a dose-dependent neutrophil infiltrate in the corneal stroma of immunocompetent mice. In marked contrast, no neutrophils were detected in the corneal stroma of MyD88−/− mice, even with the largest inoculum (Fig. 1A). Neutrophils were also absent in the corneas of MyD88−/− mice inoculated with O. volvulus extract compared with control mice (Fig. 1B). Figure 1C shows that the corneas of inoculated MyD88−/− mice were similar to saline controls.
FIG. 1.
Neutrophils in the corneal stroma of wild-type and MyD88−/− mice after injection of Wolbachia or OvAg. Wolbachia was injected into the corneal stroma of MyD88−/− mice and wild-type littermates. After 18 h, mice were sacrificed, and corneas were stained for neutrophils using monoclonal antibody NIMP-R14. (A) Total number of neutrophils per 5-μm corneal section in control and MyD88−/− mice inoculated with 20,000, 10,000, or 2,500 bacteria. (B) Total number of neutrophils in control and MyD88−/− mice inoculated intrastromally with 4 μg OvAg in 4 μl. (C) Representative sections of wild-type and MyD88−/− mice inoculated with 4 μl Hanks balanced salt solution (HBSS) or with 20,000 Wolbachia bacteria. There were statistically significant differences between wild-type and MyD88−/− mice (P < 0.0001) for all Wolbachia concentrations and for OvAg. Data points represent individual corneas. epi., epithelium; endo., endothelium.
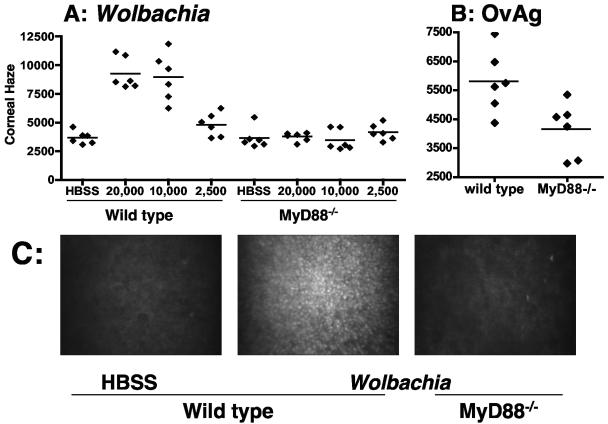
The presence of neutrophils in the corneal stroma leads to loss of corneal transparency and can be measured by determining corneal haze by in vivo confocal microscopy (11). Wolbachia or O. volvulus extract containing Wolbachia was injected into the corneal stroma of control and MyD88−/− mice as described above, and corneal haze was calculated from stromal thickness and light intensity in combined images of the corneal stroma (11). Figure 2 shows that injection of Wolbachia induced elevated levels of corneal haze in wild-type, immunocompetent mice compared with the haze in saline-inoculated mice. In contrast, there was no increase in corneal haze in Wolbachia-inoculated MyD88−/− mice compared with saline-inoculated corneas. Similar results were obtained for control and MyD88−/− mice inoculated with OvAg (Fig. 2B). Together with the impaired neutrophil recruitment to the corneas of MyD88−/− mice, these findings demonstrate that keratitis induced by Wolbachia and O. volvulus extracts is completely dependent on MyD88.
FIG. 2.
Corneal haze in Wolbachia- and O. volvulus-treated MyD88−/− mice. Wolbachia or OvAg was injected into corneas of control and MyD88−/− mice as described in the legend to Fig. 1. After 18 h, mice were examined by in vivo confocal microscopy, and corneal haze was determined. (A) Stromal haze in MyD88−/− mice and wild-type littermates inoculated with 20,000, 10,000, or 2,500 bacteria. (B) Stromal haze in MyD88−/− mice and wild-type littermates inoculated with 4 μg OvAg. The symbols indicate the values for individual corneas. (C) Representative images from the central corneal stroma, showing a cell infiltration in wild-type but not MyD88−/− mice after injection of 4 μl HBSS or 20,000 Wolbachia cells. There were statistically significant differences between wild-type and MyD88−/− mice for animals that received 20,000 Wolbachia cells (P < 0.0001) and 10,000 Wolbachia cells (P = 0.0001) but not for animals thatreceived 2,500 bacteria or OvAg (P >0.05).
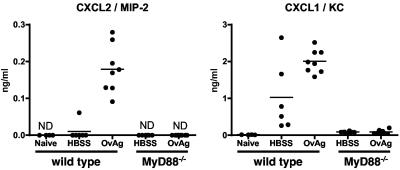
We previously demonstrated that neutrophil recruitment to the corneal stroma in immunized mice is dependent on CXCR2 and that intrastromal injection of O. volvulus stimulates CXC chemokine production by resident corneal cells (6). To determine if MyD88 is required for CXC chemokine production, O. volvulus antigen containing Wolbachia was injected into the corneal stroma of control and MyD88−/− mice. After 6 h, the peak time for CXC chemokine production and prior to neutrophil infiltration (6), corneas were dissected and sonicated, and the levels of chemokines were determined by an enzyme-linked immunosorbent assay (ELISA). As shown in Fig. 3, the levels of both KC/CXCL1 and MIP-2/CXCL2 were significantly elevated in wild-type corneas inoculated with OvAg compared with the levels in saline-treated corneas (P < 0.05). However, neither chemokine was detected in MyD88−/− mice, indicating that production of these CXC chemokines by resident cells in the cornea was completely dependent on MyD88.
FIG. 3.
Chemokine production by resident corneal cells. Corneas from MyD88−/− and wild-type littermates were inoculated with 4 μg OvAg or HBSS (4 μl). After 6 h, corneas were dissected and sonicated, and chemokine production was determined by an ELISA. The symbols indicate the values for individual corneas. For wild-type mice the P values for comparisons of saline-treated mice and OvAg-treated mice were <0.0001 for CXCL2/MIP-2 and 0.0173 for CXCL1/KC. The level of chemokine production was below the limit of detection (not detected [ND]) in MyD88−/− mice and in all corneas from naïve mice. Note that there was 10-fold more CXCL1/KC production than CXCL2/MIP-2 production. The experiment was repeated twice with similar results.
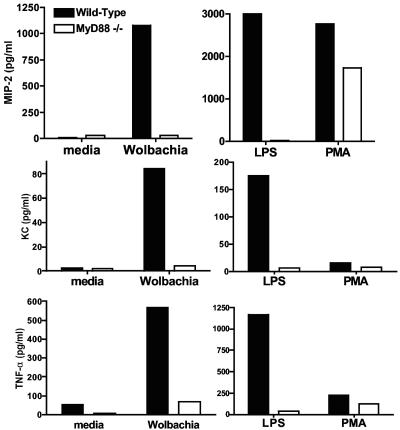
As neutrophils also produce CXC chemokines in response to filarial antigens containing Wolbachia (5), which likely exacerbates neutrophil infiltration into the cornea, we examined the role of MyD88 in neutrophil activation. Peritoneal neutrophils from control and MyD88−/− mice were isolated and incubated with isolated Wolbachia. Figure 4 shows that neutrophils incubated with Wolbachia produced KC/CXCL1, MIP-2/CXCL2, and tumor necrosis factor alpha. In contrast, neutrophils from MyD88−/− mice did not produce any of these cytokines in response to Wolbachia or lipopolysaccharide (LPS), although MyD88−/− neutrophils produced CXCL2/MIP-2 in response to phorbol myristate acetate, a non-TLR stimulus. Together, these data show that MyD88 has an essential role in Wolbachia-induced neutrophil activation and cytokine production.
FIG. 4.
Cytokine production by peritoneal neutrophils. A purified neutrophil population was isolated from peritoneal exudate cells from a pool of three control mice and MyD88−/− mice. Cells were incubated for 18 h with 10,000 Wolbachia cells, 100 ng/ml of TLR-specific ligand LPS, or 3.3 ng/ml phorbol myristate acetate (PMA) (a non-TLR ligand), and cytokine production was measured by an ELISA. Note the difference in scales. The data are the means for duplicate wells and are representative of two repeat experiments. TNF-α, tumor necrosis factor alpha.
In summary, the results of the current study show that Wolbachia- and O. volvulus-induced keratitis is completely ablated in MyD88−/− mice, demonstrating that the MyD88-dependent pathway has an essential role in this model of river blindness. Given that specific activation of TLR2, TLR4, and TLR9 in the mouse cornea requires MyD88 to induce corneal inflammation (11) and that neutrophils express TLRs and are activated by Wolbachia (5, 9), we propose that the response to Wolbachia is initiated by TLRs on resident cells in the corneal stroma. Secretion of CXC chemokines by these cells mediates neutrophil recruitment from the peripheral, limbal vessels into the avascular cornea and migration through the stromal matrix to the site of microfilaria degradation and release of Wolbachia (5). A second role for MyD88 in the inflammatory process is to initiate neutrophil activation and production of CXCL1/KC, CXCL2/MIP-2, and tumor necrosis factor alpha in response to Wolbachia, further mediating neutrophil recruitment. Neutrophil activation also leads to secretion of cytotoxic products, such as nitric oxide and oxygen radicals that disrupt the normal function of resident corneal cells, thereby leading to loss of corneal clarity.
Results of previous studies suggested that the inflammatory responses induced by Wolbachia and filaria extracts were mediated by LPS-like activity in Wolbachia (15, 18, 21); however, complete genome sequencing of Wolbachia from insects and Brugia malayi indicated that Wolbachia lacks the biosynthetic machinery for LPS (23; http://tools.neb.com/wolbachia). Although Wolbachia peptidoglycan and lipoproteins have not been tested yet, ligands for TLR2 and TLR4 are present in the Wolbachia surface protein, which is abundantly expressed in insect and filaria Wolbachia, is highly conserved among filarial species, and is recognized by filaria-infected individuals (1, 2, 17). Wolbachia surface protein from Dirofilaria immitis activates cells through TLR2 and TLR4 signaling in transfected fibroblasts, murine dendritic cells, and macrophages (2). As TLR2 and TLR4 signal through MyD88, these findings are consistent with our current findings and suggest that MyD88-independent pathways do not contribute to Wolbachia-mediated inflammatory responses.
In chronically infected individuals, adaptive immunity is established before significant microfilaria invasion of the corneal stroma; however, innate responses to Wolbachia in the cornea appear to be the triggering events for neutrophil infiltration and loss of corneal clarity, which is exacerbated in the presence of antibody and after recruitment of eosinophils (7, 8). Further studies of Wolbachia surface protein and other possible Wolbachia TLR ligands should reveal the contribution of the TLR pathways to the activation of innate and acquired immune responses to Wolbachia and filarial antigens associated with disease pathogenesis.
Acknowledgments
This work was supported by NIH grants EY10320 (to E.P.), AI-07024 (to I.G.-F.), K08 AI054652 (to A.G.H.), and EY11373, by the Research to Prevent Blindness Foundation, and by the Ohio Lions Eye Research Foundation. M.T. thanks the Wellcome Trust for Senior Fellowship support and the EC (grant ICA4-CT2002-10051).
MyD88−/− mice were generously provided by S. Akira, Osaka, Japan.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Bazzocchi, C., W. Jamnongluk, S. L. O'Neill, T. J. Anderson, C. Genchi, and C. Bandi. 2000. wsp gene sequences from the Wolbachia of filarial nematodes. Curr. Microbiol. 41:96-100. [DOI] [PubMed] [Google Scholar]
- 2.Brattig, N. W., C. Bazzocchi, C. J. Kirschning, N. Reiling, D. W. Buttner, F. Ceciliani, F. Geisinger, H. Hochrein, M. Ernst, H. Wagner, C. Bandi, and A. Hoerauf. 2004. The major surface protein of Wolbachia endosymbionts in filarial nematodes elicits immune responses through TLR2 and TLR4. J. Immunol. 173:437-445. [DOI] [PubMed] [Google Scholar]
- 3.Brattig, N. W., U. Rathjens, M. Ernst, F. Geisinger, A. Renz, and F. W. Tischendorf. 2000. Lipopolysaccharide-like molecules derived from Wolbachia endobacteria of the filaria Onchocerca volvulus are candidate mediators in the sequence of inflammatory and antiinflammatory responses of human monocytes. Microbes Infect. 2:1147-1157. [DOI] [PubMed] [Google Scholar]
- 4.Cross, H. F., M. Haarbrink, G. Egerton, M. Yazdanbakhsh, and M. J. Taylor. 2001. Severe reactions to filarial chemotherapy and release of Wolbachia endosymbionts into blood. Lancet 358:1873-1875. [DOI] [PubMed] [Google Scholar]
- 5.Gillette-Ferguson, I., A. G. Hise, H. F. McGarry, J. Turner, A. Esposito, Y. Sun, E. Diaconu, M. J. Taylor, and E. Pearlman. 2004. Wolbachia-induced neutrophil activation in a mouse model of ocular onchocerciasis (river blindness). Infect. Immun. 72:5687-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall, L. R., E. Diaconu, R. Patel, and E. Pearlman. 2001. CXC chemokine receptor 2 but not C-C chemokine receptor 1 expression is essential for neutrophil recruitment to the cornea in helminth-mediated keratitis (river blindness). J. Immunol. 166:4035-4041. [DOI] [PubMed] [Google Scholar]
- 7.Hall, L. R., E. Diaconu, and E. Pearlman. 2001. A dominant role for Fc gamma receptors in antibody-dependent corneal inflammation. J. Immunol. 167:919-925. [DOI] [PubMed] [Google Scholar]
- 8.Hall, L. R., J. H. Lass, E. Diaconu, E. R. Strine, and E. Pearlman. 1999. An essential role for antibody in neutrophil and eosinophil recruitment to the cornea: B cell-deficient (microMT) mice fail to develop Th2-dependent, helminth-mediated keratitis. J. Immunol. 163:4970-4975. [PubMed] [Google Scholar]
- 9.Hayashi, F., T. K. Means, and A. D. Luster. 2003. Toll-like receptors stimulate human neutrophil function. Blood 102:2660-2669. [DOI] [PubMed] [Google Scholar]
- 10.Henkle-Duhrsen, K., V. H. Eckelt, G. Wildenburg, M. Blaxter, and R. D. Walter. 1998. Gene structure, activity and localization of a catalase from intracellular bacteria in Onchocerca volvulus. Mol. Biochem. Parasitol. 96:69-81. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, A. C., F. P. Heinzel, E. Diaconu, Y. Sun, A. G. Hise, D. Golenbock, J. H. Lass, and E. Pearlman. 2005. Activation of Toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. IOVS (Investig. Ophthalmol. Vis. Sci.) 46:589-595. [DOI] [PubMed] [Google Scholar]
- 12.Keiser, P. B., S. M. Reynolds, K. Awadzi, E. A. Ottesen, M. J. Taylor, and T. B. Nutman. 2002. Bacterial endosymbionts of Onchocerca volvulus in the pathogenesis of posttreatment reactions. J. Infect. Dis. 185:805-811. [DOI] [PubMed] [Google Scholar]
- 13.Kozek, W. J., and H. F. Marroquin. 1977. Intracytoplasmic bacteria in Onchocerca volvulus. Am. J. Trop. Med. Hyg. 26:663-678. [DOI] [PubMed] [Google Scholar]
- 14.McGarry, H. F., G. L. Egerton, and M. J. Taylor. 2004. Population dynamics of Wolbachia bacterial endosymbionts in Brugia malayi. Mol. Biochem. Parasitol. 135:57-67. [DOI] [PubMed] [Google Scholar]
- 15.Pfarr, K. M., K. Fischer, and A. Hoerauf. 2003. Involvement of Toll-like receptor 4 in the embryogenesis of the rodent filaria Litomosoides sigmodontis. Med. Microbiol. Immunol. 192:53-56. [DOI] [PubMed] [Google Scholar]
- 16.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 17.Punkosdy, G. A., D. G. Addiss, and P. J. Lammie. 2003. Characterization of antibody responses to Wolbachia surface protein in humans with lymphatic filariasis. Infect. Immun. 71:5104-5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saint Andre, A., N. M. Blackwell, L. R. Hall, A. Hoerauf, N. W. Brattig, L. Volkmann, M. J. Taylor, L. Ford, A. G. Hise, J. H. Lass, E. Diaconu, and E. Pearlman. 2002. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science 295:1892-1895. [DOI] [PubMed] [Google Scholar]
- 19.Takeda, K., and S. Akira. 2004. Microbial recognition by Toll-like receptors. J. Dermatol. Sci. 34:73-82. [DOI] [PubMed] [Google Scholar]
- 20.Takeda, K., and S. Akira. 2004. TLR signaling pathways. Semin. Immunol. 16:3-9. [DOI] [PubMed] [Google Scholar]
- 21.Taylor, M. J., H. F. Cross, and K. Bilo. 2000. Inflammatory responses induced by the filarial nematode Brugia malayi are mediated by lipopolysaccharide-like activity from endosymbiotic Wolbachia bacteria. J. Exp. Med. 191:1429-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogel, S. N., K. A. Fitzgerald, and M. J. Fenton. 2003. TLRs: differential adapter utilization by Toll-like receptors mediates TLR-specific patterns of gene expression. Mol. Interv. 3:466-477. [DOI] [PubMed] [Google Scholar]
- 23.Wu, M., L. V. Sun, J. Vamathevan, M. Riegler, R. Deboy, J. C. Brownlie, E. A. McGraw, W. Martin, C. Esser, N. Ahmadinejad, C. Wiegand, R. Madupu, M. J. Beanan, L. M. Brinkac, S. C. Daugherty, A. S. Durkin, J. F. Kolonay, W. C. Nelson, Y. Mohamoud, P. Lee, K. Berry, M. B. Young, T. Utterback, J. Weidman, W. C. Nierman, I. T. Paulsen, K. E. Nelson, H. Tettelin, S. L. O'Neill, and J. A. Eisen. 2004. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS. Biol. 2:E69. [DOI] [PMC free article] [PubMed] [Google Scholar]