Abstract
Staphylococcus aureus is a major cause of nosocomial infections worldwide, and the rate of resistance to clinically relevant antibiotics, such as methicillin, is increasing; furthermore, there has been an increase in the number of methicillin-resistant S. aureus community-acquired infections. Effective treatment and prevention strategies are urgently needed. We investigated the potential of the S. aureus surface protein iron surface determinant B (IsdB) as a prophylactic vaccine against S. aureus infection. IsdB is an iron-sequestering protein that is conserved in diverse S. aureus clinical isolates, both methicillin resistant and methicillin sensitive, and it is expressed on the surface of all isolates tested. The vaccine was highly immunogenic in mice when it was formulated with amorphous aluminum hydroxyphosphate sulfate adjuvant, and the resulting antibody responses were associated with reproducible and significant protection in animal models of infection. The specificity of the protective immune responses in mice was demonstrated by using an S. aureus strain deficient for IsdB and HarA, a protein with a high level of identity to IsdB. We also demonstrated that IsdB is highly immunogenic in rhesus macaques, inducing a more-than-fivefold increase in antibody titers after a single immunization. Based on the data presented here, IsdB has excellent prospects for use as a vaccine against S. aureus disease in humans.
Staphylococcus aureus is a gram-positive bacterium that is notable for the frequency and severity of infections that it causes in hospitalized patients. These infections range from localized skin infections to bacteremia and septic shock. In the past 20 years there has been a dramatic increase in the incidence of nosocomial staphylococcal infections; this increase parallels the increased use of intravascular devices and invasive procedures. S. aureus has been identified as one of the three most frequent nosocomial pathogens and is responsible for approximately 25% of the 2 million nosocomial infections reported in the United States each year (38, 39). A second trend has been the increase in the incidence of methicillin-resistant S. aureus, largely due to selective antibiotic pressure. Resistant strains were initially identified in tertiary care hospitals but have been increasingly reported among infections in the community (25, 30). Resistance to methicillin is often accompanied by resistance to other antibiotics; a CDC survey showed that the proportion of methicillin-resistant isolates which were susceptible only to vancomycin rose from 22.8% to 56.2% from 1987 to 1997 (18). More recently, S. aureus strains with intermediate susceptibility or resistance to vancomycin have been reported (11, 24, 36). Infections caused by multidrug-resistant S. aureus limit therapeutic options, and they may be associated with higher mortality and higher costs than infections caused by susceptible staphylococci. There is clearly a need for new treatment and prevention strategies.
In an immunological survey of S. aureus surface antigens, human acute-phase serum was screened with S. aureus bacterial surface display libraries (8). The majority of the S. aureus genome was displayed on the surface of Escherichia coli as recombinantly expressed polypeptides fused to E. coli surface proteins. Many interesting proteins were detected, including a novel member of the LPXTG protein family. The protein LPXTG-VI reacted to convalescent-phase serum from S. aureus-infected patients. This protein had previously been described and designated iron surface determinant B (IsdB) (22), and it is regulated by the ferric uptake regulator (Fur). IsdB is expressed when there is iron limitation and has a role in the acquisition of iron (37). In this report, we describe expression of recombinant IsdB and an in vivo evaluation of this protein with a murine model of infection and a rhesus macaque (Macaca mulatta) immunogenicity study. We also characterized expression and distribution of this protein in taxonomically diverse and clinically relevant S. aureus isolates, with the aim of identifying a novel vaccine target that could provide a significant benefit by preventing invasive infections and potentially reducing the morbidity, mortality, and cost associated with such infections.
MATERIALS AND METHODS
Bacterial strains.
The S. aureus strains used in this study are listed in Table 1. An S. aureus isdB harA null mutant was constructed by sequentially knocking out the two genes from S. aureus Becker. First, isdB was replaced with the gentamicin resistance gene using standard S. aureus engineering techniques (19). One isdB null mutant was selected, and the gene that encodes HarA was replaced with the tetracycline resistance gene by using the methods of Dryla et al. (6). Transformants were confirmed by resistance to gentamicin, PCR, Southern blotting, Western analysis, and flow cytometry.
TABLE 1.
Distribution and divergence of IsdB in a collection of pathologically and taxonomically diverse S. aureus isolates
| Strain | Source | Capsule type | MLST groupa | Antibiotic resistance groupb | % Identity to immunogenc | Reactivity to anti-IsdBd | MFI end titere
|
||
|---|---|---|---|---|---|---|---|---|---|
| RPMI | TSB | TSA | |||||||
| MCL 8567 | Left forearm wound, United States | 8 | 1 | MSSA | 100 | + | 21 | 8 | 0 |
| MCL 8999 | Arm wound, United States | 8 | 1 | MSSA | 99 | + | 74 | 0 | 0 |
| MCL 8752 | Left foot, United States | 5 | 5 | MRSA | 99 | + | 0 | 0 | 2 |
| MCL 8072 | Wound, United States | 5 | 5 | MRSA | 99 | + | 22 | 23 | 0 |
| MCL 8933 | Right leg wound, United States | 5 | 8 | MRSA | 100 | + | 38 | 0 | 0 |
| ME9f | Ireland, hospital acquired | 5 | 8 | MRSA | + | 43 | 0 | 0 | |
| ME10f | United Kingdom, hospital acquired, nares | 5 | 9 | MSSA | 97 | + | 21 | 0 | 0 |
| ME11f,g | United Kingdom, blood | 5 | 9 | MSSA | 97 | + | 87 | 9 | 19 |
| ME13f | The Netherlands, blood | 8 | 12 | MSSA | 97 | + | 93 | 0 | 37 |
| ME14f | United Kingdom, nares | 8 | 12 | MSSA | 97 | + | 1 | 0 | 3 |
| MCL 8538g | Left finger skin, United States | 8 | 15 | MSSA | 98 | + | 78 | 19 | 13 |
| MCL 8714 | Pus left anaresle, United States | 8 | 15 | MSSA | 98 | + | 22 | 0 | 0 |
| ME24f | United States, blood | 5 | 22 | MRSA | + | 47 | 0 | 0 | |
| ME25f | Germany, Barnin MRSA | 5 | 22 | MRSA | 98 | + | 121 | 0 | 0 |
| ME4f | United States, blood | 5 | 25 | MRSA/VISA | 98 | + | 87 | 0 | 0 |
| ME27f,g | Canada, blood | 5 | 25 | MRSA | 97 | + | 58 | 19 | 1 |
| ME31f,g | Sweden, wound | 8 | 30 | MRSA | 94 | + | 48 | 0 | 35 |
| ME32f | Germany, source not known | 8 | 30 | MRSA | 94 | + | 4 | 15 | 6 |
| MCL 8781 | Left leg stump pus, United States | 8 | 45 | MRSA | 95 | + | 96 | 0 | 0 |
| Beckerg,h | United States prototype capsule strain | 8 | 45 | MSSA | 95 | + | 192 | 0 | 0 |
| ME54f | The Netherlands, blood | 5 | 97 | MSSA | 98 | + | 20 | 0 | 0 |
| ME55f | Canada, blood | 5 | 97 | MSSA | 99 | + | 58 | 0 | 0 |
| ME59f | United Kingdom, nares, community disease | 8 | 121 | MSSA | 96 | + | 93 | 0 | 0 |
| ME60f,g | United Kingdom, blood | 8 | 121 | MSSA | 96 | + | 73 | NDi | 0 |
MLST group, multilocus sequence typing taxonomic group.
MRSA, methicillin-resistant S. aureus; MSSA, methicillin-sensitive S. aureus; VISA, S. aureus resistant to intermediate levels of vancomycin.
Level of identity of the IsdB amino acid sequence to the amino acid sequence of IsdB from S. aureus COL, the source of the vaccine sequence used in the immunization experiments.
S. aureus strains were grown in RPMI and screened for reactivity to IsdB antiserum from IsdB-immunized mice. +, binding to serum as detected by flow cytometry.
MFI, mean fluorescene intensity when an anti-IsdB monoclonal antibody dilution of 1:500 was used.
Clinical strain provided by Mark Enright, Imperial College, London, United Kingdom.
Strain tested in the murine sepsis model using mice immunized with the IsdB vaccine.
Strain provided by Chia Lee, University of Arkansas for Medical Sciences, Little Rock.
ND, not determined.
Cloning, expression, and sequencing of proteins.
The isdB gene was amplified by PCR from S. aureus COL using the following PCR primers and standard PCR amplification conditions: isdBF (5′-AACCGGTTTTCCATGGGGAACAAACAGCAAAAAGAATTT-3′) and isdBR (5′-ACCGGTTTCTCGAGGTTTTTACGTTTTCTAGGTAATAC-3′). The PCR product was cloned into pET28 using the protocol described by the manufacturer (Novagen). Plasmids were used to transform E. coli HMS174(DE3) cells (Novagen) using the manufacturer's protocols to produce a His-tagged protein with isopropyl-β-d-thiogalactoside (IPTG) (1 mM) induction. IsdB was also expressed in Saccharomyces cerevisiae by using the protocols described by Jansen et al. (13). Sequencing was performed using BigDye (ABI). All experiments with BALB/c mice were performed using the E. coli construct. The S. cerevisiae IsdB expression system was used to immunize ICR mice. The two cloning strategies resulted in comparable proteins.
IsdB vaccine preparation.
Large quantities of cells producing IsdB were produced in stirred tank fermentors (75 liters). Cells were grown to an A600 of 0.8 and induced with IPTG (1 mM). Cells were harvested 3 h after IPTG induction by centrifugation (9,000 × g, 4°C, 20 min). Cell pellets were suspended in a solution containing 50 mM Tris-HCl (pH 8.0), 0.1 M NaCl, 2 mM MgCl2, 10 mM imidazole, 0.1% Tween 80, 6 M guanidine-HCl, protease inhibitors (P8849; Sigma), lysozyme (1 mg/ml), and Benzonase (250 U/ml; EM Industries). Cells were lysed with a microfluidizer, and the lysate was clarified by centrifugation (11,000 × g, 4°C, 30 min).
His-tagged IsdB was purified from the clarified lysate by Ni+-nitrilotriacetic acid agarose (QIAGEN) chromatography. The column was washed with 20 mM Tris-HCl (pH 8.0)-0.15 M NaCl-0.1% Tween 80, and His-tagged IsdB was eluted with a step gradient consisting of 300 mM imidazole, 20 mM Tris-HCl (pH 7.5), 0.15 M NaCl, and 0.1% Tween 80.
The Ni+-nitrilotriacetic acid agarose eluate was fractionated by size exclusion chromatography (Sephacryl S-300 26/60 column; Amersham Biosciences) in 10 mM morpholinepropanesulfonic acid (MOPS) (pH 7.1)-150 mM NaCl. Fractions containing His-tagged IsdB were pooled, and endotoxin was removed by filtration through a Zeta-Plus Biofilter (CUNO).
Murine sepsis model.
Groups of 20 BALB/c or ICR mice (Taconic, Germantown, NY) were immunized three times with IsdB (20 μg per dose) formulated with amorphous aluminum hydroxyphosphate sulfate adjuvant (AAHSA) (450 μg per dose) or were sham immunized with AAHSA alone. The doses were administered as two 50-μl intramuscular injections on days 0, 7, and 21. The mice were bled on day 28, and their sera were screened by an enzyme-linked immunosorbent assay (ELISA) for reactivity to IsdB. On day 35 the mice were challenged by intravenous tail vein injection of S. aureus, and survival was monitored for 10 days. Bacterial challenges were prepared as described by Kuklin et al. (15).
IsdB ELISA.
Medium-binding microtiter plates (Costar) were coated overnight at 2 to 8°C with 50 ng/well of IsdB in phosphate-buffered saline (PBS). Each plate was washed three times with PBS-0.05% Tween 20 and blocked with 1% bovine serum albumin—PBS-0.05% Tween 20 (assay diluent) for at least 1 h. The plate was washed as described above, and diluted serum from immunized animals or tissue culture supernatants from the fusion wells or cloned hybridomas were added; the preparations were then incubated for 2 h at room temperature. The plate was washed as described above, a goat anti-mouse immunoglobulin G(heavy plus light chains) [IgG(H+L)]-horseradish peroxidase (HRP) conjugate (Zymed) (1:25,000 dilution in assay diluent for mouse serum; 1:8,000 dilution for tissue culture supernatant) was added, and the preparation was incubated for 1 h at room temperature. Assay plates were developed with the TMB (3,3′,5,5′-tetramethylbenzidine) substrate, the reaction was stopped with 2.0 N H2SO4, and the results were read at an optical density at 450 nm (OD450) with a plate reader. Mouse serum was examined at several dilutions, and the titer was defined as the lowest dilution that resulted in a value that was three times greater than the background value for wells containing only conjugate. To compare the survival of mice to the serum responses, the OD450 values obtained for the 1:4000 serum dilution were used.
Hybridoma wells that were considered positive had an optical density at 450 nm of >0.1. For the rhesus macaque studies the goat anti-mouse IgG(H+L)-HRP conjugate was replaced with goat anti-rhesus macaque IgG(H+L)-HRP conjugate (Southern Biotechnologies). Rhesus macaque serum was examined at several dilutions, and the titer was defined as the lowest dilution that resulted in value that was three times greater than the background value for wells containing only conjugate.
Generation of monoclonal antibody to IsdB.
Female BALB/c mice (Taconic, Germantown, NY) were immunized intramuscularly on days 0, 7, and 21 with 20 μg IsdB protein formulated with AAHSA. A final intravenous injection of 20 μg of protein in PBS was administered 3 days prior to cell fusion. Mice were sacrificed, and the spleens were removed for cell fusion. Lymphocytes prepared from spleens were fused with the mouse myeloma partner SP2/0-Ag14 (ATTC 1581) by using polyethylene glycol 1500 (Boehringer Mannheim) at a ratio of 3:1. The fused cells were plated into 96-well flat-bottom microtiter plates in Dulbecco's modified Eagle's medium with a high glucose concentration and pyruvate containing 20% fetal bovine serum, hypoxanthine (10−4 M), thymidine (10−5 M), 10% Origen hybridoma cloning factor, and OPI medium supplement. Aminopterin (4 × 10−7 M) was added 24 h later. Supernatants from growing hybridomas were screened by an ELISA for reactivity to IsdB as previously described. Positive wells were cloned by limiting dilution and retested for ELISA reactivity. Monoclonal antibodies were classified with an antibody-isotyping kit (Roche Diagnostics Corporation, Indianapolis, IN). Single-cell cloned hybridomas were grown in static cultures in T150 vented flasks in Dulbecco's modified Eagle's medium with a high glucose concentration and pyruvate supplemented with 10% fetal bovine serum (HyClone) and 4 mM l-glutamine (Mediatech). Cells were incubated at 37°C in the presence of 5% CO2 for 2 weeks. Supernatants were combined and harvested by centrifugation and then kept at 4°C until purification. The antibody was purified on a protein A column (Pharmacia Protein A, Fast Flow) in a final buffer containing 20 mM sodium phosphate and 150 mM sodium chloride (pH 7.2).
Evaluation of in vitro IsdB expression by S. aureus clinical isolates.
For evaluation of in vitro expression of IsdB, bacteria were grown in nutrient-rich media, including tryptone soy broth (TSB) (BD, San Jose, CA) and tryptone soy agar (TSA) (BD), or in nutrient-limited media, including RPMI (BD) and RPMI supplemented with 20 μM FeSO4 (Sigma, St. Louis, MO), 20 μM FeCl3 (Sigma), or 10 μM heme (catalog no. H5533; Sigma). For the liquid cultures the broth was inoculated with the S. aureus isolate at a low concentration (≤106 CFU/ml) and grown to the stationary phase at 37°C. Cells were then collected by centrifugation. For the agar cultures plates were streaked with the strain and grown overnight at 37°C. The bacteria were then scraped off and resuspended in PBS to obtain a final OD600 of 1.0. Approximately 107 CFU suspended in 50 μl of PAAG (PBS containing 1% bovine serum albumin, 0.1% NaN3, and 0.2% pig IgG) was added to 50 μl of the first antibody (∼40 ng IsdB monoclonal antibody IgG/μl in PAAG) and incubated at room temperature for 1 h. Cells were collected by centrifugation, washed with 1 ml of PAAG, resuspended in 100 μl of the second antibody (fluorescein isothiocyanate-conjugated goat anti-mouse IgG [0.5 μg/μl in PAAG]), and incubated at room temperature for 1 h. After washing, the bacteria were resuspended in 1 ml of PBS, and binding to surface-expressed IsdB was measured by flow cytometry.
Preparation of in vivo-grown S. aureus cells for expression analysis studies.
S. aureus Becker from a −70°C glycerol stock was streaked on TSA plates and grown overnight at 37°C. Cells were scraped from the plates and resuspended in PBS, and the A650 was determined. The suspension was diluted into TSB to obtain an A650 of 0.05 and incubated with shaking at 37°C for approximately 4 h. The A650 was determined, and the culture was diluted into sterile saline to obtain a final A650 of 0.0005 (approximately 106 CFU/ml). An aliquot of this dilution was plated to determine the actual number of CFU. The suspension of bacteria was transferred into a bag prepared from hydrated, 18-mm, 3,500-kDa dialysis tubing (Spectra/Por) that had been sterilized by soaking for 30 min in 70% ethanol and rinsed extensively with sterile water and then sterile saline. The bag was closed with a knot and rinsed extensively with sterile saline. Each bag contained approximately 5 ml of culture. Bags were implanted subcutaneously on the backs of male Sprague-Dawley rats. A 4- to 5-cm incision was made along the dorsal midline of anesthetized rats. Pockets on either side of the incision were made by gently separating the skin from the underlying tissue. One bag was inserted on either side of the midline, and the incision was closed with surgical adhesive. After 24 h, the rats were euthanized, the bags were removed, and the bacteria were recovered. Bacteria were washed with PBS and then prepared for flow cytometry analysis as described above for the cells grown in vitro. Culture densities greater than 5 × 109 CFU/ml were routinely obtained, control bags with sterile saline remained sterile throughout the procedure, and pilot experiments using Xen 8.1 (Xenogen Corp., Almeda, CA), a luminescently marked S. aureus strain (15), showed that the bacteria recovered were 100% luminescent, which indicated that pure cultures were obtained.
Flow cytometry.
Bacteria were prepared as described above, collected by centrifugation at 3,500 × g for 10 min at room temperature, and washed once with PBS. Approximately 5 × 107 CFU bacteria suspended in 100 μl PAAG (Sigma, St. Louis, Mo) were stained with either 200 ng anti-IsdB monoclonal IgG, murine anti-IsdB immune serum, or murine convalescent-phase serum at room temperature for 1 h. The stained bacterial cells were washed, resuspended in 100 μl PAAG, and subsequently stained with 500 ng fluorescein isothiocyanate-labeled goat anti-mouse Ig (BD Biosciences) at room temperature for 1 h. After washing, the bacteria were resuspended in 1 ml of PBS and analyzed by flow cytometry. An irrelevant isotype control antibody was used as an additional control. The antibody was grown and purified using the same methods that were used for the anti-IsdB monoclonal antibody.
Immunization of rhesus macaques.
Two groups of rhesus macaques (n = 3) were immunized intramuscularly with either IsdB-AAHSA (50 μg IsdB) or AAHSA alone at day 0 and week 4. Serum was collected on days 0 and 9 and every 4 weeks up to week 24. IsdB responses were measured by ELISA as described above.
Statistical analysis.
Logistic models were used to compare the final survival data with SAS PROC LOGISTIC. The P values were based on 20 to 30 mice per group.
The animal experiments were reviewed and approved by the Merck Research Laboratories-West Point Institutional Animal Care and Use Committee in accordance with all applicable regulations and guidelines.
RESULTS
Mice immunized with IsdB formulated with APA are protected against lethal S. aureus Becker infection.
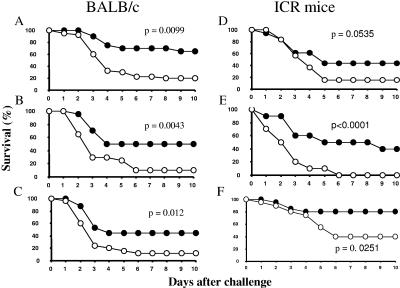
We were interested in investigating whether IsdB protects animals in animal models of S. aureus infection and thus is a suitable candidate for an S. aureus vaccine. The gene encoding IsdB was cloned from S. aureus COL and expressed either in E. coli or in yeast (S. cerevisiae). Purified antigen was formulated using Merck amorphous aluminum hydroxyphosphate sulfate adjuvant and was used to immunize mice (inbred BALB/c and outbred ICR mice) (n = 20). Control groups of mice were sham immunized with AAHSA alone (n = 20 to 30). All mice immunized with IsdB exhibited substantial immune responses to the protein (ELISA titers, >100,000) compared to the baseline titers and the titers of mice sham immunized with AAHSA alone (<5,000). The mice were challenged with an 80 to 90% lethal dose (LD80-90) of S. aureus Becker (4.9 × 108 to 8.7 × 108 CFU for BALB/c mice and 1.0 × 109 to 2.0 × 109 CFU for ICR mice), and survival was monitored for 10 days. The results of three representative experiments with 20 BALB/c mice per group and three experiments with the same number of ICR mice per group are shown in Fig. 1. In all six experiments, the mice immunized with IsdB exhibited greater survival (45, 40, 32, 29, 40, and 40%) than the mice in the sham-immunized control group (Fig. 1). A logistical analysis was performed to compare the survival in the IsdB-treated groups to the survival in the AAHSA-treated groups. P values of 0.05 or less were obtained for five of the six experiments.
FIG. 1.
Improved survival of BALB/c and ICR mice immunized with IsdB and challenged with S. aureus Becker. (A, B, and C) Survival curves for three independent experiments performed with 20 to 30 BALB/c mice per group. (D, E, and F) Survival curves for three independent experiments performed with 10 to 20 ICR mice. •, survival of mice immunized with IsdB-APA; ○, survival of mice immunized with AAHSA.
Correlation between antibody responses and survival in mice immunized with IsdB.
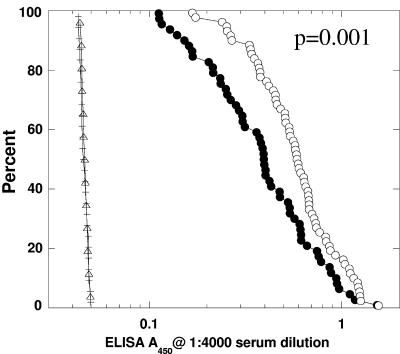
A reverse cumulative distribution function (32) survival analysis of mice immunized with either IsdB (n = 120) or AAHSA alone (n = 40) was performed to compare the titers to IsdB with survival. Mice were grouped according to whether they survived through 10 days after S. aureus challenge (alive) or died before day 10 after challenge (dead). It was evident from this analysis (Fig. 2) that mice with higher anti-IsdB antibody titers had a higher probability of surviving a challenge with a lethal dose of S. aureus (P = 0.001). Antibody titers to this vaccine therefore correlated with survival in the murine sepsis model.
FIG. 2.
Correlation of the survival of S. aureus lethal challenge to IsdB serum titer in IsdB- and sham-immunized mice (n = 160). The data are presented as a reverse cumulative distribution-frequency plot. IsdB serum titers from 160 mice were used for this plot; 120 of the serum samples were from mice immunized with IsdB (circles), and 40 were from naïve adjuvant-immunized mice (triangles and horizontal lines). All serum samples were diluted 1:4,000 and analyzed by ELISA. The x axis indicates the optical density measured at A450, and the y axis indicates the percent probability of survival of mice with a specific optical density value. Open circles indicate immunized mice that survived the challenge (n = 65), and filled circles indicate immunized mice that succumbed to infection (n = 55). Likewise, mice that survived in the sham-immunized group are plotted as triangles (n = 13), and mice that died are plotted as horizontal lines (n = 27).
Distribution and homology of IsdB in S. aureus clinical isolates.
To determine the distribution of isdB, we screened a collection of 25 S. aureus strains that represented a diverse range of S. aureus clonal lineages, as determined by multilocus sequence typing (7), capsule type, and antibiotic resistance profiles. These isolates were obtained from different geographic locations, as well as clinical diseases; invasive community-acquired isolates were also included. The complete IsdB sequences were determined for these strains. For the 645 amino acids encoded, we found a total of 87 amino acid differences among all 25 sequences. The sequences segregated according to taxonomic type and did not seem to be subject to a high degree of variation. The sequence that was most divergent from the sequence of the vaccine antigen (IsdB from S. aureus COL) was still 94.5% identical at the amino acid level. The laboratory strain S. aureus Becker, which was the challenge strain in the immunization experiments, had 32 amino acid differences (95% identity) compared to the immunization protein from COL (Table 1). Epidemiological analysis of S. aureus COL and S. aureus Becker using multilocus sequence typing, capsule typing, and antibiotic resistance profiles revealed that these two strains were derived from different lineages. Therefore, we concluded that it is highly likely that immunization with IsdB could confer protection against a broad spectrum of S. aureus isolates. To test this hypothesis, we adapted the mouse sepsis model to investigate the efficacy of IsdB-AAHSA immunization against different S. aureus clinical isolates.
Protection against lethal infection with various S. aureus clinical isolates.
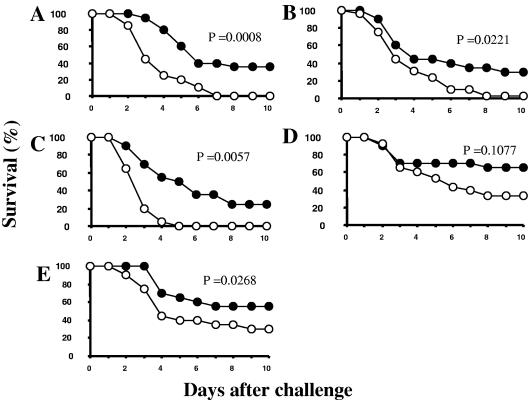
To investigate the efficacy of IsdB against infection with different S. aureus clinical isolates, five additional strains were tested with the murine sepsis model (Table 1). The isolates that were selected were taxonomically diverse, had been obtained from different types of S. aureus infections, and had different capsule types, and there were differences between their IsdB sequences and the vaccine sequence (Table 1). The variation ranged from five amino acid differences in S. aureus MCL8538 (99% identical) to 38 amino acid differences in S. aureus ME31 (94% identical). The approximate LD80-100 was determined for each isolate, and the values ranged from 1.0 × 108 to 3.1 × 108. Interestingly, the LD80-100 of the two methicillin-resistant S. aureus strains were the lowest LD80-100, and therefore these strains the most virulent. IsdB-immunized mice were challenged with the appropriate dose for each isolate, and the survival was monitored for 10 days. Improved survival was observed for both methicillin-sensitive and methicillin-resistant strains (Fig. 3); the levels of survival were 35%, 21%, and 32% greater than the level of survival of the controls with methicillin-sensitive S. aureus isolates ME60, MCL8538, and ME11, respectively, (Fig. 3A, B, and E) and 25% greater than the level of survival of the controls following infection with either of the methicillin-resistant S. aureus strains, ME27 or ME31 (Fig. 3C and D). P values of 0.05 or less were obtained for four of the five S. aureus strains tested.
FIG. 3.
Improved survival of mice immunized with three doses of IsdB and challenged with diverse clinical isolates of S. aureus. (A) S. aureus ME60 (median lethal challenge dose, 3.1 × 108 CFU); (B) S. aureus MCL8538 (median lethal challenge dose, 2.9 × 108 CFU); (C) S. aureus ME27 (median lethal challenge dose, 1.0 × 108 CFU; (D) S. aureus ME31 (median lethal challenge dose, 1.6 × 108 CFU); (E) S. aureus ME11 (median lethal challenge dose, 2.1 × 108 CFU). •, survival of mice immunized with IsdB-AAHSA; ○, survival of mice immunized with AAHSA.
Universal expression and characterization of IsdB.
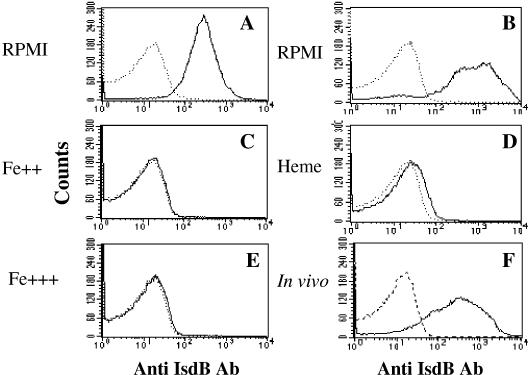
To determine if IsdB was surface expressed on all of the isolates used in this study, we conducted a flow cytometry analysis of bacteria grown in an iron-deficient defined medium (RPMI 1640). Murine IsdB hyperimmune mouse sera yielded positive staining results for all strains tested (Fig. 4 and Table 1). Since S. aureus has several proteins that exhibit sequence identity to IsdB (2), including HarA (6) and IsdA (21), the analysis was repeated using a monoclonal antibody that was shown to be specific for IsdB (Fig. 4B); expression of IsdB was detected in all but two of the isolates. In complex iron-enriched media (either broth cultures in TSB or plate-grown cultures on TSA), little or no expression of IsdB was detected (Table 1). To confirm that expression of the protein was regulated by iron, we also demonstrated that the protein could not be detected after addition of iron to RPMI medium in the form of iron sulfate, heme, or iron chloride (Fig. 4C, D, and E, respectively). We further explored expression of this antigen in vivo by implanting sterile chambers inoculated with S. aureus cells cultured in an iron-rich medium into rats. IsdB was readily detected on the surface of the recovered bacteria by flow cytometry (Fig. 4 F). No binding was observed for the irrelevant isotype control monoclonal antibody (data not shown).
FIG. 4.
Surface expression of IsdB by S. aureus Becker grown under different conditions. The histograms indicate the IsdB-specific fluorescence intensities (solid line) and the baseline signals obtained with the second antibody only (dotted line). IsdB expression was measured using murine IsdB immune serum (A) and an IsdB-specific monoclonal antibody (B to F). Cultures were grown overnight in vitro in RPMI 1640 (RPMI) (A and B) or RPMI 1640 supplemented with 20 μM FeSO4 (Fe++) (C), 10 μM heme (Heme) (D), or 20 μM FeCl3 (Fe+++) (E). In vivo cultures were generated from dialysis tubes inoculated with S. aureus cells and implanted into rats (F).
Protection in the mouse sepsis model is specific for the immune response generated against surface-expressed IsdB antigen.
To correlate the protective effect of the IsdB vaccine with a direct anti-IsdB antibody-IsdB protein interaction in the murine sepsis model, we genetically engineered an S. aureus Becker isdB deletion strain. The genes that encode IsdB and HarA (a related cross-reactive protein) were removed. Lack of expression of IsdB in the deletion strain was confirmed by flow cytometry; bacterial cells were not reactive to IsdB antibodies (Fig. 5A) when they were grown under the same growth conditions as the IsdB-expressing wild-type strain (Fig. 5B). As positive control we demonstrated that both the deletion and wild-type strains reacted with convalescent S. aureus mouse serum (Fig. 5C and 5D).
FIG. 5.
Lack of IsdB surface expression on S. aureus Becker isdB harA as measured by flow cytometry. (A) IsdB-specific monoclonal antibody staining of S. aureus Becker isdB harA. (B) IsdB-specific monoclonal antibody staining of S. aureus Becker wild type. (C) Convalescent S. aureus mouse serum used for staining S. aureus Becker isdB harA. (D) Convalescent S. aureus mouse serum used for staining wild-type S. aureus Becker. The thick solid line indicates the results for IsdB-specific antibodies, the dotted line shows the results for naïve serum, and the thin solid line shows the results for the second-stage control (without primary antibody). Ab, antibody.
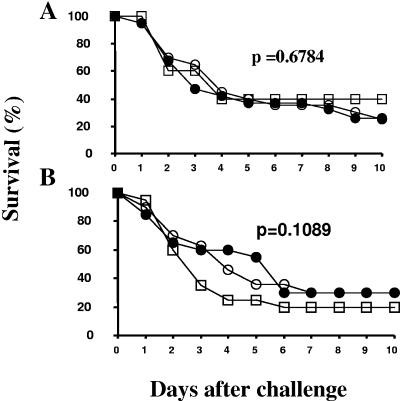
Titration of S. aureus Becker and S. aureus Becker isdB harA inocula was performed in ICR mice to determine whether deletion of the two associated proteins had an effect on the infectivity or virulence of S. aureus in vivo. The deletion strain was virulent in mice; however, higher doses of this strain than of the wild-type strain were needed to obtain an LD80-100 (P = <0.01) (data not shown). Once the LD80-100 was determined for the deletion strain, vaccine-immunized, AAHSA control, and naïve mice were challenged with S. aureus Becker isdB harA. The antibody responses to the vaccine in all IsdB-APA-immunized mice were determined at day 35 (7 days prior to challenge), and the results confirmed that the challenge groups were immunized successfully (data not shown). Reduced survival was observed for mice immunized with IsdB vaccine and challenged with S. aureus Becker isdB harA in two independent experiments (Fig. 6) compared to the results for challenges with the wild-type strain (Fig. 1), which provided evidence for the specificity of protection provided by the IsdB vaccine.
FIG. 6.
Protection is specific for an immune response against surface-expressed IsdB: survival of mice immunized three times with 20 μg of IsdB formulated with AAHSA after challenge with S. aureus Becker isdB harA (n = 20 for the IsdB- and AAHSA-immunized groups; n = 10 for the naïve group). Panels A and B show the results for two independent experiments. •, IsdB-AAHSA-immunized mice; □, naïve mice; ○, AAHSA-immunized mice.
IsdB formulated with AAHSA is immunogenic in rhesus macaques.
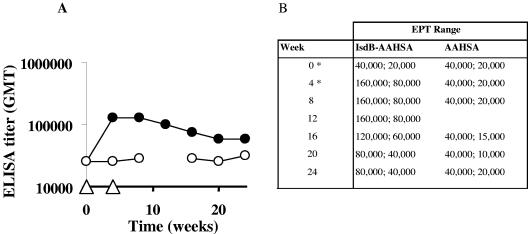
The presence of antibody titers to IsdB in human acute-phase serum (8) led us to believe that our vaccine candidate would be immunogenic in primates. To evaluate this, we investigated the immunological response to IsdB in nonhuman primates. We immunized two groups of rhesus macaques with either IsdB or adjuvant alone (AAHSA) via the intramuscular route, using recombinant protein expressed from E. coli. Animals were immunized at zero time and 4 weeks. Prior to vaccination it was noted that the animals had preexisting titers to IsdB, and a single dose of the vaccine resulted in a fivefold increase in the titer to IsdB that was long lasting. The titer did not increase after a subsequent boost at 4 weeks (Fig. 7).
FIG. 7.
Anamnestic and long-lasting response in rhesus macaques immunized with IsdB. (A) Geometric mean titer (GMT) obtained for the rhesus macaque immunization group (n = 3). •, IsdB-AAHSA-immunized rhesus macaques; ○, AAHSA-immunized rhesus macaques. The immunization schedule is indicated by open triangles. (B) End point titer (EPT) range obtained for each group at each time. *, vaccine administration.
DISCUSSION
A major challenge for healthcare in the 21st century is the increasing levels of resistance to antimicrobial compounds of S. aureus. While the search for novel antibacterial drugs continues, isolates that are resistant even to novel antibiotics, such as linazolid, are quickly found (1). An effective vaccine to prevent the often life-threatening infections is urgently needed. S. aureus strains possess many antigens on their surfaces that have been investigated for their potential as either therapeutic or prophylactic vaccines. A vaccine to capsular polysaccharide conjugate is currently being tested in clinics and was found to reduce infection by 56% over a 10-month period in hemodialysis patients (35). This vaccine targets two S. aureus capsule serotypes that represent approximately 70% of S. aureus clinical isolates (29). There are also ongoing studies to investigate S. aureus surface proteins, including clumping factor A (ClfA) (10, 14), fibronectin binding proteins (34), fibrinogen binding proteins (20), collagen binding protein (Cna) (27), and secreted toxins (12, 23, 28). Some of these antigens have been tested in murine sepsis models similar to the one used in this study. Cna (27), toxic shock syndrome toxin (12), and enterotoxin have been tested in active immunization studies, and ClfA (10) has been tested in passive immunization studies using a ClfA-specific monoclonal antibody. The overall survival rates for vaccinated groups for which positive data were reported ranged from 20 to 87%. In our hands the survival rates for IsdB-immunized animals in which significant levels of protection were observed ranged from 20 to 80%. The differences in survival between vaccine and sham groups ranged from 15 to 74%, compared to 20 to 40% for IsdB-vaccinated animals. The reduced efficacy window and the lack of 100% efficacy for all of these antigens in the murine sepsis model are believed to be artifacts of this model. Extremely high challenge doses of S. aureus (107 to 109 cells) are required to overcome the natural innate immunity of mice, even with naïve animals. The S. aureus vaccine candidates studied to date all play important roles in vivo; however, none is essential, and they may not be expressed in all phases of infection and may have variable distribution among S. aureus clinical isolates. The Cna antigen showed excellent potential with the murine sepsis model, and the survival rates were 64 and 74% greater than the control survival rates (27); however, epidemiology studies have shown that this antigen is present in only a small proportion of clinical isolates (33). In S. aureus there is a high level of redundancy in the virulence protein repertoire, so loss of a specific protein may not be fatal. This built-in redundancy makes the search for successful vaccines a challenge, and for current investigational targets it is clear that multiple approaches are needed to combat disease in the long term. We demonstrated that although IsdB is not an essential protein for S. aureus in vitro, loss of this protein results in a reduction in virulence in vivo, which makes it an attractive vaccine candidate.
We demonstrated that IsdB, when formulated with AAHSA, is highly immunogenic. The induction of IsdB-specific antibody responses correlated with reproducible and significant protection in a mouse model of infection with broad coverage against different S. aureus clinical isolates, including methicillin-resistant strains. Specifically, we demonstrated that mice immunized with IsdB and challenged with a strain which had isdB harA deletions were not protected from death, thus identifying the specificity of protection provided by an immune response targeted against surface-expressed IsdB.
IsdB is conserved among diverse S. aureus clinical isolates, both methicillin resistant and methicillin sensitive, and it is expressed on the surface of all isolates tested. It is interesting that the bacteria used to challenge animals were routinely prepared from plates containing TSA, a medium which represses IsdB expression. S. aureus Becker does not have detectable levels of IsdB on its surface under these growth conditions. However, the ability to detect surface expression of the IsdB protein on bacteria grown in vivo, as well as the protection data, suggest that the protein is expressed very quickly during infection.
Finally, we demonstrated that the IsdB-immunized mice which survived the lethal challenge had higher antibody responses than the mice that succumbed to the infection. This underscores the role of IsdB antibody responses in protection in the sepsis model. One question that remains to be answered is the mechanism of action for the protection mediated by this vaccine. We demonstrated that there is a protective effect mediated by expression of this protein by S. aureus cells and that the vaccine induces high antibody responses to the protein. Human clearance of bacterial infections is expected to be via opsonic killing which is mediated after phagocyte uptake (40). There are many convincing examples for this from studies using gram-positive polysaccharide antigens, such as Streptococcus pneumoniae capsular polysaccharide (3-5, 16, 17) and S. aureus capsular polysaccharide. The S. aureus capsular polysaccharide vaccine that is currently undergoing clinical trials has demonstrated that there is a correlation between opsonophagocytic killing and human postimmune capsule titers (9). There is not such clarity for gram-positive protein antigens. Uptake by phagocytes has been observed (26), but direct killing has been harder to demonstrate. Despite these results, monoclonal antibodies to proteins have been shown to confer protection against S. aureus challenge in animal models of infection (31); however, mechanisms other than opsonophagocytic killing may account for the protection observed.
One of the most striking observations in this study was the brisk immune response to IsdB in rhesus macaques, which gave us reason to hypothesize that the vaccine described here may generate an anamnestic response in humans, who, like monkeys, have preexisting titers to the antigen, thus providing rapid protection against the vaccine. This suggests that it may be possible to develop a truly nosocomial vaccine that can prevent S. aureus infection in the hospital setting without a lengthy immunization schedule. Humans are constantly exposed to S. aureus, a natural colonizer of the skin and nares, which may account for the presence of preexisting titers. We found that in a murine sepsis model, survival is correlated with the magnitude of the titer to IsdB. We believe that an IsdB-based vaccine should be an effective antigen for the prevention of S. aureus infection that boosts preexisting antibody titers to this protein to a threshold that can provide protection from infection.
Editor: D. L. Burns
REFERENCES
- 1.Anderegg, T. R., H. S. Sader, T. R. Fritsche, J. E. Ross, and R. N. Jones. 2005. Trends in linezolid susceptibility patterns: report from the 2002-2003 worldwide Zyvox Annual Appraisal of Potency and Spectrum (ZAAPS) Program. Int. J. Antimicrob. Agents 26:13-21. [DOI] [PubMed] [Google Scholar]
- 2.Andrade, M. A., F. D. Ciccarelli, C. Perez-Iratxeta, and P. Bork. 2002. NEAT: a domain duplicated in genes near the components of a putative Fe3+ siderophore transporter from Gram-positive pathogenic bacteria. Genome Biol. 3:RESEARCH0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett, D. J., and E. M. Ayoub. 1986. IgG2 subclass restriction of antibody to pneumococcal polysaccharides. Clin. Exp. Immunol. 63:127-134. [PMC free article] [PubMed] [Google Scholar]
- 4.Briles, D. E., C. Forman, S. Hudak, and J. L. Claflin. 1984. The effects of subclass on the ability of anti-phosphocholine antibodies to protect mice from fatal infection with Streptococcus pneumoniae. J. Mol. Cell. Immunol. 1:305-309. [PubMed] [Google Scholar]
- 5.Chu, R. S., T. McCool, N. S. Greenspan, J. R. Schreiber, and C. V. Harding. 2000. CpG oligodeoxynucleotides act as adjuvants for pneumococcal polysaccharide-protein conjugate vaccines and enhance antipolysaccharide immunoglobulin G2a (IgG2a) and IgG3 antibodies. Infect. Immun. 68:1450-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dryla, A., D. Gelbmann, A. von Gabain, and E. Nagy. 2003. Identification of a novel iron regulated staphylococcal surface protein with haptoglobin-haemoglobin binding activity. Mol. Microbiol. 49:37-53. [DOI] [PubMed] [Google Scholar]
- 7.Enright, M. C., N. P. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etz, H., D. B. Minh, T. Henics, A. Dryla, B. Winkler, C. Triska, A. P. Boyd, J. Sollner, W. Schmidt, U. von Ahsen, M. Buschle, S. R. Gill, J. Kolonay, H. Khalak, C. M. Fraser, A. von Gabain, E. Nagy, and A. Meinke. 2002. Identification of in vivo expressed vaccine candidate antigens from Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 99:6573-6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fattom, A., S. Fuller, M. Propst, S. Winston, L. Muenz, D. He, R. Naso, and G. Horwith. 2004. Safety and immunogenicity of a booster dose of Staphylococcus aureus types 5 and 8 capsular polysaccharide conjugate vaccine (StaphVAX) in hemodialysis patients. Vaccine 23:656-663. [DOI] [PubMed] [Google Scholar]
- 10.Hall, A. E., P. J. Domanski, P. R. Patel, J. H. Vernachio, P. J. Syribeys, E. L. Gorovits, M. A. Johnson, J. M. Ross, J. T. Hutchins, and J. M. Patti. 2003. Characterization of a protective monoclonal antibody recognizing Staphylococcus aureus MSCRAMM protein clumping factor A. Infect. Immun. 71:6864-6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 12.Hu, D. L., K. Omoe, S. Sasaki, H. Sashinami, H. Sakuraba, Y. Yokomizo, K. Shinagawa, and A. Nakane. 2003. Vaccination with nontoxic mutant toxic shock syndrome toxin 1 protects against Staphylococcus aureus infection. J. Infect. Dis. 188:743-752. [DOI] [PubMed] [Google Scholar]
- 13.Jansen, K. U., M. Rosolowsky, L. D. Schultz, H. Z. Markus, J. C. Cook, J. J. Donnelly, D. Martinez, R. W. Ellis, and A. R. Shaw. 1995. Vaccination with yeast-expressed cottontail rabbit papillomavirus (CRPV) virus-like particles protects rabbits from CRPV-induced papilloma formation. Vaccine 13:1509-1514. [DOI] [PubMed] [Google Scholar]
- 14.Josefsson, E., O. Hartford, L. O'Brien, J. M. Patti, and T. Foster. 2001. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J. Infect. Dis. 184:1572-1580. [DOI] [PubMed] [Google Scholar]
- 15.Kuklin, N. A., G. D. Pancari, T. W. Tobery, L. Cope, J. Jackson, C. Gill, K. Overbye, K. P. Francis, J. Yu, D. Montgomery, A. S. Anderson, W. McClements, and K. U. Jansen. 2003. Real-time monitoring of bacterial infection in vivo: development of bioluminescent staphylococcal foreign-body and deep-thigh-wound mouse infection models. Antimicrob. Agents Chemother. 47:2740-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, C. J., L. H. Lee, and C. E. Frasch. 2003. Protective immunity of pneumococcal glycoconjugates. Crit. Rev. Microbiol. 29:333-349. [DOI] [PubMed] [Google Scholar]
- 17.Lee, C. J., T. R. Wang, and C. E. Frasch. 2001. Immunogenicity in mice of pneumococcal glycoconjugate vaccines using pneumococcal protein carriers. Vaccine 19:3216-3225. [DOI] [PubMed] [Google Scholar]
- 18.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 19.Luong, T. T., and C. Y. Lee. 2002. Overproduction of type 8 capsular polysaccharide augments Staphylococcus aureus virulence. Infect. Immun. 70:3389-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mamo, W., M. Boden, and J. I. Flock. 1994. Vaccination with Staphylococcus aureus fibrinogen binding proteins (FgBPs) reduces colonisation of S. aureus in a mouse mastitis model. FEMS Immunol. Med. Microbiol. 10:47-53. [DOI] [PubMed] [Google Scholar]
- 21.Mazmanian, S. K., E. P. Skaar, A. H. Gaspar, M. Humayun, P. Gornicki, J. Jelenska, A. Joachmiak, D. M. Missiakas, and O. Schneewind. 2003. Passage of heme-iron across the envelope of Staphylococcus aureus. Science 299:906-909. [DOI] [PubMed] [Google Scholar]
- 22.Mazmanian, S. K., H. Ton-That, K. Su, and O. Schneewind. 2002. An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc. Natl. Acad. Sci. USA 99:2293-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menzies, B. E., and D. S. Kernodle. 1996. Passive immunization with antiserum to a nontoxic alpha-toxin mutant from Staphylococcus aureus is protective in a murine model. Infect. Immun. 64:1839-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, D., V. Urdaneta, and S. Park. 2002. Vancomycin-resistant Staphylococcus aureus—Pennsylvania, 2002. Morb. Mortal. Wkly. Rep. 51:902. [PubMed] [Google Scholar]
- 25.Morin, C. A., and J. L. Hadler. 2001. Population-based incidence and characteristics of community-onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998. J. Infect. Dis. 184:1029-1034. [DOI] [PubMed] [Google Scholar]
- 26.Neth, O., D. L. Jack, M. Johnson, N. J. Klein, and M. W. Turner. 2002. Enhancement of complement activation and opsonophagocytosis by complexes of mannose-binding lectin with mannose-binding lectin-associated serine protease after binding to Staphylococcus aureus. J. Immunol. 169:4430-4436. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson, I. M., J. M. Patti, T. Bremell, M. Hook, and A. Tarkowski. 1998. Vaccination with a recombinant fragment of collagen adhesin provides protection against Staphylococcus aureus-mediated septic death. J. Clin. Investig. 101:2640-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson, I. M., M. Verdrengh, R. G. Ulrich, S. Bavari, and A. Tarkowski. 1999. Protection against Staphylococcus aureus sepsis by vaccination with recombinant staphylococcal enterotoxin A devoid of superantigenicity. J. Infect. Dis. 180:1370-1373. [DOI] [PubMed] [Google Scholar]
- 29.O'Riordan, K., and J. C. Lee. 2004. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 17:218-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palavecino, E. 2004. Community-acquired methicillin-resistant Staphylococcus aureus infections. Clin. Lab. Med. 24:403-418. [DOI] [PubMed] [Google Scholar]
- 31.Patti, J. M. 2004. A humanized monoclonal antibody targeting Staphylococcus aureus. Vaccine 22(Suppl. 1):S39-S43. [DOI] [PubMed] [Google Scholar]
- 32.Reed, G. F., B. D. Meade, and M. C. Steinhoff. 1995. The reverse cumulative distribution plot: a graphic method for exploratory analysis of antibody data. Pediatrics 96:600-603. [PubMed] [Google Scholar]
- 33.Ryding, U., J. I. Flock, M. Flock, B. Soderquist, and B. Christensson. 1997. Expression of collagen-binding protein and types 5 and 8 capsular polysaccharide in clinical isolates of Staphylococcus aureus. J. Infect. Dis. 176:1096-1099. [DOI] [PubMed] [Google Scholar]
- 34.Schennings, T., A. Heimdahl, K. Coster, and J. I. Flock. 1993. Immunization with fibronectin binding protein from Staphylococcus aureus protects against experimental endocarditis in rats. Microb. Pathog. 15:227-236. [DOI] [PubMed] [Google Scholar]
- 35.Shinefield, H., S. Black, A. Fattom, G. Horwith, S. Rasgon, J. Ordonez, H. Yeoh, D. Law, J. B. Robbins, R. Schneerson, L. Muenz, S. Fuller, J. Johnson, B. Fireman, H. Alcorn, and R. Naso. 2002. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 346:491-496. [DOI] [PubMed] [Google Scholar]
- 36.Sievert, D. M., M. L. Boulton, G. Stoltman, D. Johnson, M. G. Stobierski, F. P. Downes, P. A. Somsel, J. T. Rudrik, W. Brown, W. Hafeez, T. Lundstrom, E. Flanagan, R. Johnson, J. Mitchell, and S. Chang. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. Morb. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 37.Skaar, E. P., and O. Schneewind. 2004. Iron-regulated surface determinants (Isd) of Staphylococcus aureus: stealing iron from heme. Microbes Infect. 6:390-397. [DOI] [PubMed] [Google Scholar]
- 38.Solomon, S., T. Horan, M. Andrus, J. Edwards, G. Emori, S. Fridkin, G. Peavy, J. Tolson, S. Upadhyayula, and B. Yi. 2002. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 to June 2002, issued August 2002. Am. J. Infect. Control. 30:458-475. [DOI] [PubMed] [Google Scholar]
- 39.Solomon, S., T. Horan, M. Andrus, J. Edwards, S. Fridkin, J. Koganti, G. Peavy, and J. Tolson. 2003. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 through June 2003, issued August 2003. Am. J. Infect. Control. 31:481-498. [DOI] [PubMed] [Google Scholar]
- 40.Underhill, D. M., and A. Ozinsky. 2002. Phagocytosis of microbes: complexity in action. Annu. Rev. Immunol. 20:825-852. [DOI] [PubMed] [Google Scholar]