Abstract
Aspergillus fumigatus induces the release of innate immune-related molecules from phagocytic cells early in the course of infection. Little is known, however, about the complex expression profiles of the multiple genes involved in this response. We therefore investigated the kinetics of early gene expression in human monocytes (HMCs) infected with conidia of A. fumigatus using DNA microarray analysis. Total RNA from HMCs at 0, 2, 4, and 6 h was extracted, linearly amplified, hybridized onto Affymetrix HG133 Plus 2.0 gene chips, and analyzed with an Affymetrix scanner. Changes in gene expression were calculated as a ratio of those expressed by infected versus control HMCs. Aspergillus fumigatus induced differential regulation of expression in 1,827 genes (P < 0.05). Genes encoding cytokines and chemokines involved in host defense against A. fumigatus, including interleukin-1β (IL-1β), IL-8, CXCL2, CCL4, CCL3, and CCL20, as well as the opsonin long pentraxin 3, were up-regulated during the first 2 to 6 h, coinciding with an increase in phagocytosis. Simultaneously, genes encoding CD14, ficolin1, and MARCO were down-regulated, and genes encoding IL-10 and matrix metalloproteinase 1 were up-regulated. Up-regulation of the genes encoding heat shock proteins 40 and 110 and connexins 26 and 30 may point to novel molecules whose role in the pathogenesis of aspergillosis has not been previously reported. Verification of the transcriptional profiling was obtained for selected genes by reverse transcription-PCR and enzyme immunoassay. Thus, A. fumigatus conidia induced a coordinated expression of genes important in host defense and immunomodulation.
Invasive aspergillosis is an important cause of morbidity and mortality among immunocompromised patients. Risk factors for developing aspergillosis include aplastic anemia (46), hematopoietic stem cell transplantation (32), solid organ transplantation (44), and phagocytic deficiencies, such as chronic granulomatous disease and human immunodeficiency virus infection (40, 43). Aspergillus fumigatus is the species most commonly identified as the cause of invasive aspergillosis.
Monocytes/macrophages play a pivotal role in normal host defense against A. fumigatus. The innate host response against this pathogen is mediated by release of immune-related molecules from phagocytic cells. However, little is known about the complex expression kinetic profiles of the multiple genes mediating the initial immune response to A. fumigatus. Thus, we hypothesized that conidia of A. fumigatus would induce reciprocal changes in expression of genes encoding products related to innate immunity that may provide insight into early host-parasite interaction.
In this study, we used microarray analysis to elucidate the initial kinetics of the transcriptional response of genes encoding innate host defense molecules in normal human monocytes after in vitro stimulation/infection with A. fumigatus conidia. We report herein the changes in expression of genes whose products are involved in the initial innate immune response, including proteins involved in phagocytosis, endocytosis, cytokine and chemokine signaling and their receptors; proteins involved in cell adhesion and cell communication; scavenger receptors, Toll-like receptors, and other immunological receptors; matrix metalloproteinases; pattern recognition receptors; and heat shock proteins. On a functional genomic level, these studies elucidate the intricate interaction between effector cells, their mediators, and the microorganism over the initial 6 h of infection with A. fumigatus.
MATERIALS AND METHODS
Aspergillus fumigatus preparation.
A well-characterized strain of Aspergillus fumigatus (NIH 4215, ATCC MYA-1163), originally isolated from a cancer patient with invasive aspergillosis, was used throughout these studies. The isolate was preserved on frozen potato dextrose agar slants at −70°C. Working stocks of A. fumigatus conidia were harvested from freshly prepared 5-day-old slants and suspended in phosphate-buffered saline as described previously (42). Conidia were stored at 4°C for up to 1 week.
Monocyte isolation.
Human monocytes (HMCs) were isolated from five healthy volunteers by automated leukapheresis of blood and subsequently separated by counterflow centrifugal elutriation (model J-6 M centrifuge; Beckman Instruments, Fullerton, CA). Cells were washed in Hanks' balanced salt solution (HBSS) without Ca2+ and Mg2+ (HBSS−) and resuspended to a final concentration of 1 × 107 cells/ml in HBSS with Ca2+ and Mg2+ (HBSS+). Viability was assessed as greater than 98% by trypan blue exclusion, and the cell population isolated was >90% monocytes by Wright-Giemsa stain and/or nonspecific esterase stain.
Incubation of monocytes with A. fumigatus conidia.
Monocytes from five different donors and conidia of A. fumigatus were incubated and processed in five separate experiments. For each donor, HMCs and A. fumigatus conidia were prepared in HBSS+ at concentrations of 1 × 107 cells/ml and 1 × 106 conidia/ml, respectively, for an E:T ratio of 10:1. Using a polystyrene six-well flat-bottomed plate (Becton Dickinson, Franklin Lakes, NJ), two wells were designated for cells alone (control HMCs) and four wells were set up for cells plus conidia (stimulated HMCs) for each time point. The wells containing control HMCs and two of the wells containing HMCs plus conidia were used for RNA extraction, while the remaining two wells with stimulated cells were used for phagocytosis and viability determinations.
Time points were established at 0, 2, 4, and 6 h. The plates were incubated at 37°C and 5% CO2 on a plate shaker (Delfia 1296-004; Wallac, Turku, Finland) for gentle mixing. For the zero hour time point (T0), HMCs and A. fumigatus conidia were maintained on ice and mixed immediately prior to centrifuging at 4°C, followed by RNA extraction (see below). Supernatants from aliquots from all time points were collected and frozen at −70°C for protein analysis.
Two of the wells containing cells plus conidia also contained 18-mm circular glass coverslips that were used to determine phagocytosis of conidia and viability of the HMCs at each time point. The coverglass slides were gently removed from the two designated wells at each time point. One of the slides was examined immediately using trypan blue exclusion to determine viability of HMCs. The second slide was allowed to dry at room temperature, fixed, and stained with a modified Wright-Giemsa stain. The percent phagocytosis was calculated by determining microscopically the percent HMCs containing organisms. The phagocytic index was calculated as the average number of conidia per phagocytosing cell (39).
RNA preparation and Affymetrix microarray analysis.
Immediately following each time point, total RNA from 2 × 107 stimulated and unstimulated HMCs was extracted using the TRIzol method (Invitrogen, Carlsbad, CA). cRNA synthesis using 15 μg total RNA and hybridization to the HG-U133 Plus 2.0 GeneChip was performed following the manufacturer's (Affymetrix, Santa Clara, CA) recommended protocol. Affymetrix Microarray Analysis Suite (MAS) 5.0 was used for chip-to-chip normalization and gene expression value determination. For each of the five donors and four time points, a separate two-sample comparison between unstimulated control HMCs and A. fumigatus infected-HMCs was performed using GCOS software, and log2 ratio data for treated/untreated cells were collected for statistical analysis.
Statistical determination of significant differential expression.
Two-way analysis of variance (Partek Pro 5.1 Statistical Package, St. Charles, MO) was performed with donors set as a random effect, time as the main effect, and contrast set to compare time points only to T0. Since the T0 comparison is actually a technical replicate within donors (no treatment was applied in the treatment group), the data at T0 were used to remove noisy probe sets by filtering out those probe sets that showed average changes among the five donors greater than 1.3-fold in either direction. Probe sets with mean expression values <40 for both comparison groups in the analysis of variance (near the limits of detection) were eliminated from further consideration. Differentially expressed genes were defined as those having a P value of ≤0.05 and an absolute change of greater than 1.5-fold for each time point compared to T0. A 1.5-fold change previously has been shown to determine significance of differences in gene expression (3, 24, 28). Moreover, a number of reports have shown that change (fold) calculated from Affymetrix microarrays often underestimates the true change (fold) in expression (5, 6, 21, 37).
Genes were grouped by K-Means clustering (Spotfire DecisionSite, Somerville, MA) of log2 ratio data. The data were plotted as the mean gene expression ratio of five healthy donors over time: for each of the five donors, the change (log2 fold) for each time point relative to T0 was determined with and without incubation with Aspergillus. Functional annotation of the genes was performed using DAVID version 2. (http://david.niaid.nih.gov/david/version2/index.htm).
Real-time PCR.
HMCs from an independent group of three healthy donors were studied on three separate days. These cells were treated or untreated with A. fumigatus conidia for 0 and 6 h as described above. Total RNA was isolated from 2 × 107 cells using TRIzol, and 1 μg of total RNA was reverse transcribed with random hexamer primers using the TaqMan reverse transcription (RT) kit system (Applied Biosystems, Foster City, CA). For quantitative real-time PCR, one-eightieth of the first strand cDNA was used as a template for detection of each gene of interest. GBJ2, PTGS2, and SOD2 were selected as target genes because each belonged to a different biological category and each displayed a magnitude of change in expression by microarray that was more likely to be detected by RT-PCR. B2M was used as a control gene. GJB2, PTGS2, SOD2, and B2M levels were detected using the Assay-on-Demand System (Applied Biosystems) and the TaqMan Universal Master Mix (Applied Biosystems). The fluorescence signals were measured in real time during the extension step using the iCycler iQ Real Time Detection System (Bio-Rad, Hercules, CA). The ratio change in target genes relative to the B2M control gene was determined within each sample by the 2−ΔΔCT method (27).
Determination of protein levels.
One-milliliter aliquots of cell-free supernatants from HMCs alone and HMCs plus A. fumigatus conidia were collected from at least three separate experiments for each time point. Samples were stored at −70°C in curved-bottom cryotubes until processed. CXCL8/interleukin-8 (IL-8), CCL4/MIP1β, CCL5/RANTES, and MMP-1 protein levels were evaluated in duplicate serial dilutions of supernatants using commercially available quantitative sandwich enzyme immunoassay (EIA) technique (EIA kits) for CXCL8/IL-8, CCL4/MIP1β, CCL5/RANTES, and MMP-1 (R&D Systems, Inc., Minneapolis, MN), according to protocols specified by the manufacturer. The plates were read in an EIA reader (Titertek Multiskan MCC/340; Lab Systems, Finland) at a wavelength of 450 nm with wavelength correction set at 570 nm. The concentrations of cytokines in the samples were calculated from a standard curve of CXCL8/IL-8, CCL4/MIP1β, CCL5/RANTES, and MMP-1, respectively. Subsequently, the differences between protein levels in HMCs with A. fumigatus and control HMCs were obtained and plotted versus log2 expression ratios of the genes over time.
RESULTS
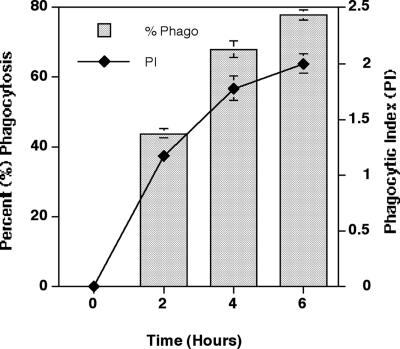
The viability of HMCs was greater than 80% for all donors at all time points evaluated. The percent phagocytosis increased progressively over the 6-h time course from 0% to 80% (Fig. 1). The phagocytic index also increased progressively over time.
FIG. 1.
Kinetics of phagocytosis of A. fumigatus conidia. Results are expressed as mean ± standard error % phagocytosis (number of HMCs ingesting ≥1 conidia of A. fumigatus per 100 counted cells) and as mean ± standard error phagocytic index (average number of conidia of A. fumigatus per phagocytosing HMC).
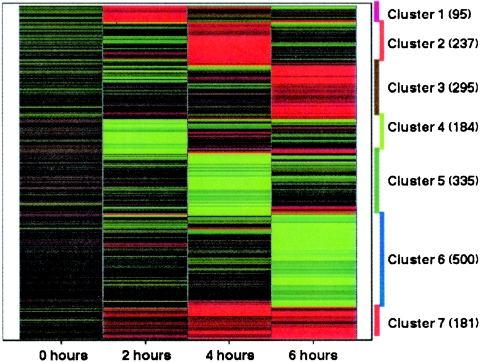
As expected, 99.9% of genes in normal HMCs were unchanged at the zero-hour time point. There were a total of 1,827 differentially expressed genes with signal/log ratio (log2) of ≥1.5 measured over the 2- to 6-h time course (see Table S1 in the supplemental material). A heat map of the 1,827 differentially expressed genes arranged into seven distinct clusters according to the time of maximal up- or down-regulation is shown in Fig. 2. Cluster 1 represents a group of 95 genes that were maximally up-regulated at 2 h of incubation. Cluster 2 comprises 237 genes with maximal up-regulation at 4 h. Cluster 3 has a total of 295 genes with maximal up-regulation at 6 h of incubation. In contrast, cluster 4 displays 184 genes with maximal down-regulation at 2 h, while cluster 5 includes 335 genes with maximal down-regulation at 4 h, and cluster 6 has 500 genes displaying maximal down-regulation at 6 h. There was a group of 181 genes, cluster 7, that was up-regulated at 2 h and remained elevated throughout the 6-h time course with peak elevation at approximately 4 h. Of the 1,827 genes differentially expressed, 239 were related to previously documented or suspected immune functions according to Gene Ontology consortium and its databases GO Biological, GO Molecular Function, and GO Cellular Component (http://www.geneontology.org/) and the database SwissProt (http://www.pir.uniprot.org/) keyword. Of these 239 genes with suspected or previously documented immune-related function, 125 were up-regulated and 114 were down-regulated.
FIG. 2.
Heat map of gene expression profile in HMCs exposed to conidia of A. fumigatus. The results show K-Means clustering of 1,827 genes significantly differentially expressed following the maximal up-regulation (red) or down-regulation (green) over time. Cluster 1, maximal up-regulation at 2 h; cluster 2, maximal up-regulation at 4 h; cluster 3, maximal up-regulation at 6 h; cluster 4, maximal down-regulation at 2 h; cluster 5, maximal down-regulation at 4 h; cluster 6, maximal down-regulation at 6 h; cluster 7, up-regulation over 2 to 6 h.
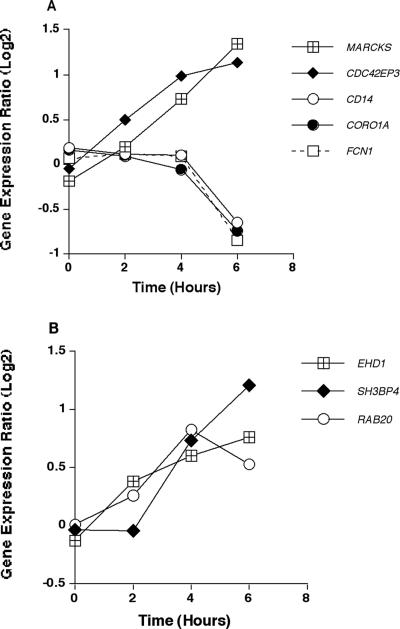
Genes encoding molecules related to phagocytic function of monocytes were examined in response to A. fumigatus. Figure 3A shows the kinetic profiles of those genes that were differentially expressed in response to the conidia. The phagocytosis-related genes that were up-regulated in response to A. fumigatus conidia included MARCKS (myristoylated alanine-rich protein kinase C substrate, a protein thought to be involved in cell motility, phagocytosis, membrane trafficking, and mitogenesis) and CDC42EP3 (Rho GTPase binding 3). The kinetics of gene expression of MARCKS and CDC42EP3 showed steep upward trends, peaking at 6 h.
FIG. 3.
Expression of phagocytosis- and endocytosis-related genes. (A) Mean expression ratio (log2) kinetics of genes involved in phagocytosis in HMCs in response to conidia of A. fumigatus (P ≤ 0.05 for peak change compared to T0). Results for the following genes are shown: MARCKS (myristoylated alanine-rich protein kinase C substrate), CDC42EP3 (Rho GTPase binding 3), CD14 (cluster designation antigen 14), CORO1A (coronin 1A; also known as TACO); FCN1 (M-ficolin precursor). (B) Mean expression ratio (log2) kinetics of genes involved in endocytosis in HMCs in response to conidia of A. fumigatus (P ≤ 0.05 for peak change compared to T0). Results for the following genes are shown: EHD1 (EH domain-containing 1), SH3BP4 (SH3-domain binding protein 4), and RAB20 (RAB20 member of RAS oncogene family).
Among the genes related to phagocytosis, CD14, CORO1A, and FCN1 were down-regulated in response to A. fumigatus conidia. All of these displayed similar kinetic profiles (Fig. 3A). CD14 (a glycosylphosphatidyl inositol-linked glycoprotein present on mononuclear and polymorphonuclear phagocytes), CORO1A (the gene encoding an actin binding protein involved in the fusion of phagosomes and lysosomes), and FCN1 (the gene encoding a precursor of M-ficolin) were all significantly down-regulated by 6 h in response to A. fumigatus.
Genes encoding proteins involved in endocytosis also were examined. The kinetic profiles of those genes found to be significantly differentially expressed in response to A. fumigatus are shown in Fig. 3B. EHD1, SH3BP4, and RAB20 all encode proteins involved in endocytosis of particles by human monocytes. The protein encoded by EHD1 is thought to play a role in the endocytosis of IGF1 receptors and to reside in the endocytic recycling compartment. SH3BP4 homologies and consensus sequence sites indicate that it may be involved in a newly identified cascade of proteins involved in endocytosis, intracellular sorting, and the cell cycle. The protein encoded by RAB20 is involved in apical endocytosis/recycling. The three genes followed similar kinetic profiles, with significant up-regulation in gene expression by 4 h and peak expression occurring between 4 and 6 h.
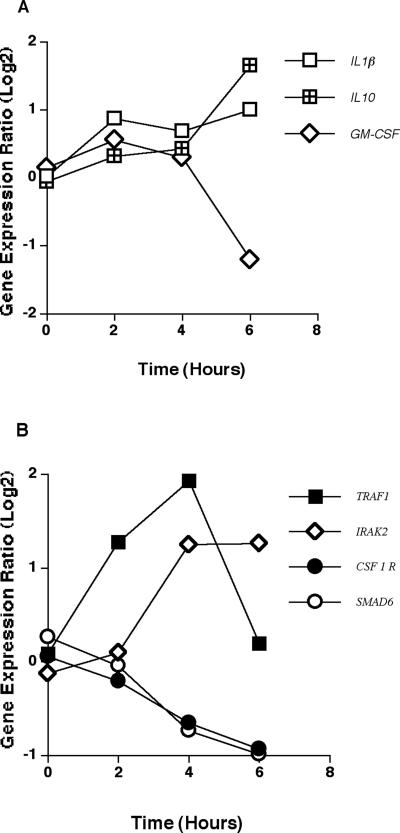
Cytokine-encoding genes that were significantly differentially expressed in response to A. fumigatus are shown in Fig. 4A. The genes encoding interleukin-1β (IL1β) and interleukin-10 (IL10) were up-regulated, beginning at 2 h and peaking at 6 h. In contrast, expression of the gene encoding granulocyte-macrophage colony-stimulating factor (CSF2) was not significantly changed until 6 h, when there was a significant down-regulation compared to baseline. Genes encoding other molecules with cytokine activity that were significantly differentially expressed in response to A. fumigatus are shown in Table 1.
FIG. 4.
Expression of cytokine- and cytokine receptor-encoding genes. (A) Mean expression ratio (log2) kinetics of genes encoding cytokines in HMCs in response to A. fumigatus conidia (P ≤ 0.05 for peak change compared to T0). Results for the following genes are shown: IL1β (interleukin-1β), IL10 (interleukin-10), CSF2 (granulocyte-macrophage colony-stimulator factor). (B) Mean expression ratio (log2) kinetics of genes encoding cytokine receptors in HMCs in response to conidia of A. fumigatus (P ≤ 0.05 for peak change compared to T0). Results for the following genes are shown: TRAF1 (tumor necrosis factor receptor-associated factor 1), IRAK2 (interleukin-1 receptor-associated kinase 2), CSF1R (colony-stimulating factor 1 receptor; formerly McDonough feline sarcoma viral [v-fms] oncogene homolog; CD115), and SMAD6 (SMAD; mothers against DPP homolog 6 [Drosophila]).
TABLE 1.
Kinetic profiles of expression of genes encoding molecules with cytokine activity
| Gene expression and product | Gene | Mean log2 expression ratio ata:
|
|||
|---|---|---|---|---|---|
| 0 h | 2 h | 4 h | 6 h | ||
| Up-regulated | |||||
| TNF-α-induced protein 6 | TNFAIP6 | −0.27 | 0.38 | 0.35 | 0.96 |
| TNF-α-induced protein 3 | TNFAIP3 | −0.03 | 0.43 | 0.73 | 0.80 |
| Oncostatin M | OSM | 0.12 | 0.44 | 0.67 | 0.58 |
| Inhibin β A (activin) | INHBA | 0.11 | 0.45 | 1.43 | 0.97 |
| Amphiregulin | AREG | −0.09 | 0.41 | 0.75 | 0.82 |
| Down-regulated | |||||
| TNF (ligand) superfamily member 8 | TNFSF8 | −0.02 | 0.11 | 0.15 | −1.46 |
| TNF (ligand) superfamily member 13 | TNFSF13 | 0.14 | 0.11 | −0.08 | −0.61 |
Quantitative expression of genes.
Cytokine receptor-associated genes also were evaluated. The kinetic profiles of those genes that were differentially expressed in HMCs in response to A. fumigatus conidia are shown in Fig. 4B. Genes encoding TRAF1 (tumor necrosis factor receptor[TNFR]-associated factor 1; TRAF proteins associate with and mediate the signal transduction from various receptors of the TNFR superfamily) and IRAK2 (interleukin-1 receptor-associated kinase 2; IRAK2 is reported to participate in the IL-1-induced up-regulation of NF-κB) were significantly up-regulated by 4 h. Up-regulation of IRAK2 was sustained at 6 h, while expression of TRAF1 returned to baseline levels by 6 h. In contrast, expression of CSF1R (the gene encoding the colony-stimulating factor 1 receptor), and SMAD6 (the gene encoding a protein that acts as a second messenger distal to the transforming growth factor-β [TGF-β] family of receptors) were significantly down-regulated by 4 h with down-regulation persisting at 6 h.
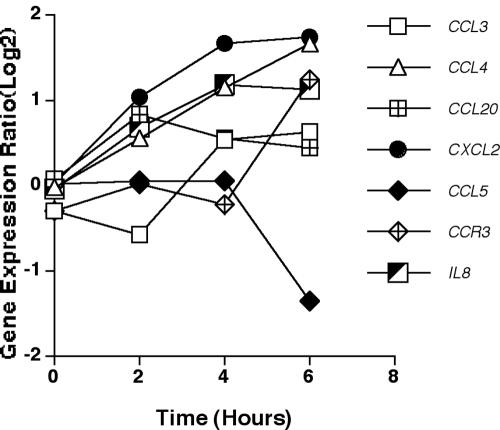
Chemokine and chemokine receptor-encoding genes that were significantly differentially expressed in response to A. fumigatus conidia are shown in Fig. 5. There were several distinct expression profiles observed among these genes. Expression of the genes CXCL8/IL8, CXCL2/Groβ, CCL4/MIP1β, and CCL20/MIP3α was significantly up-regulated by 2 h in response to A. fumigatus. Expression of CCL4/MIP1β and CXCL2/Groβ continued to be up-regulated over the 6-h time course, while expression of CCL20/MIP3α peaked at 2 h and expression of CXCL8/IL8 peaked at 4 h. Expression of the gene encoding CCL3/MIP1α was initially down-regulated at 2 h but significantly up-regulated at 4 to 6 h. CCL5/RANTES was the only chemokine-encoding gene whose expression was significantly down-regulated in response to A. fumigatus, with maximal down-regulation at 6 h (Fig. 5).
FIG. 5.
Expression of chemokine and chemokine receptor genes. Mean expression ratio (log2) kinetics of genes encoding chemokines and chemokine receptors in HMCs in response to conidia of A. fumigatus (P ≤ 0.05 for peak change compared to T0). Results for the following genes are shown: CCL3/MIP1α (chemokine C-C motif ligand 3), CCL4/MIP1β (chemokine C-C motif ligand 4), CCL20/MIP3α (chemokine C-C motif ligand 20), CXCL2/Groβ (chemokine C-X-C motif ligand 2), CCL5/RANTES (regulated upon activation, normally T-cell expressed and secreted), CCR3 (chemokine C-C motif receptor 3), and IL8 (interleukin-8).
The only chemokine receptor-encoding gene found to be significantly differentially expressed in response to A. fumigatus conidia was CCR3. As shown in Fig. 5, there was little change in gene expression over the first 4 h but a significant up-regulation in expression at 6 h.
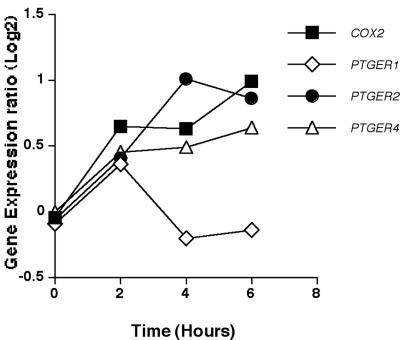
The kinetic expression profiles of genes encoding prostaglandin-related molecules that significantly changed in response to conidia of A. fumigatus are shown in Fig. 6. COX2 (the gene encoding the inducible cyclooxygenase) was up-regulated significantly by 2 h with increased expression over the 6-h time course. The genes encoding prostaglandin E2 receptors 2 and 4 (PTGER2 and PTGER4) also were up-regulated. By comparison, expression of the gene encoding receptor 1 (PTGER1) was up-regulated significantly at 2 h, but returned to baseline by 4 h.
FIG. 6.
Expression of prostaglandin-related genes. Mean expression ratio (log2) kinetics of genes encoding prostaglandin synthesis molecules and receptors in HMCs in response to A. fumigatus conidia (P ≤ 0.05 for peak change compared to T0). Results for the following genes are shown: COX2 (prostaglandin G/H synthase and cyclooxygenase), PTGER1 (prostaglandin E receptor 1), PTGER2 (prostaglandin E receptor 2), and PTGER4 (prostaglandin E receptor 4).
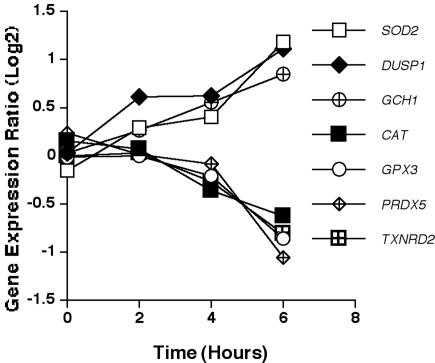
Figure 7 illustrates the expression kinetic profiles of genes involved in the oxidative response of HMCs to A. fumigatus. There was concomitant up-regulation of the gene encoding superoxide dismutase (SOD2) and down-regulation of the gene encoding catalase (CAT), both maximally changed at 6 h. Additionally, other genes encoding oxidative stress and response molecules, including GPX3, PRDX5, and TXNRD2, were down-regulated, with maximal down-regulation at 6 h. The gene encoding dual phosphatase 1 (DUSP1), also involved in the response to oxidative stress, was found to be maximally up-regulated at 6 h in response to A. fumigatus. Other genes involved in the oxidative burst, including those encoding NADPH oxidase and myeloperoxidase, were not significantly differentially expressed in response to conidia of A. fumigatus.
FIG. 7.
Expression of oxidative response genes. Mean expression ratio (log2) kinetics of genes encoding oxidative stress response molecules in HMCs in response to conidia of A. fumigatus (P ≤ 0.05 for peak change compared to T0). Results for the following genes are shown: SOD2 (superoxide dismutase 2), DUSP1 (dual-specificity phosphatase 1), GCH1 (GTP cyclohydrolase 1), CAT (catalase), GPX3 (glutathione peroxidase 3), PRDX5 (peroxiredoxin 5), and TXNRD2 (thioredoxin reductase 2).
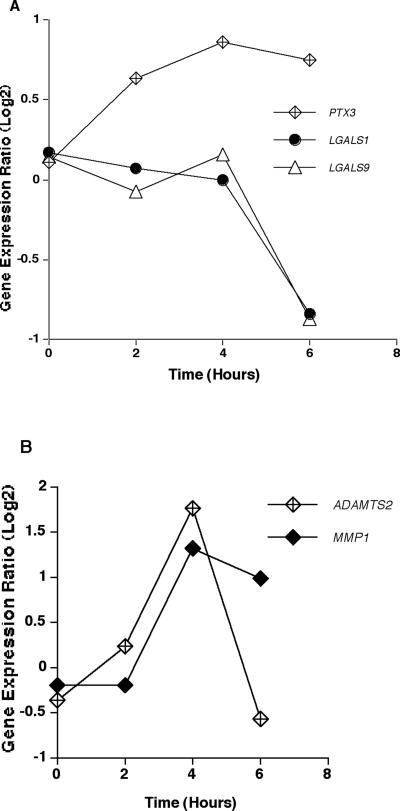
Among genes encoding for pattern recognition receptors, only PTX3 (long pentraxin 3) had a sustained up-regulation in response to A. fumigatus throughout the 6-h time course, peaking at 4 h (Fig. 8A). Conversely, genes encoding other lectins, such as galectin 1 (LGALS1) and galectin 9 (LGALS9), were down-regulated. There were several genes encoding non-lectin cell adhesion molecules that also were significantly differentially expressed in HMCs in response to A. fumigatus (Table 2).
FIG. 8.
Expression of genes encoding pattern recognition receptors and matrix metalloproteinases. (A) Mean expression ratio (log2) kinetics of genes encoding pattern recognition receptors in HMCs infected with conidia of A. fumigatus (P ≤ 0.05 for peak change compared to T0). Results for the following genes are shown: PTX3 (long pentraxin 3), LGALS1 (galectin 1), and LGALS9 (galectin 9). (B) Mean expression ratio (log2) kinetics of genes encoding matrix metalloproteinases in HMCs infected with conidia of A. fumigatus (P ≤ 0.05 for peak change compared to T0). Results for the following genes are shown: ADAMTS2 (a desintegrin-like metalloproteinase with thrombospondin type 1 motif 2) and MMP1 (matrix metalloproteinase 1; also known as interstitial collagenase).
TABLE 2.
Kinetic profiles of expression of genes encoding molecules with cell adhesion capability
| Gene expression and product | Gene | Mean log2 expression ratio ata:
|
|||
|---|---|---|---|---|---|
| 0 h | 2 h | 4 h | 6 h | ||
| Up-regulated | |||||
| CD47 antigen immunoglobulin | CD47 | −0.12 | 2.25 | 0.15 | 0.32 |
| SF, member 4 | IGSF4 | −0.34 | −0.48 | 0.96 | −0.01 |
| Protocadherin 11 X-linked | PCDH11X | −0.3 | −1.43 | 2.01 | −0.83 |
| Cell adhesion molecule 1 | CEACAM1 | −0.23 | −0.72 | 0.23 | 1.5 |
| Catenin (cadherin-associated protein) | CTNNB1 | −0.06 | 0.24 | 0.10 | 0.66 |
| Metallopeptidase M8 family | LMLN | −0.03 | −1 | −0.82 | 1.22 |
| Protocadherin 1 (cadherin-like 1) | PCDH1 | −0.03 | −0.48 | −0.12 | 1.43 |
| Immunoglobulin SF member 1 | IGSF1 | −0.35 | 0.24 | 1.29 | 0.94 |
| Lymphocyte antigen 9 | LY9 | 0.21 | 0.4 | 0.84 | 1.38 |
| Thrombospondin 1 | THBS1 | −0.07 | 0.36 | 1.81 | 0.89 |
| Down-regulated | |||||
| G-protein-coupled receptor 4 | EDG4 | −0.13 | −0.93 | −0.12 | −0.32 |
| Hyaluronan and proteoglycan link protein 1 | CRTL1 | −0.1 | −1.08 | 0.03 | −0.40 |
| Platelet/endothelial cell adhesion molecule (CD31 antigen) | PECAM1 | 0.13 | −0.14 | −0.76 | −0.24 |
| Sialic acid binding Ig-like lectin 7 | SIGLEC7 | −0.03 | −0.55 | −1.32 | −0.87 |
| Rho GDP dissociation inhibitor (GDI) β | ARHGDIB | 0.15 | 0.07 | −0.83 | −0.21 |
| CD151 antigen | CD151 | 0.18 | −0.11 | 0.14 | −1.01 |
| Flotillin 2 | FLOT2 | 0.2 | 0.23 | −0.24 | −0.59 |
| Paired Ig-like type 2 receptor α | PILRA | 0.11 | 0.01 | −0.10 | −0.59 |
| Selectin P ligand | SELPLG | 0.12 | −0.09 | −0.21 | −0.76 |
Quantitative expression of genes.
Genes encoding other immune receptors, including Toll-like receptors (TLRs) and scavenger receptors, also were examined (Table 3). Among genes encoding the TLRs, only TLR1 was significantly changed, with down-regulation being maximal at 6 h. Among the scavenger receptors, only MARCO (macrophage receptor with collagenous structure) was significantly differentially expressed in response to A. fumigatus, with maximal down-regulation at 6 h.
TABLE 3.
Kinetic profiles of expression of genes encoding leukocyte antigens and receptors
| Gene producta | Gene | Mean log2 expression ratio atb:
|
|||
|---|---|---|---|---|---|
| 0 h | 2 h | 4 h | 6 h | ||
| Toll-like receptor 1 | TLR1 | 0.10 | −0.29 | −0.84 | −0.26 |
| Macrophage receptor with collagenous structure | MARCO | 0.37 | −0.20 | −0.30 | −1.38 |
| CD56 (Campath-1 Ag) | CDW52 | 0.18 | 0.03 | −0.59 | 0.08 |
| CD14 | CD14 | 0.19 | 0.12 | 0.10 | −0.65 |
| Leukocyte immunoglobulin receptor subfamily B (with TM and ITM domains), member 1 | LILRB1 | 0.15 | 0.08 | −0.16 | −0.76 |
| Lymphocyte-specific protein 1 | LSP1 | 0.15 | 0.02 | 0.17 | −0.85 |
All are down-regulated.
Quantitative expression of genes.
Of genes encoding metalloproteinases, MMP1 (matrix metalloproteinase 1, interstitial collagenase) and ADAMTS2 (a desintegrin-like metalloproteinase with thrombospondin type 1 motif 2) were significantly differentially expressed in response to conidia of A. fumigatus. As shown in Fig. 8B, MMP1 and ADAMTS2 were up-regulated, with peak up-regulation occurring at 4 h. By 6 h, ADAMTS2 expression returned to baseline, whereas, MMP1 expression remained significantly elevated over baseline.
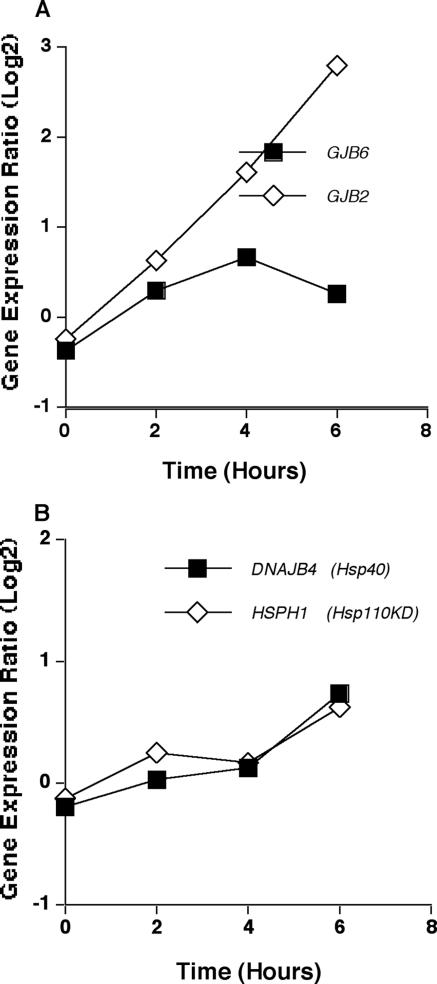
As cell-cell communication is a critical function of cells involved in the immune response, expression of genes encoding connexins in HMCs in response to conidia of A. fumigatus was explored. As shown in Fig. 9A, genes encoding connexins 26 (GJB2) and 30 (GJB6) were significantly differentially expressed in response to A. fumigatus. GJB2 expression increased continuously over the 6-h time course, while expression of GJB6 peaked at 4 h with expression declining thereafter. As shown in Fig. 9B, genes encoding heat shock proteins Hsp40 and Hsp110 in HMCs were significantly differentially expressed in response to A. fumigatus, with maximal up-regulation occurring at 6 h.
FIG. 9.
Expression of genes encoding connexins and heat shock proteins. (A) Mean expression ratio (log2) kinetics of genes encoding connexins in HMCs infected with conidia of A. fumigatus (P ≤ 0.05 for peak change compared to T0). Results for the following genes are shown: GJB6 (gap junction protein B6, connexin 30) and GJB2 (gap junction protein B2, connexin 26). (B) Mean expression ratio (log2) kinetics of genes encoding heat shock proteins in HMCs infected with conidia of A. fumigatus (P ≤ 0.05 for peak change compared to T0). Results for the following genes are shown: DNAJB4 (heat shock protein 40 kDa). HSPH1 (heat shock protein 110 kDa).
Verification of microarray data in selected up-regulated genes was performed by real-time reverse transcription-PCR (Table 4). Increases in mRNA levels by RT-PCR were consistent with increased expression found using microarray analysis.
TABLE 4.
Reverse transcriptase analysis of selected genes at peak 6 h
| Gene product | Gene | Log2 fold changea |
|---|---|---|
| Gap junction protein, beta-2 (connexin 26) | GJB2 | 3.7 ± 0.74 |
| Prostaglandin-endoperoxide synthase 2 | PTGS2 | 2.07 ± 0.73 |
| Superoxide dismutase 2 | SOD2 | 2.61 ± 0.63 |
Data are expressed as mean ± standard error of the mean log2 change of gene expression for three separate donors displayed.
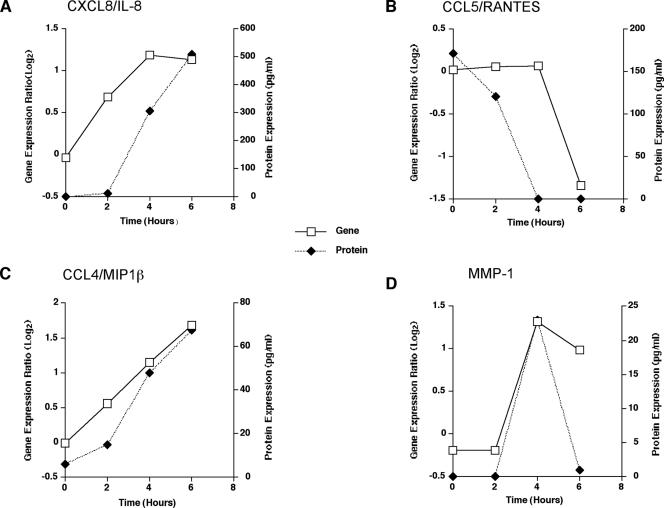
End product protein levels were determined for selected representative up- and down-regulated genes by EIAs. Kinetics of the mean protein levels (pg/ml) of CXCL8/IL-8, CCL4/MIP1β, CCL5/RANTES, and MMP-1 and their respective gene expression ratios (log2) over time are shown in Fig. 10. The release of these protein products paralleled the expression of their respective genes.
FIG. 10.
Genomic expression and protein secretion of selected genes in HMCs in response to A. fumigatus conidia. (A) Mean expression ratio (log2) kinetics of genes encoding CXCL8/IL-8 and simultaneous determination of IL-8 (end product) in supernatants of HMCs infected with conidia of A. fumigatus. (B) Mean expression ratio (log2) kinetics of genes encoding CCL5/RANTES and simultaneous determination of RANTES protein in supernatants of HMCs infected with conidia of A. fumigatus. (C) Mean expression ratio (log2) kinetics of genes encoding CCL4/MIP1β and simultaneous determination of CCL4/MIP1β (end product) in supernatants of HMCs infected with conidia of A. fumigatus. (D) Mean expression ratio (log2) kinetics of genes encoding matrix metalloproteinase-1 (MMP-1) and simultaneous determination of MMP-1 (end product) in supernatants of HMCs infected with conidia of A. fumigatus.
DISCUSSION
To our knowledge, this is the first study to investigate the kinetic profile of the coordinated gene expression of innate host defense molecules of normal human monocytes in response to A. fumigatus. The functional genomic profile elicited in HMCs upon exposure to conidia of A. fumigatus demonstrated several important processes related to the pathogenesis of human aspergillosis. First, A. fumigatus conidia induced in HMCs the differential expression of genes encoding products known to be involved in the innate immune response, including cytokines, chemokines, cell adhesion molecules, and immune receptors. Additionally, several genes differentially expressed in HMCs in response to conidia of A. fumigatus highlighted potential mechanisms through which this organism initially may circumvent the host immune system. Genes regulating the inflammatory response also were differentially expressed. Moreover, expression of genes not previously appreciated to be involved in monocyte-mediated host response to A. fumigatus, such as those coding for heat shock proteins (Hsp40 and Hsp110) and connexins (connexins 26 and 30), also were significantly affected. The data presented herein support the original hypothesis that the majority of genes that were enriched (up- or down-regulated in expression) were clustered in biological categories that are recognized to have or potentially have a role in innate immunity (see Table S2 in the supplemental material).
Aspergillus fumigatus induced significant changes in the expression of several genes in HMCs involved in phagocytosis. In this regard, there was significant up-regulation of the gene encoding CDC42EP3, a Rho GTPase involved in the regulation of the formation of F-actin-containing structures through its interaction with downstream effector proteins that are known to induce pseudopodia formation (18). Similarly, the three genes encoding endocytosis-related molecules that were differentially expressed in HMCs in response to A. fumigatus (EHD1, SH3BP4, and RAB20), were up-regulated by 4 h, suggesting an early attempt by the cells to ingest the invading organisms.
Concomitantly, there was down-regulation of other genes possibly involved in phagocytosis: CD14, CORO1A, and FCN1. Down-regulation of the gene encoding CD14, a surface protein expressed on monocytes and macrophages, has been reported previously in human monocytes in response to the obligatory intracellular parasite Ehrlichia chaffeensis (25). E. chaffeensis down-regulated mRNA expression of TLR2, TLR4, and CD14, genes coding for key pattern recognition receptors. These authors suggest that down-regulation of these receptors allows for survival of E. chaffeensis in the host cells. Down-regulation of CD14 in response to A. fumigatus also may represent a survival mechanism triggered by the organism. Down-regulation of CORO1A, the gene encoding the actin-binding protein coronin, also may represent a potential escape mechanism for Aspergillus. Coronin is a phagosome-coating protein (13) that has previously been shown to be required for efficient phagocytosis in Dictyostelium (30). There was 70% reduction in yeast uptake demonstrated with deletion of this protein.
Ficolins are a family of proteins serving as receptors, opsonins, and complement activators (19). The protein encoded by FCN1 is the precursor of M-ficolin, a phagocytic receptor expressed on the surface of HMCs. Down-regulation of FCN1 in HMCs in the initial 6 h of interaction with A. fumigatus also may represent an escape mechanism induced by the fungus, similar to the down-regulation of CD14 and CORO1A.
There were a limited number of genes encoding cytokines or cytokine-related molecules that were significantly differentially expressed within the first 6 h in response to conidia of A. fumigatus. The gene encoding interleukin-1β (IL1β) was significantly up-regulated. IL-1β is an important proinflammatory cytokine involved in a variety of cellular activities, including phagocyte activation, cell proliferation, differentiation, and apoptosis (47). With similar kinetics, the gene encoding IL-10, a cytokine known to be important in the control of the inflammatory response, also was up-regulated in HMCs early in response to conidia of A. fumigatus. Our laboratory has previously shown that although IL-10 suppresses HMC-mediated hyphal damage of A. fumigatus, it enhances phagocytosis of conidia but with a net negative effect on host response to A. fumigatus (39). In a recent murine model, Netea et al. have proposed that IL-10 production may be a host defense evasion mechanism utilized by Candida albicans (35). Del Sero et al. have shown that IL-10 depletion leads to increased protection against invasive aspergillosis and candidiasis in C57b mice and that normalization of IL-10 levels suppresses host response to these infections (8). Consistent with this suppression of host response, increased levels of circulating IL-10 in neutropenic patients with invasive aspergillosis also are associated with progressive infection (41). Brouard et al. found that increased levels of IL-10 were associated with increased risk of aspergillosis in patients with cystic fibrosis (4). Furthermore, IL-10 is known to inhibit the synthesis of TNF-α, IL-6, IL-8, IL-12, and granulocyte-macrophage colony-stimulating factor induced by gamma interferon (IFN-γ) or lipopolysaccharide and down-regulate production of reactive oxygen and nitrogen intermediates (11). Consistent with these observations, we observed that expression of the TNFα, IL6, and IL12 genes remained unchanged and that CSF2 was down-regulated during the initial 6 h of interaction between HMCs and conidia of A. fumigatus.
Expression of genes encoding cytokine-receptor-associated proteins in HMCs in response to A. fumigatus also was evaluated. TRAF1, the gene encoding a member of the TRAF protein family, was up-regulated by 4 h. TRAF proteins associate with and mediate the signal transduction from various receptors of the TNFR superfamily (9). This protein, along with TRAF2, is required for TNF-α-mediated activation of MAPK8/JNK and NF-κB. IRAK2, whose gene expression also was up-regulated early in response to A. fumigatus, has been shown to be a proximal mediator of IL-1, a component of the IL-1 receptor signaling complex, and like TRAF1 is involved in activation of NF-κB (34).
The protein encoded by CSF1R is the receptor for colony-stimulating factor 1, a cytokine involved in the production, differentiation, and activation of macrophages. SMAD6 encodes a protein that acts as second messenger distal to the TGF-β family of receptors. These two genes were significantly down-regulated during the 6-h time course, suggesting a balance between activation and suppression of the inflammatory response, possibly to control damage inflicted to the host by the inflammatory response.
Genes encoding several chemokines associated with the recruitment and activation of phagocytes were significantly differentially expressed in HMCs in response to conidia of A. fumigatus. Our data showed significantly increased expression of CCL3/MIP1α, CCL4/MIP1β, and CCL20/MIP3α during the 6-h time course. These chemokines have previously been shown to be important in the early immune response to the yeast phase of C. albicans (23). The CXC chemokines, CXCL2/Groβ and CXCL8/IL8, which also were up-regulated, play a role in attracting neutrophils and monocytes to the site of an acute inflammatory response.
CXCL8/IL-8 appears to be an important immunoregulatory cytokine in response to A. fumigatus. Richardson et al. found that CXCL8/IL-8 enhanced the phagocytosis of conidia by polymorphonuclear neutrophils (38). Winn et al. also demonstrated that IL-15-stimulated polymorphonuclear leukocytes exhibit enhanced release of CXCL8/IL-8 in response to the hyphae of A. fumigatus (49). This current study further contributes to our understanding of IL-8 in the phagocytic response of HMCs to conidia of A. fumigatus. Expression of IL8 was significantly up-regulated in parallel with release of its protein during HMC phagocytosis of Aspergillus conidia. While this interaction may augment the initial inflammatory response to A. fumigatus by recruitment of polymorphonuclear leukocytes, there also may be a deleterious component to this response. Studies of tumor cell migration reveal that IL-8 increases transcriptional expression of matrix metalloproteinases and promotes tumor invasion (22, 29). Similarly, matrix metalloproteinases appear to be up-regulated in HMCs, which may contribute to enhanced invasion by A. fumigatus. This relationship is further discussed below in regulation of genes encoding matrix metalloproteinases.
In contrast, the gene encoding CCL5/RANTES was down-regulated during this early phase of infection with A. fumigatus conidia. CCL5/RANTES is a potent chemotaxin and is considered to be a type 1 T-cell chemokine (12). In a recent study, Marques et al. reported that IL-10 inhibited the mRNA expression of CCL5/RANTES in microglia infected with herpes simplex virus type 1 (31). Similarly, the increase in IL-10 induced by A. fumigatus in our studies may be responsible for the decreased expression of CCL5. The significance of down-regulation of CCL5/RANTES in response to A. fumigatus warrants further investigation as another potential mechanism of organism-mediated suppression of innate host responses.
CCR3 is a known receptor used by CCL5/RANTES (12). Of the genes encoding chemokine receptors, only CCR3 was up-regulated in HMCs in response to A. fumigatus conidia. The up-regulation of this receptor may be a paracrine response to the down-regulation of CCL5/RANTES, as they both occur at the 6-h time point. Furthermore, this up-regulation of CCR3 may allow for selective amplification of a Th2-type response to A. fumigatus.
Toll-like receptors are important pattern-recognition receptors involved in sensing pathogenic microorganisms (45). Recognition of A. fumigatus conidia by HMCs involves both TLR2 and TLR4 (36). During the early 6-h time course, we found significant down-regulation in TLR1, with no significant change in expression of the genes encoding TLR2 and TLR4. Changes in expression of TLR2 and TLR4 most likely occur following longer exposure to the organism, or these results may differ due to variation in the experimental design. Further research is warranted to clarify the significance of TLR1 in response to A. fumigatus.
The gene encoding the macrophage scavenger receptor MARCO also was significantly down-regulated in HMCs in response to A. fumigatus. MARCO is a scavenger receptor that has an important role in clearance of inert particles and in mounting an efficient and appropriately regulated innate immune response against Streptococcus pneumoniae (1). That MARCO is down-regulated in the presence of conidia of A. fumigatus may be yet another potential mechanism utilized by A. fumigatus to evade innate immunity or of the host to modulate the initial inflammatory response.
HMCs also may recognize conidia of A. fumigatus through the use of soluble receptors such as collectins. This group of receptors is involved in cell adhesion, opsonization, complement activation, and apoptosis (14). The gene encoding the long pentraxin 3 (PTX3) was up-regulated at all time points. This early up-regulation of PTX3 is consistent with its role in innate pulmonary host defense specific to A. fumigatus (15). PTX3 opsonizes A. fumigatus conidia and contributes significantly to improved survival in experimental pulmonary aspergillosis (16). Down-regulation of expression of genes encoding other lectins, galectin-1 and galectin-9, may represent the cell's attempt at prolonging its own survival, since both galectins are known to induce apoptosis (26).
Non-lectin cell adhesion molecules also may be important in early host response to fungi. Expression of CD47, a gene encoding a membrane protein involved in the increase of intracellular calcium concentrations that occur upon cell adhesion to extracellular matrices, was up-regulated in response to A. fumigatus. The early influx of Ca2+ through this membrane protein may lead to early cell activation.
The cell adhesion molecule LMLN has been shown to suppress cell-mediated immune responses through its ability to down-regulate IL-12 production, and thus inhibit classical activation of macrophages (33). In our experiments, MSP/LMLN was significantly up-regulated, a response that may constitute another potential mechanism utilized by A. fumigatus to evade host defense or to dampen the initial inflammatory response.
Aspergillus fumigatus also may deleteriously alter host interaction through up-regulation of host matrix metalloproteinases, which may facilitate subsequent fungal invasion. Among the genes encoding matrix metalloproteinases, ADAMTS2 and MMP1 were significantly differentially expressed in response to A. fumigatus. ADAMTS2 encodes a desintegrin-like metalloproteinase with thrombospondin type 1 motif 2, while MMP1 encodes matrix metalloproteinase 1, also known as collagenase 1. This increased expression of metalloproteinases may provide a mechanism by which A. fumigatus induces lysis of the extracellular matrix and in this way facilitates local invasion in connective tissue, parenchyma, and blood vessels. These observations are consistent with those observed in the pathogenesis of Coccidioides posadasii (48). Coccidioides induces expression of matrix metalloproteinase 9 (MMP-9) in rabbits with experimental coccidioidal meningitis. Production of MMP-9 is considered related to the inflammatory vasculitis associated with this infection. Additionally, in an in vitro model of oral epithelial cells and basement membrane, C. albicans induces the production of MMP-9 and matrix metalloproteinase 2 (7). There also is a striking analogy to tumor cell invasion, wherein malignant cells induce expression of MMP1 from fibroblasts (2). This increased expression of MMP1 promotes invasion by neoplastic epithelial cells into surrounding stroma.
Production of reactive oxygen species, such as superoxide anion, hydrogen peroxide (H2O2), and hydroxyl radicals, is an important component of the oxidative host response in phagocytes. While killing invasive microorganisms is the primary function of these reactive oxygen species, their production and release are coordinately controlled to prevent cell and tissue damage. In this regard, while genes encoding superoxide dismutase 2 (SOD2) and dual phosphatase 1 (DUSP1) were significantly up-regulated, a concomitant and similar down-regulation occurred in the group of genes encoding catalase (CAT), glutathione peroxidase 3 (GPX3), and peroxiredoxin 5 (PRDX5). The up-regulation of SOD2 and the down regulation of CAT may result in the persistence of H2O2 in the extracellular environment. This persistence may enhance fungicidal activity as well as serve as a second messenger (17). Thus, there appears to be a well-polarized and coordinated gene expression in oxidative response genes induced by the interaction of HMCs with A. fumigatus conidia in order to eliminate the organisms while simultaneously preserving the cell from excessive inflammatory damage.
HMCs may coordinate their response to A. fumigatus through intercellular communication via gap junctions. Osteoclasts, which are derived from monocytes, coordinate their function through hemi-channel gap junctions (20). Vertebrate gap junctions are made up of a multigene family called connexins. Gap junctions are assumed to modulate various biological processes through mediating the massive movement of ions and second messengers, allowing for a form of “quorum sensing.” However, little is known about their role in innate host defenses. We found that connexin-26 and connexin-30 were significantly differentially expressed in our study. Kim et al. also observed this pattern of up-regulation of connexins where genes encoding connexin-26 and connexin-31 were up-regulated in monocytes in response to C. albicans (23).
Eicosanoids are synthesized from arachidonic acid by enzymes called cyclooxygenases (COX), which exist in two isoforms, a constitutive enzyme, type 1 (COX-1), and type 2 (COX-2), which is induced by inflammatory mediators, mainly cytokines. Of the genes encoding prostaglandin-related molecules, the gene encoding prostaglandin endoperoxidase H synthase (PTGS2/COX2), the inducible isoform involved in the regulation of inflammation, was significantly up-regulated. Candida albicans induces PTGS2/COX2 through TLR2 and TLR4 followed by phosphokinase C, and/or NF-κB (10). While the role of COX2 expression in response to A. fumigatus is unknown, it may serve as a potent immunoregulator in the tracheobronchial tree.
These data demonstrate the utility of the microarray as a powerful tool for assessing kinetic changes in expression of innate host response genes in monocytes induced by A. fumigatus. A potential limitation in microarray analysis, however, is that expression of mRNA may not coincide with extracellular protein release either quantitatively or qualitatively. In our studies, gene expression patterns for three selected genes did in fact mirror protein release. Another potential limitation of this study is the use of HMCs as opposed to pulmonary alveolar macrophages in determining the initial host response to conidia of A. fumigatus. However, given the lack of availability of human pulmonary alveolar macrophages, circulating HMCs that are precursors to tissue macrophages offer insight into the genetic profile of the initial response to Aspergillus.
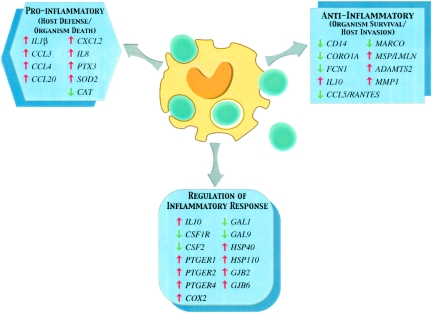
These microarray data reveal a reciprocal regulatory control and coordinated kinetic expression pattern of multiple genes involved in the early host response to A. fumigatus conidia. As illustrated in Fig. 11, genes whose products are involved in organism death and injury are simultaneously regulated along with genes whose products may facilitate host invasion by dampening the inflammatory response. Furthermore, these microarray data reveal the coordinated temporal response of genes heretofore not known to be involved in the immunopathogenesis of human aspergillosis. Thus, this early kinetic profile of gene expression in response to A. fumigatus offers a greater understanding of the early host-pathogen interaction of this medically important mycosis.
FIG. 11.
Model of early gene expression of innate host defense molecules in normal human monocytes in response to conidia of A. fumigatus. The functional genomic response of normal human monocytes against A. fumigatus may fit a dichotomous model that is modulated by genes encoding immunoregulatory proteins. The dichotomous response involves genes coding for proteins mediating host defense and organism death or organism survival and host invasion. The latter may be induced by the organism or may be the result of immunomodulation causing down-regulation of host response. The third arm depicted in this model represents the complex and multifaceted immunoregulatory component of monocytes that may affect both arms of the reciprocal response elicited by the organism. The outcome of survival or death of A. fumigatus may be determined by specific host derangements that alter this finely regulated response. Results for the following genes are shown: IL1β (interleukin-1β); CCL3, CCL4, and CCL20 (C-C chemokine ligands 3, 4, and 20); CXCL2 (C-X-C chemokine ligand 2); IL8 (interleukin-8); PTX3 (pentraxin 3); SOD2 (dismutase 2); CAT (catalase); CD14 (cluster designation 14); CORO1A (coronin 1A); FCN1 (ficolin 1 precursor); IL10 (interleukin-10); CCL5/RANTES (C-C chemokine ligand 5); MARCO (macrophage receptor with collagenous structure); LMLN (leishmanolysin-like [metallopeptidase M8 family]); ADAMTS2 (desintegrin-like and metalloprotease [reprolysin type] with thrombospondin type 1 motif 2); MMP1 (matrix metalloproteinase 1); CSF1R (colony-stimulating factor receptor 1); PTGER1, PTGER2, and PTGER4 (prostaglandin E receptors 1, 2, and 4), COX2 (inducible cyclooxygenase 2); GAL1 and GAL9 (galectins 1 and 9); HSP40 and HSP110 (heat shock proteins 40 and 110 kDa); and GJB2 and GJB6 (connexins 26 and 30).
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Programs of the NCI and NIAID of the National Institutes of Health.
Editor: A. Casadevall
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Arredouani, M., Z. Yang, Y. Ning, G. Qin, R. Soininen, K. Tryggvason, and L. Kobzik. 2004. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J. Exp. Med. 200:267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arribas, J. 2005. Matrix metalloproteases and tumor invasion. N. Engl. J. Med. 352:2020-2021. [DOI] [PubMed] [Google Scholar]
- 3.Brocke-Heidrich, K., A. K. Kretzschmar, G. Pfeifer, C. Henze, D. Loffler, D. Koczan, H. J. Thiesen, R. Burger, M. Gramatzki, and F. Horn. 2004. Interleukin-6-dependent gene expression profiles in multiple myeloma INA-6 cells reveal a Bcl-2 family-independent survival pathway closely associated with Stat3 activation. Blood 103:242-251. [DOI] [PubMed] [Google Scholar]
- 4.Brouard, J., N. Knauer, P. Y. Boelle, H. Corvol, A. Henrion-Caude, C. Flamant, F. Bremont, B. Delaisi, J. F. Duhamel, C. Marguet, M. Roussey, M. C. Miesch, K. Chadelat, M. Boule, B. Fauroux, F. Ratjen, H. Grasemann, and A. Clement. 2005. Influence of interleukin-10 on Aspergillus fumigatus infection in patients with cystic fibrosis. J. Infect. Dis. 191:1988-1991. [DOI] [PubMed] [Google Scholar]
- 5.Choe, S. E., M. Boutros, A. M. Michelson, G. M. Church, and M. S. Halfon. 2005. Preferred analysis methods for Affymetrix GeneChips revealed by a wholly defined control dataset. Genome Biol. 6:R16. [Online.] http://genomebiology.com/2005/6/2/R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chudin, E., R. Walker, A. Kosaka, S. X. Wu, D. Rabert, T. K. Chang, and D. E. Kreder. 2002. Assessment of the relationship between signal intensities and transcript concentration for Affymetrix GeneChip arrays. Genome Biol. 3:RESEARCH0005. [Online.] http://genomebiology.com/2001/3/1/RESEARCH/0005. [DOI] [PMC free article] [PubMed]
- 7.Claveau, I., Y. Mostefaoui, and M. Rouabhia. 2004. Basement membrane protein and matrix metalloproteinase deregulation in engineered human oral mucosa following infection with Candida albicans. Matrix Biol. 23:477-486. [DOI] [PubMed] [Google Scholar]
- 8.Del Sero, G., A. Mencacci, E. Cenci, C. F. d'Ostiani, C. Montagnoli, A. Bacci, P. Mosci, M. Kopf, and L. Romani. 1999. Antifungal type 1 responses are upregulated in IL-10-deficient mice. Microbes Infect. 1:1169-1180. [DOI] [PubMed] [Google Scholar]
- 9.Dempsey, P. W., S. E. Doyle, J. Q. He, and G. Cheng. 2003. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 14:193-209. [DOI] [PubMed] [Google Scholar]
- 10.Deva, R., P. Shankaranarayanan, R. Ciccoli, and S. Nigam. 2003. Candida albicans induces selectively transcriptional activation of cyclooxygenase-2 in HeLa cells: pivotal roles of Toll-like receptors, p38 mitogen-activated protein kinase, and NF-kappa B. J. Immunol. 171:3047-3055. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly, R. P., H. Dickensheets, and D. S. Finbloom. 1999. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J. Interferon Cytokine Res. 19:563-573. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez, E. J., and E. Lolis. 2002. Structure, function, and inhibition of chemokines. Annu. Rev. Pharmacol. Toxicol. 42:469-499. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari, G., H. Langen, M. Naito, and J. Pieters. 1999. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell 97:435-447. [DOI] [PubMed] [Google Scholar]
- 14.Garlanda, C., B. Bottazzi, A. Bastone, and A. Mantovani. 2005. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu. Rev. Immunol. 23:337-366. [DOI] [PubMed] [Google Scholar]
- 15.Garlanda, C., E. Hirsch, S. Bozza, A. Salustri, M. De Acetis, R. Nota, A. Maccagno, F. Riva, B. Bottazzi, G. Peri, A. Doni, L. Vago, M. Botto, R. De Santis, P. Carminati, G. Siracusa, F. Altruda, A. Vecchi, L. Romani, and A. Mantovani. 2002. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 420:182-186. [DOI] [PubMed] [Google Scholar]
- 16.Gaziano, R., S. Bozza, S. Bellocchio, K. Perruccio, C. Montagnoli, L. Pitzurra, G. Salvatori, R. De Santis, P. Carminati, A. Mantovani, and L. Romani. 2004. Anti-Aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals. Antimicrob. Agents Chemother. 48:4414-4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giron-Calle, J., and H. J. Forman. 2000. Phospholipase D and priming of the respiratory burst by H2O2 in NR8383 alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 23:748-754. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch, D. S., D. M. Pirone, and P. D. Burbelo. 2001. A new family of Cdc42 effector proteins, CEPs, function in fibroblast and epithelial cell shape changes. J. Biol. Chem. 276:875-883. [DOI] [PubMed] [Google Scholar]
- 19.Holmskov, U., S. Thiel, and J. C. Jensenius. 2003. Collectins and ficolins: humoral lectins of the innate immune defense. Annu. Rev. Immunol. 21:547-578. [DOI] [PubMed] [Google Scholar]
- 20.Ilvesaro, J., and J. Tuukkanen. 2003. Gap-junctional regulation of osteoclast function. Crit. Rev. Eukaryot. Gene Expr. 13:133-146. [DOI] [PubMed] [Google Scholar]
- 21.Irizarry, R. A., B. Hobbs, F. Collin, Y. D. Beazer-Barclay, K. J. Antonellis, U. Scherf, and T. P. Speed. 2003. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249-264. [DOI] [PubMed] [Google Scholar]
- 22.Janowska-Wieczorek, A., L. A. Marquez, J. M. Nabholtz, M. L. Cabuhat, J. Montano, H. Chang, J. Rozmus, J. A. Russell, D. R. Edwards, and A. R. Turner. 1999. Growth factors and cytokines upregulate gelatinase expression in bone marrow CD34+ cells and their transmigration through reconstituted basement membrane. Blood 93:3379-3390. [PubMed] [Google Scholar]
- 23.Kim, H. S., E. H. Choi, J. Khan, E. Roilides, A. Francesconi, M. Kasai, T. Sein, R. L. Schaufele, K. Sakurai, C. G. Son, B. T. Greer, S. Chanock, C. A. Lyman, and T. J. Walsh. 2005. Expression of genes encoding innate host defense molecules in normal human monocytes in response to Candida albicans. Infect. Immun. 73:3714-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kogel, D., R. Schomburg, E. Copanaki, and J. H. Prehn. 2005. Regulation of gene expression by the amyloid precursor protein: inhibition of the JNK/c-Jun pathway. Cell Death Differ. 12:1-9. [DOI] [PubMed] [Google Scholar]
- 25.Lin, M., and Y. Rikihisa. 2004. Ehrlichia chaffeensis downregulates surface Toll-like receptors 2/4, CD14 and transcription factors PU.1 and inhibits lipopolysaccharide activation of NF-kappa B, ERK 1/2 and p38 MAPK in host monocytes. Cell Microbiol. 6:175-186. [DOI] [PubMed] [Google Scholar]
- 26.Liu, F. T. 2000. Galectins: a new family of regulators of inflammation. Clin. Immunol. 97:79-88. [DOI] [PubMed] [Google Scholar]
- 27.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−Delta Delta C(T) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 28.Lu, T., Y. Pan, S. Y. Kao, C. Li, I. Kohane, J. Chan, and B. A. Yankner. 2004. Gene regulation and DNA damage in the ageing human brain. Nature 429:883-891. [DOI] [PubMed] [Google Scholar]
- 29.Luca, M., S. Huang, J. E. Gershenwald, R. K. Singh, R. Reich, and M. Bar-Eli. 1997. Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am. J. Pathol. 151:1105-1113. [PMC free article] [PubMed] [Google Scholar]
- 30.Maniak, M., R. Rauchenberger, R. Albrecht, J. Murphy, and G. Gerisch. 1995. Coronin involved in phagocytosis: dynamics of particle-induced relocalization visualized by a green fluorescent protein Tag. Cell 83:915-924. [DOI] [PubMed] [Google Scholar]
- 31.Marques, C. P., S. Hu, W. Sheng, M. C. Cheeran, D. Cox, and J. R. Lokensgard. 2004. Interleukin-10 attenuates production of HSV-induced inflammatory mediators by human microglia. Glia 47:358-366. [DOI] [PubMed] [Google Scholar]
- 32.Marr, K. A., R. A. Carter, F. Crippa, A. Wald, and L. Corey. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34:909-917. [DOI] [PubMed] [Google Scholar]
- 33.Morrison, A. C., C. B. Wilson, M. Ray, and P. H. Correll. 2004. Macrophage-stimulating protein, the ligand for the stem cell-derived tyrosine kinase/RON receptor tyrosine kinase, inhibits IL-12 production by primary peritoneal macrophages stimulated with IFN-gamma and lipopolysaccharide. J. Immunol. 172:1825-1832. [DOI] [PubMed] [Google Scholar]
- 34.Muzio, M., J. Ni, P. Feng, and V. M. Dixit. 1997. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 278:1612-1615. [DOI] [PubMed] [Google Scholar]
- 35.Netea, M. G., R. Sutmuller, C. Hermann, C. A. Van der Graaf, J. W. Van der Meer, J. H. van Krieken, T. Hartung, G. Adema, and B. J. Kullberg. 2004. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J. Immunol. 172:3712-3718. [DOI] [PubMed] [Google Scholar]
- 36.Netea, M. G., A. Warris, J. W. Van der Meer, M. J. Fenton, T. J. Verver-Janssen, L. E. Jacobs, T. Andresen, P. E. Verweij, and B. J. Kullberg. 2003. Aspergillus fumigatus evades immune recognition during germination through loss of toll-like receptor-4-mediated signal transduction. J. Infect. Dis. 188:320-326. [DOI] [PubMed] [Google Scholar]
- 37.Rajagopalan, D. 2003. A comparison of statistical methods for analysis of high density oligonucleotide array data. Bioinformatics 19:1469-1476. [DOI] [PubMed] [Google Scholar]
- 38.Richardson, M. D., and M. Patel. 1995. Stimulation of neutrophil phagocytosis of Aspergillus fumigatus conidia by interleukin-8 and N-formylmethionyl-leucylphenylalanine. J. Med. Vet. Mycol. 33:99-104. [PubMed] [Google Scholar]
- 39.Roilides, E., A. Dimitriadou, I. Kadiltsoglou, T. Sein, J. Karpouzas, P. A. Pizzo, and T. J. Walsh. 1997. IL-10 exerts suppressive and enhancing effects on antifungal activity of mononuclear phagocytes against Aspergillus fumigatus. J. Immunol. 158:322-329. [PubMed] [Google Scholar]
- 40.Roilides, E., A. Holmes, C. Blake, P. A. Pizzo, and T. J. Walsh. 1993. Impairment of neutrophil antifungal activity against hyphae of Aspergillus fumigatus in children infected with human immunodeficiency virus. J. Infect. Dis. 167:905-911. [DOI] [PubMed] [Google Scholar]
- 41.Roilides, E., T. Sein, M. Roden, R. L. Schaufele, and T. J. Walsh. 2001. Elevated serum concentrations of interleukin-10 in nonneutropenic patients with invasive aspergillosis. J. Infect. Dis. 183:518-520. [DOI] [PubMed] [Google Scholar]
- 42.Roilides, E., K. Uhlig, D. Venzon, P. A. Pizzo, and T. J. Walsh. 1993. Enhancement of oxidative response and damage caused by human neutrophils to Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect. Immun. 61:1185-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenzweig, S. D., and S. M. Holland. 2004. Phagocyte immunodeficiencies and their infections. J. Allergy Clin. Immunol. 113:620-626. [DOI] [PubMed] [Google Scholar]
- 44.Singh, N. 2003. Fungal infections in the recipients of solid organ transplantation. Infect. Dis. Clin. N. Am. 17:113-134, viii. [DOI] [PubMed] [Google Scholar]
- 45.Staros, E. B. 2005. Innate immunity: new approaches to understanding its clinical significance. Am. J. Clin. Pathol. 123:305-312. [DOI] [PubMed] [Google Scholar]
- 46.Torres, H. A., G. P. Bodey, K. V. Rolston, H. M. Kantarjian, I. I. Raad, and D. P. Kontoyiannis. 2003. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer 98:86-93. [DOI] [PubMed] [Google Scholar]
- 47.Wewers, M. D. 2004. IL-1beta: an endosomal exit. Proc. Natl. Acad. Sci. USA 101:10241-10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams, P. L., S. L. Leib, P. Kamberi, D. Leppert, R. A. Sobel, Y. D. Bifrare, K. V. Clemons, and D. A. Stevens. 2002. Levels of matrix metalloproteinase-9 within cerebrospinal fluid in a rabbit model of coccidioidal meningitis and vasculitis. J. Infect. Dis. 186:1692-1695. [DOI] [PubMed] [Google Scholar]
- 49.Winn, R. M., C. Gil-Lamaignere, E. Roilides, M. Simitsopoulou, C. A. Lyman, A. Maloukou, and T. J. Walsh. 2003. Selective effects of interleukin (IL)-15 on antifungal activity and IL-8 release by polymorphonuclear leukocytes in response to hyphae of Aspergillus species. J. Infect. Dis. 188:585-590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.