Abstract
Humans infected with Giardia exhibit intestinal hypermotility, but the underlying mechanisms and functional significance are uncertain. Here we show in murine models of giardiasis that small-intestinal hypermotility occurs in a delayed fashion relative to peak parasite burden, is dependent on adaptive immune defenses, and contributes to giardial clearance.
Infection with Giardia lamblia is one of the most common causes of diarrheal disease worldwide (22). This protozoan pathogen colonizes the small intestine and can attach to the epithelium but does not invade the mucosa. Infections are normally self-limiting, since immunocompetent hosts can control and typically eradicate G. lamblia, a process that involves CD4 T cells and the generation of secretory immunoglobulin A (IgA) and other, poorly understood effectors (6, 8, 9, 18). Despite the frequently severe clinical symptoms, diarrhea, abdominal pain, malabsorption, and weight loss, infection is not accompanied by significant mucosal inflammation (12). These observations suggest that inflammatory mediators may not be important for parasite-induced diarrhea, although the mechanisms governing diarrhea in giardiasis are poorly understood. Giardia has not been shown to release enterotoxins that might account for the disturbance of intestinal fluid absorption or secretion. A reduction in absorptive surface due to a loss of epithelial microvilli occurs upon Giardia infection in mice (16), which could lead to osmotically driven diarrhea associated with malabsorption, but the absolute surface reduction is modest compared to the predicted anatomical reserve of the small intestine. Humans infected with G. lamblia exhibit signs of intestinal hypermotility upon radiological examination (15), a phenomenon also observed in experimentally infected Mongolian gerbils (5). The underlying mechanisms and functional significance of these findings are presently unclear. Therefore, the goal of the present study was to test the hypothesis that intestinal hypermotility represents a host defense mechanism against Giardia, using murine models of giardiasis.
Adult C57BL/6, SCID, and neuronal nitric oxide synthase (nNOS)-deficient mice were obtained from The Jackson Laboratory (Bar Harbor, ME). For infections with Giardia muris, cysts were purified by sucrose flotation, counted in a hemocytometer under a phase-contrast microscope, and given by oral gavage in water at 104 cysts/mouse in 0.2 ml (9). For G. lamblia infections, trophozoites of the GS/M strain (ATCC 50580) (11) were grown in TYIS-33 medium and given by oral gavage at 107/mouse in 0.2 ml of the same medium (9). Small-intestinal motility was determined by a modified test meal method. Mice were fasted overnight and given 0.2 ml of a suspension of 106 fluorescent polystyrene beads (10-μm-diameter Fluoresbrite YG carboxylate microspheres; Polysciences, Inc., Warrington, PA) (19) and 6% carmine dye in 5% gum arabic in phosphate-buffered saline (PBS). After 20 min, the small intestine was removed rapidly, and the position of the carmine dye front and the entire length of the small intestine were recorded. The intestine was then divided into eight equal-sized pieces, each of which was opened longitudinally, placed into 2 ml PBS, and cooled on ice for 10 min. The mixtures were shaken vigorously to detach the trophozoites and beads, which were then counted separately by using phase-contrast and fluorescence microscopes, respectively. The distance traveled by the carmine dye front was expressed as a percentage of the entire length of the small intestine. Bead numbers per segment were expressed as a percentage of the total number of beads in the small intestine. To assess the consequences of inhibiting small-intestinal motility on giardial clearance, mice were first infected orally with G. muris or G. lamblia GS/M and treated by oral gavage every other day, beginning on day 7 or day 4, respectively, with 30 mg/kg loperamide or with PBS as a control. Small-intestinal trophozoite numbers were determined on day 21 for G. muris and on day 9 for G. lamblia.
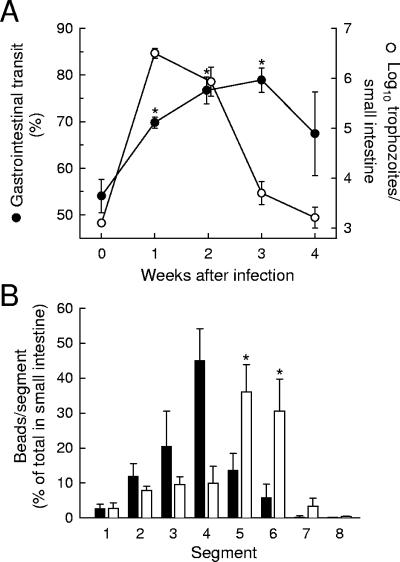
To determine whether normal adult mice are a suitable model for studying the role of intestinal motility in controlling giardial infection, we infected 8- to 10-week-old C57BL/6 mice with cysts of the naturally occurring murine pathogen G. muris. Small-intestinal motility was determined by a test meal method, using carmine dye as a liquid phase marker (14) and 10-μm polystyrene beads as a marker of particulates comparable in size to Giardia trophozoites (19). Infection with G. muris accelerated small-intestinal transit, since the fronts of both carmine dye (Fig. 1A) and polystyrene beads (Fig. 1B) had traveled significantly farther in infected mice than in uninfected controls. A time course analysis of this phenomenon revealed that hypermotility occurred within a week after infection but peaked at 2 to 3 weeks, at a time when trophozoite numbers were decreased compared to the numbers at the time of maximal infection at 1 week (Fig. 1A). Thus, the maximal changes in small-intestinal motility were delayed relative to the peak in the trophozoite burden, suggesting that these changes may be caused by a mechanism other than direct giardial stimulation.
FIG. 1.
G. muris infection of wild-type mice induces small-intestinal hypermotility. (A) Adult C57BL/6 mice were infected orally with 104 G. muris cysts (weeks 1 to 4) or left uninfected as controls (week 0). At the indicated times after infection, small-intestinal motility (•) and trophozoite load (○) were examined. Motility data are shown as the distance traveled by a carmine dye-containing test meal relative to the length of the entire small intestine over a 20-min period. Values are means ± standard errors of the means (SEM) of results for four to seven animals. Asterisks represent a significant (P < 0.05, t test) increase in motility relative to uninfected controls. (B) Mice infected for 2 weeks with G. muris (empty bars) and uninfected controls (filled bars) were given a test meal containing 10-μm fluorescent polystyrene beads, and bead transit was assessed after 20 min. Bead numbers in each of eight equally sized small-intestinal segments (which are numbered in order from proximal duodenum to distalileum) are given as percentages of the total number of beads in the small intestine. No significant difference was found between the numbers of residual beads in the stomachs of uninfected and infected mice (14.2% ± 5.7% versus 18.5% ± 3.4%, respectively). Values are means ± SEM for six to seven animals. Asterisks represent a significant (P < 0.05, t test) increase relative to uninfected mice.
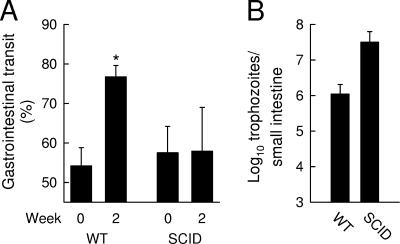
This finding is reminiscent of reports of Giardia-induced loss of intestinal epithelial microvilli in which the host adaptive immune response was found to be responsible (16, 17). To evaluate whether similar mechanisms may be involved in causing small-intestinal hypermotility, we evaluated mice with severe combined immunodeficiency (SCID mice). These mice lack functional T and B cells due to a defect in the catalytic subunit of DNA-dependent protein kinase, PRKDC, which is required for normal V(D)J recombination, and cannot eradicate Giardia (9, 18). G. muris infection of SCID mice did not alter small-intestinal transit, which contrasted sharply with the observations in the immunocompetent controls (Fig. 2). These results indicate that giardiasis-associated small-intestinal hypermotility was dependent on the induction of a normal adaptive immune response to the pathogen.
FIG. 2.
Dependence of intestinal hypermotility on intact T- and B-cell functions. Adult C57BL/6 (wild-type [WT]) and SCID mice were infected orally with G. muris cysts or left uninfected as controls, and small-intestinal motility (A) and trophozoite numbers (B) were evaluated 2 weeks after infection. Values are mean ± SEM for four to six animals. The asterisk designates a significant increase (P < 0.05, t test) relative to uninfected controls.
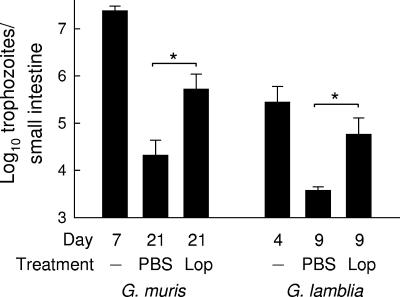
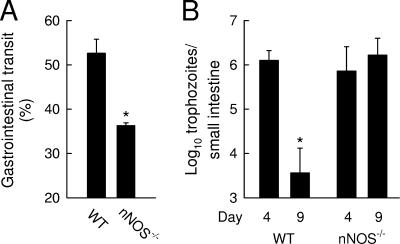
To test whether the observed small-intestinal hypermotility contributed to the clearance of Giardia, we treated mice with loperamide, a drug that inhibits intestinal transit by activating μ-opioid receptors in the gastrointestinal tract (2, 13, 21). Drug treatment was started at the time of peak G. muris infection (day 7) to ensure that pharmacologically induced changes in motility would not interfere with the initial establishment of the infection. The inhibition of small-intestinal motility by loperamide markedly compromised giardial clearance, with 25-fold-higher trophozoite numbers in loperamide-treated mice than in PBS-treated controls at 21 days (Fig. 3). Loperamide treatment had no effect on the development of adaptive immunity, since titers of antigiardial IgA in intestinal mucosal secretions were not affected by the treatment (data not shown). Furthermore, this experimental strategy revealed a similar inhibitory effect on murine infection with G. lamblia GS/M, a human giardial pathogen that can infect normal adult mice (3, 9). Mice treated with PBS had largely cleared the infection by 9 days, while mice treated with loperamide from day 4 onwards continued to have significant numbers of G. lamblia trophozoites in the small intestine (Fig. 3). Thus, inhibition of small-intestinal motility compromised the clearance of Giardia in the murine host, irrespective of the giardial species. As an additional approach to determine the importance of intestinal motility in antigiardial host defense, we used a genetic approach in which disruption of the gene for nNOS interferes with effective propulsion in the intestine in mice (20). Motility analysis confirmed that nNOS-deficient mice exhibited a constitutive delay in gastrointestinal transit compared to their wild-type littermates (Fig. 4A). In parallel, the knockout mice failed to clear G. lamblia infection normally (Fig. 4B). Thus, using pharmacologic and genetic approaches, we found that decreased intestinal motility was associated with impairment of host defense against Giardia.
FIG. 3.
Pharmacologic inhibition of intestinal motility compromises giardial clearance. Adult C57BL/6 mice were infected orally with 104 G. muris cysts or with 107 G. lamblia GS/M trophozoites. After 7 days (G. muris) or 4 days (G. lamblia), mice were given loperamide (Lop) or PBS orally every other day. Trophozoite numbers in the small intestine were determined at the indicated times after infection. Values are means ± SEM for eight to ten animals. *, P < 0.05, as determined by t test.
FIG. 4.
Mice lacking nNOS exhibit decreased gastrointestinal transit and increased susceptibility to Giardia infection. (A) Adult mice deficient for nNOS (nNOS−/−) and their wild-type littermate controls (WT) were tested for gastrointestinal motility using a carmine dye test meal. Values are means ± SEM for at least three animals. *, P < 0.05 (t test), relative to the results for wild-type mice. (B) Mice were infected orally with 107 trophozoites of G. lamblia GS/M, and trophozoite numbers in the small intestine were determined at the indicated times after infection. Values are means ± SEM for three to four animals. *, P < 0.05, relative to the counts on day 4.
Our study shows that intestinal hypermotility is an important host defense against Giardia, a conclusion also reached in another recent report (10). This defense appears to depend on the development of a normal adaptive immune response against the parasite, as it did not occur in mice lacking T and B cells, although it is possible, in principle, that T or B cells contribute to hypermotility independent of their role in adaptive antigiardial immunity. Immune-dependent hypermotility operates in host defense against other enteric parasites, particularly helminths. For example, eradication of the roundworm Trichinella spiralis, which spends a significant portion of its life cycle in the small intestine, is highly correlated with enhanced intestinal motility (4, 23). Likewise, expulsion of the hookworm Nippostrongylus brasiliensis in rats is accompanied by small-intestinal hypermotility, suggesting a role in host defense against this helminth (7). Common to all these enteric pathogens is their primary, if not exclusive, localization in the intestinal lumen. Viewed anatomically, this site of infection is located outside the epithelium-lined body proper and hence is not readily accessible to many immune effector cells and molecules, such as neutrophils or complement, which operate effectively within the body. In fact, effective antimicrobial defense in the intestinal lumen poses a special challenge to the host, which has a limited repertoire of defenses at this site. Of these, secretory IgA is regarded commonly as a prime luminal defense mechanism, but its actual importance in giardial clearance appears to be variable and may depend on poorly defined host and parasite factors (6, 9, 18). Our data and prior work with helminths (4, 23) indicate that intestinal hypermotility is another important defense mechanism against colonization of the intestinal lumen.
Immune-dependent hypermotility may provide a mechanistic explanation for the diarrhea associated with giardiasis, as noted in prior reports (5, 15). In principle, diarrhea can be caused by decreased fluid absorption or increased secretion or a combination of these two mechanisms. Little evidence exists for enhanced ion and fluid secretion in giardiasis, leaving impaired fluid absorption as the likely cause. Shortened contact time with luminal fluids, which may be ingested or derived from gastric or pancreatic secretions, would be expected to compromise the effectiveness of ion transport across the epithelium, particularly when hypermotility is combined with the reported loss of absorptive epithelial surfaces (16). It must be noted, however, that mice do not exhibit frank diarrhea upon Giardia infection. Nonetheless, it is possible that an intestinal fluid imbalance occurs in both human and animal hosts and that it remains compensated in mice but not in humans. If hypermotility indeed contributes to the pathogenesis of diarrhea, our finding that SCID mice failed to exhibit Giardia-induced hypermotility would imply that patients with cellular immunodeficiencies associated with increased susceptibility to Giardia infections (e.g., chronic variable immunodeficiency) may be less likely to develop infection-associated diarrhea. Furthermore, our results suggest that caution is indicated when considering the use of intestinal motility inhibitors in the treatment of Giardia-induced diarrhea (1), as such treatment may prolong the underlying infection.
Acknowledgments
This work was supported by NIH grants DK35108 and RR17030.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Al-Abri, S. S., N. J. Beeching, and F. J. Nye. 2005. Traveller's diarrhoea. Lancet Infect. Dis. 5:349-360. [DOI] [PubMed] [Google Scholar]
- 2.Awouters, F., A. Megens, M. Verlinden, J. Schuurkes, C. Niemegeers, and P. A. Janssen. 1993. Loperamide. Survey of studies on mechanism of its antidiarrheal activity. Dig. Dis. Sci. 38:977-995. [DOI] [PubMed] [Google Scholar]
- 3.Byrd, L. G., J. T. Conrad, and T. E. Nash. 1994. Giardia lamblia infections in adult mice. Infect. Immun. 62:3583-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castro, G. A., F. Badial-Aceves, J. W. Smith, S. J. Dudrick, and N. W. Weisbrodt. 1976. Altered small bowel propulsion associated with parasitism. Gastroenterology 71:620-625. [PubMed] [Google Scholar]
- 5.Deselliers, L. P., D. T. Tan, R. B. Scott, and M. E. Olson. 1997. Effects of Giardia lamblia infection on gastrointestinal transit and contractility in Mongolian gerbils. Dig. Dis. Sci. 42:2411-2419. [DOI] [PubMed] [Google Scholar]
- 6.Eckmann, L. 2003. Mucosal defences against Giardia. Parasite Immunol. 25:259-270. [DOI] [PubMed] [Google Scholar]
- 7.Farmer, S. G. 1981. Propulsive activity of the rat small intestine during infection with the nematode Nippostrongylus brasiliensis. Parasite Immunol. 3:227-234. [DOI] [PubMed] [Google Scholar]
- 8.Faubert, G. 2000. Immune response to Giardia duodenalis. Clin. Microbiol. Rev. 13:35-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langford, T. D., M. P. Housley, M. Boes, J. Chen, M. F. Kagnoff, F. D. Gillin, and L. Eckmann. 2002. Central importance of immunoglobulin A in host defense against Giardia spp. Infect. Immun. 70:11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, E., P. Zhou, and S. M. Singer. 2006. Neuronal nitric oxide synthase is necessary for elimination of Giardia lamblia infections in mice. J. Immunol. 176:516-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nash, T. E., D. A. Herrington, G. A. Losonsky, and M. M. Levine. 1987. Experimental human infections with Giardia lamblia. J. Infect. Dis. 156:974-984. [DOI] [PubMed] [Google Scholar]
- 12.Oberhuber, G., N. Kastner, and M. Stolte. 1997. Giardiasis: a histologic analysis of 567 cases. Scand. J. Gastroenterol. 32:48-51. [DOI] [PubMed] [Google Scholar]
- 13.Puig, M. M., and O. Pol. 1998. Peripheral effects of opioids in a model of chronic intestinal inflammation in mice. J. Pharmacol. Exp. Ther. 287:1068-1075. [PubMed] [Google Scholar]
- 14.Read, N. W., C. A. Miles, D. Fisher, A. M. Holgate, N. D. Kime, M. A. Mitchell, A. M. Reeve, T. B. Roche, and M. Walker. 1980. Transit of a meal through the stomach, small intestine, and colon in normal subjects and its role in the pathogenesis of diarrhea. Gastroenterology 79:1276-1282. [PubMed] [Google Scholar]
- 15.Reeder, M. M. 1997. Radiological diagnosis of giardiasis. Semin. Roentgenol. 32:291-300. [DOI] [PubMed] [Google Scholar]
- 16.Scott, K. G.-E., M. R. Logan, G. M. Klammer, D. A. Teoh, and A. G. Buret. 2000. Jejunal brush border microvillous alterations in Giardia muris-infected mice: role of T lymphocytes and interleukin-6. Infect. Immun. 68:3412-3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott, K. G.-E., L. C. H. Yu, and A. G. Buret. 2004. Role of CD8+ and CD4+ T lymphocytes in jejunal mucosal injury during murine giardiasis. Infect. Immun. 72:3536-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singer, S. M., and T. E. Nash. 2000. T-cell-dependent control of acute Giardia lamblia infections in mice. Infect. Immun. 68:170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sukhdeo, M. V., and N. A. Croll. 1981. Gut propulsion in mice infected with Trichinella spiralis. J. Parasitol. 67:906-910. [PubMed] [Google Scholar]
- 20.Takahashi, T. 2003. Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract. J. Gastroenterol. 38:421-430. [DOI] [PubMed] [Google Scholar]
- 21.Tan-No, K., F. Niijima, O. Nakagawasai, T. Sato, S. Satoh, and T. Tadano. 2003. Development of tolerance to the inhibitory effect of loperamide on gastrointestinal transit in mice. Eur. J. Pharm. Sci. 20:357-363. [DOI] [PubMed] [Google Scholar]
- 22.Thompson, R. C. 2000. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int. J. Parasitol. 30:1259-1267. [DOI] [PubMed] [Google Scholar]
- 23.Vallance, B. A., and S. M. Collins. 1998. The effect of nematode infection upon intestinal smooth muscle function. Parasite Immunol. 20:249-253. [DOI] [PubMed] [Google Scholar]