Abstract
CD8+ T cells induced by Plasmodium yoelii sporozoites develop into protective memory cells without undergoing changes in interleukin-7 receptor α (IL-7Rα) expression, differing from the development of memory CD8+ T cells against viruses, which is associated with enhanced IL-7Rα expression. This suggests a microbe-dependent diversity in the signals determining the development of memory populations.
Memory CD8+ T cells develop following activation of naïve CD8+ T cells induced by microbial infection and establish a pool of cells capable of responding in greater numbers and with greater efficacy than naïve precursors (1). The formation of this stable pool of memory CD8+ T cells is vital to establish protective immunity upon reexposure to infectious intracellular pathogens. While significant advances have been made in our understanding of the induction of CD8+ T-cell responses, the molecular mechanisms controlling the development of memory cells and the markers that identify cells that may undergo memory differentiation still remain poorly characterized. A recent study using CD8+ T cells specific for lymphocytic choriomeningitis virus (LCMV) showed that expression of interleukin-7 receptor α (IL-7Rα, CD127) among a small percentage of CD8+ T-cell effectors identified those cells that would become memory cells (4). Furthermore, naïve mice that received IL-7Rαhigh CD8+ T cells from immunized mice 11 days after immunization went on to develop into memory cells, whereas those that received IL-7Rαlow CD8+ T cells did not. These observations led the authors to conclude that the selective expression of IL-7Rα identified memory cell precursors, thus suggesting that this marker may be useful in predicting the number of memory T cells generated after infection or immunization (4). Given this finding, we characterized the expression of IL-7Rα among CD8+ T cells in a rodent malaria model. Numerous studies have demonstrated that the induction of effector and memory CD8+ T cells specific for the pre-erythrocytic stage of Plasmodium is critical to establish protective immunity against malaria parasite infection (5, 7, 9, 10). The use of a malaria T-cell receptor (TCR) transgenic system, in which most CD8+ T cells express TCR specific for the H2Kd-restricted epitope SYVPSAEQI from the circumsporozoite protein of Plasmodium yoelii, has provided new insights into the development and tissue distribution of memory cells. We have recently shown that IL-4-IL-4R interactions are critical to the development and maintenance of protective memory CD8+ T cells in nonlymphoid tissue (3, 6).
Using this P. yoelii-specific TCR transgenic model, we studied the modulation of IL-7Rα expression after immunization with either P. yoelii sporozoites or recombinant vaccinia virus expressing the parasite epitope (rec-VV) (8). In this system, effector and memory CD8+ T cells express the SYVPSAEQI-specific TCR, which will have the same affinity and strength for the recognition of peptide-major histocompatibility complex complexes expressed after infection with the different microbes.
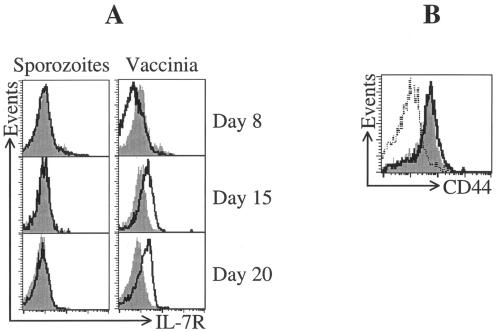
To examine the expression of activated IL-7Rα on T cells, we used an in vivo system in which TCR-transgenic CD8+ T cells were adoptively transferred to normal mice, which were then immunized with either irradiated P. yoelii sporozoites or rec-VV. In mice immunized with rec-VV, antigen-specific CD8+ T cells expressed lower levels of IL-7Rα at 8 days postimmunization compared to naïve cells (Fig. 1A). As the transition from effector to memory cells evolved, the expression of IL-7Rα increased on the antigen-specific CD8+ T cells, and by day 15, the majority of the cells became IL-7Rαhigh (Fig. 1A). Thus, the response elicited by rec-VV regarding the expression of IL-7Rα on effector and memory CD8+ T cells follows the pattern described for CD8+ T cells after infection with LCMV. In contrast to the shifting pattern of IL-7Rα expression after infection with this virus, CD8+ T cells from mice immunized with irradiated sporozoites exhibited no modulation of IL-7Rα expression throughout the development of this T-cell response (Fig. 1A). Indeed, the expression of IL-7Rα on these cells remained unchanged throughout the effector phase and continued through the memory transition phase at levels similar to those found in naïve cells. Importantly, this lack of modulation in the surface expression of IL-7Rα in parasite-immunized mice was not due to a lack of activation of these cells, as both immunogens were equally efficient at driving CD8+ T-cell activation, because essentially all SYVPSAEQI-specific CD8+ T cells induced by either sporozoites or rec-VV expressed identical, high levels of CD44, the most reliable surface marker of cell activation (Fig. 1B).
FIG. 1.
Expression profile of IL-7Rα on CD8+ T cells induced by immunization with sporozoites and recombinant vaccinia virus. Expression of IL-7Rα on activated CD8+ T cells was assessed using splenocytes obtained from mice that received 1.2 × 106 transgenic CD8+ T cells and subsequently immunized intravenously with either 0.5 × 106 irradiated sporozoites or 5 × 106 PFU recombinant vaccinia virus expressing the parasite epitope SYVPSAQEI (rec-VV). (A) At days 8, 15, and 20 after immunization, fluorescence-activated cell sorting analysis was performed using anti-CD8-allophycocyanin (APC), H2Kd-tetramer-phycoerythrin (PE), and anti-IL-7Rα-fluorescein isothiocyanate (FITC). The histograms are gated on tetramer+ CD8+ cells. Sporozoite-induced cells and rec-VV-induced cells are shown with a dark line, and naïve CD8+ T cells are represented in a solid gray histogram. (B) Expression of CD44 on activated CD8+ T cells was performed 20 days after immunization by using anti-CD8-APC, H2Kd-tetramer-PE, and anti-CD44-FITC. The dotted line represents naïve CD8+ T cells; sporozoite- and rec-VV-immunized cells are represented by a dark line and a solid gray histogram, respectively.
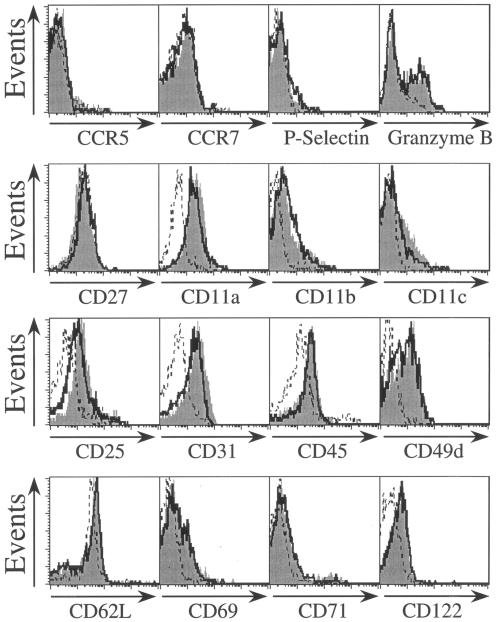
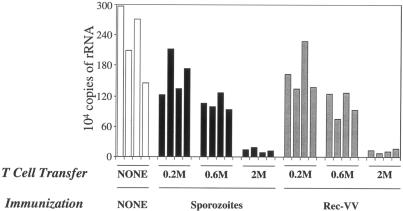
We wished to confirm that the cells present 20 days after irradiated-sporozoite immunization represent a bona fide memory population despite their lack of increased IL-7R expression. Our previous work has shown that irradiated sporozoites induce a classical pattern of activation, expansion, and contraction in the CD8 T-cell response, which leaves a residual population of memory cells. These cells express a surface phenotype typical of antigen-experienced memory cells with respect to CD11a, CD11b, CD11c, CD25, CD27, CD31, CD45RB, CD49d, CD62L, CD69, CD71, CD122, CCR5, CCR7, P-selectin, and granzyme B (6). For all these markers, the phenotypes of parasite-induced memory cells and rec-VV-induced cells are identical (Fig. 2). The notable exception to this is IL-7R. We wanted to know if this difference in IL-7R expression marked some wider defect in the function of these cells. To address this question, we performed adoptive transfer experiments in which naïve mice received equal numbers of memory CD8+ T cells obtained from mice immunized with either irradiated sporozoites or rec-VV. Memory CD8+ T cells used in these experiments were obtained from spleens 25 days after immunization and were purified by depleting CD4+ T and B cells by magnetic sorting, as described previously (6). After adoptive transfer into naïve mice, the protective capacity of these cells was assessed by determining their ability to inhibit the development of the parasites in the liver, measured 34 h postchallenge using real-time PCR (2, 6). The results from these protection experiments showed that CD8+ T cells primed by either rec-VV or irradiated sporozoites inhibited parasite development with equivalent efficacies and followed a similar dependence on effector T-cell frequency (Fig. 3). Thus, the differential expression of IL-7Rα on memory antigen-specific CD8+ T cells did not affect their protective qualities.
FIG. 2.
Phenotypic characterization of spleen memory CD8+ T cells induced by immunization with sporozoites and recombinant vaccinia virus. Mice were immunized with either 0.5 × 106 irradiated sporozoites or 5 × 106 PFU recombinant vaccinia virus expressing the parasite epitope SYVPSAEQI (rec-VV). Twenty-five days later we isolated lymphocytes from the spleens. The cells were stained with H2Kd-tetramer-phycoerythrin, CD8-antigen-presenting cells, and marker-specific fluorescein isothiocyanate-labeled antibodies prior to analysis by fluorescence-activated cell sorting. The histograms represent the expression of the markers on CD8+ tetramer+ T cells. Gray histograms show expression on rec-VV-induced cells, and dark-line histograms show sporozoite-induced cells. Expression of markers on control naïve cells is represented by the dotted line.
FIG. 3.
Comparison of anti-parasite activities of memory CD8+ T cells induced by sporozoites or by recombinant vaccinia virus. SYVPSAEQI-specific memory CD8+ T cells were generated from mice that received 1.0 × 106 naïve transgenic CD8+ T cells, and the mice were subsequently immunized with either 0.5 × 106 irradiated (γ source; 20 krad) sporozoites or 5 × 106 PFU rec-VV. Twenty-five days after immunization, purified memory CD8+ T cells obtained from spleens were adoptively transferred into naïve mice. Recipient and control mice were challenged 2 days later with 3 × 104 viable sporozoites. Thirty-four hours after challenge, the livers of infected mice were excised, and parasite load was measured using real-time PCR. The bar graph represents the number of P. yoelii 18S rRNA copies per liver. These data are representative of two independent experiments using four mice per group.
Taken together, our data and previous work suggest that memory cells can develop by following different pathways of differentiation. Despite the fact that CD8+ T cells responding to sporozoites and vaccinia virus may differ in their requirements for IL-7 during differentiation, the cells induced by both microbes were able to protect against live sporozoite challenge. Thus, while it is likely that the expression of IL-7R is important for memory development in certain situations, IL-7R cannot be considered a universal marker for protective immunity. It is perhaps not unexpected that CD8+ T-cell responses to the same epitope may follow different activation pathways. The innate immune responses to sporozoites and vaccinia virus may vary widely in terms of the stimulation of Toll-like receptors, the role of dendritic cells and NK cells, production of cytokines, and the strength of costimulation. All these things may alter the development of the acquired immune response. The present results indicate that multiple mechanisms may exist that lead to the development of memory CD8+ T cells and provide insights into the relationships between pathogens and their ability to generate lymphocyte memory.
Acknowledgments
This work was supported by National Institutes of Health grant AI44375.
Flow cytometry was performed in the Becton Dickinson Immune Function Laboratory at the Johns Hopkins Bloomberg School of Public Health.
We have no conflicting financial interests.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science 272:54-60. [DOI] [PubMed] [Google Scholar]
- 2.Bruna-Romero, O., J. C. Hafalla, G. Gonzalez-Aseguinolaza, G. Sano, M. Tsuji, and F. Zavala. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 31:1499-1502. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho, L. H., G. Sano, J. C. Haffala, A. Morrot, M. A. Curotto de Lafaille, and F. Zavala. 2002. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat. Med. 8:166-170. [DOI] [PubMed] [Google Scholar]
- 4.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191-1198. [DOI] [PubMed] [Google Scholar]
- 5.Morrot, A., and F. Zavala. 2002. Effector and memory CD8+ T cells as seen in immunity to malaria. Immunol. Rev. 201:291-303. [DOI] [PubMed] [Google Scholar]
- 6.Morrot, A., J. C. Hafalla, I. A. Cockburn, L. H. Carvalho, and F. Zavala. 2005. IL-4 receptor expression on CD8+ T cells is required for the development of protective memory responses against liver stages of malaria parasites. J. Exp. Med. 202:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues, M. M., A. S. Cordey, G. Arreaza, G. Corradin, P. Romero, J. L. Maryanski, R. S. Nussenzweig, and F. Zavala. 1991. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int. Immunol. 3:982-987. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues, M., S. Li, K. Murata, D. Rodriguez, J. R. Rodriguez, I. Bacik, J. R. Bennink, J. W. Yewdell, A. Garcia-Sastre, R. S. Nussenzweig, M. Esteban, P. Palese, and F. Zavala. 1994. Influenza and vaccinia viruses expressing malaria CD8+ T and B cell epitopes. Comparison of their immunogenicity and capacity to induce protective immunity. J. Immunol. 153:4636-4648. [PubMed] [Google Scholar]
- 9.Schofield, L., J. Villaquiran, A. Ferreira, H. Schellekens, R. Nussenzweig, and V. Nussenzweig. 1987. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330:664-666. [DOI] [PubMed] [Google Scholar]
- 10.Weiss, W. R., M. Sedegah, R. L. Beaudoin, L. H. Miller, and M. F. Good. 1988. CD8+ T cells (cytotoxic/supressors) are required for protection in mice immunized with malaria sporozoites. Proc. Natl. Acad. Sci. USA 85:573-576. [DOI] [PMC free article] [PubMed] [Google Scholar]