Abstract
Despite being central to parasite establishment and subsequent host pathological and immunologic responses, host-parasite interactions during early third-stage filarial larva (L3) migration are poorly understood. These studies aimed to define early tissue migration of Brugia pahangi L3 in the gerbil (Meriones unguiculatus) and measure host cellular responses during this period. Gerbils were intradermally inoculated in the hind limb with 100 B. pahangi L3, and necropsies were performed at various times. At 3 h, most L3 (96.3%) were recovered from tissues associated with the infection site, with marked L3 migration occurring by 24 h. Larvae were dispersed throughout the lymphatics at 7 days postinfection (dpi), and at 28 dpi, most parasites were recovered from the spermatic cord lymphatics. Parasites were identified histologically at all time points. Inflammatory cells, primarily neutrophils, were frequently observed around larvae in the dermis and muscle near the injection site at 3 h and 24 h. Levels of interleukin-6 (IL-6) and tumor necrosis factor-α mRNA peaked at 3 h in all tissues, with IL-6 levels also high in the spleen at 28 dpi. Levels of IL-4 mRNA were elevated in all tissues at 28 dpi. These observations demonstrate that L3 migrate quickly through various tissues and into lymph nodes in a predictable pattern. Migrating L3 induce an early acute inflammatory response that is modulated as parasites establish in the lymphatics. Polarization of the host response towards a dominant Th2-like profile is present at 7 dpi and is well established by 28 dpi in this permissive host.
It is generally accepted that third-stage filarial nematode infective larvae (L3) emerge from the mosquito labia during feeding, accumulate in a pool of hemolymph, and enter the host through the wound left by the mosquito vector (13). While the L3 are apparently unable to penetrate intact skin, their early migrations require movements through the connective tissues of the skin to lymphatic vessels and, in some cases, the blood vascular system. These events have been demonstrated experimentally by feeding Brugia-infected mosquitoes on cats (14, 17) and gerbils (3) and by in vivo studies involving the application of L3 to punctured skin (14). Furthermore, previous studies by Ah et al. (1) have clearly shown that L3 can actively and rapidly migrate through a variety of complex tissues following placement on the surface of the gerbil eyeball. The mechanisms utilized by the larvae during this early migratory phase through these various tissues have not been defined.
Early migrations of L3 through the tissues of the skin not only are central to the successful establishment of parasites but are also likely to be important in the initial induction of the host response. It is expected that these responses are involved in the elimination or inhibition of incoming L3 during early migrations. The migration of L3 into the skin may also be uniquely involved in defining the nature of subsequent immune responses to parasite antigens. Based on various in vitro studies, it is hypothesized that L3 interactions with antigen-presenting cells within the skin, such as Langerhans cells, macrophages, keratinocytes, and dendritic cells, may be at least partly responsible for the initial induction of the down-regulated condition of the immune response seen in the majority of filariasis patients (31). Other in vitro experiments have demonstrated altered responses of T cells when cultured with vector-derived L3. In these experiments, naïve peripheral blood mononuclear cells cultured with L3 showed an increase at 24 h in T-cell-derived proinflammatory cytokines but not Th2 cytokines. This response was dependent on the presence of antigen-presenting cells and contact with live L3 (6). While these in vitro studies are intriguing, correlative in vivo experiments on the effect of early L3 migrations on the induction of immune or inflammatory responses are limited. Brugia pahangi L3 inoculations in the footpads of BALB/c mice have been shown to induce a dramatic increase in interleukin-4 (IL-4) mRNA in popliteal lymph nodes at 24 h postinfection (26). This response was apparently elicited only by L3, as similar inoculations of microfilariae induced increases in gamma interferon (IFN-γ) in the absence of IL-4 or other Th2 cytokines. While the recovery of parasites or definition of any associated inflammatory responses was not reported, these in vivo results are in clear contrast to the more recent in vitro studies utilizing human cells briefly noted above.
In the current experiments, we define the migration pattern of B. pahangi L3 in the permissive gerbil (Meriones unguicualtus) host by inoculating known numbers of L3 into the dermis of the lower hind leg. This placement mandates a migration of L3 into skin tissues prior to entry into lymphatic vessels and localizes the infection site to an accessible area. It also allows for the use of a quantifiable inoculum, which is not possible with either mosquito feeding or skin prick methods. The cytokine mRNA profiles and histological characterization of inflammatory responses between 3 h and 7 days following L3 inoculation were described and compared to these responses at 28 days, when immature adults are established in the lymphatic system. The data suggest that there is a very early inflammatory response dominated by neutrophils in the presence of a mixed cytokine profile. This is quickly down-regulated and replaced by a dominant Th2 cytokine response reflected by increased levels of IL-4 mRNA after 7 days postinfection (dpi).
Data indicate that this model system of early L3 migration will be useful for a variety of studies.
MATERIALS AND METHODS
Gerbils and parasites.
Male Mongolian gerbils (Meriones unguiculatus), approximately 8 weeks of age, were obtained from Charles River (Wilmington, MA) and were maintained on standard rodent chow and water ad libitum. Brugia pahangi third-stage larvae (L3) were recovered from infected Aedes aegypti (Black eye strain) mosquitoes by Baermannization (24). Motile L3 were washed twice in RPMI 1640 medium, pH 7.2 (Louisiana State University School of Veterinary Medicine Media Services, Baton Rouge, LA), containing penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin B (0.025 μg/ml). All infections were given by intradermal (i.d.) injection into the medial aspect of the distal hind limb using a 28-gauge needle and consisted of 100 B. pahangi L3 suspended in 50 μl RPMI medium. Control (uninfected) animals received i.d. injections of 50 μl RPMI medium from the final larval wash.
Larval migration studies.
Gerbils were infected in the left distal hind limb with 100 B. pahangi L3 and then selected at random, and necropsies were performed at 3 h, 24 h, 72 h, 7 days, and 28 days postinfection (dpi). Six gerbils were examined at each time point. Larvae were recovered from the animals by dissection and teasing of the left (injected) hind limb skin and muscle, major lymph nodes (popliteal lymph nodes [PLN], inguinal lymph nodes, subinguinal lymph nodes, ileac lymph nodes, and renal lymph nodes [RLN]) and lymphatic vessels, heart, lungs, testicles, and spermatic cord lymphatics. Tissues were then soaked in phosphate-buffered saline for at least 1 h before larvae were recovered with the aid of a stereomicroscope. Each dissected tissue was examined twice. Larval recoveries from the skin and muscle of the left hind limb were pooled.
Histologic identification of larvae.
Fifteen gerbils were injected i.d. with 100 B. pahangi L3 in 50 μl RPMI medium in the left distal hind limb. Five control gerbils were injected with 50 μl RPMI medium from the final larval wash after Baermannization in the left and right distal hind limbs. Immediately after infection (0 h) and at 3 h, 24 h, 72 h, and 7 dpi, three infected animals and one control animal were selected at random and euthanized. The hind limbs (intact), RLN, PLN, and subinguinal lymph nodes were removed and immersed in 10% neutral buffered formalin to fix for a minimum of 7 days. Hind limbs were then deboned and cut into five to seven cross-sectional pieces. Tissue pieces were embedded in paraffin. Two sections, 10 μm apart, were cut from the anterior face of the paraffin block, and two sections were then cut from the posterior face of the paraffin block. Lymph nodes were embedded intact in paraffin, and two to four serial sections were made. All sections were stained with hematoxylin and eosin.
Cytokine quantitation.
Forty gerbils were randomly divided into two groups. One group (30 gerbils) was infected i.d. with 200 B. pahangi L3 (100 L3 in 50 μl RPMI medium in each hind limb). The second (control) group (10 gerbils) was injected i.d. in the right and left distal hind limbs with RPMI medium from the final larval wash. At 3 h, 24 h, 72 h, 7 dpi, and 28 dpi, six infected and two control animals were selected at random and euthanized, and the spleen, RLN, and PLN were collected for cytokine mRNA quantification. Individual PLN and RLN were pooled from each animal. Single-cell suspensions were prepared using a Falcon 70-μm cell strainer (BD-Biosciences, Paramus, NJ) and stored in 0.5 ml of RNAStat 60 (Tel-test, Friendship, TX) at −70°C (10). Total RNA was isolated using chloroform extraction according to the manufacturer's instructions (Tel-test) and reverse transcribed to cDNA (10) for use as a template for the ABI PRISM Sequence Detection System 7700 (TaqMan; Applied Biosystems, Foster City, CA).
Levels of gerbil hypoxanthine phosphoribosyltransferase (GenBank accession number L37778), IL-4 (accession number L37779), IFN-γ (accession number L37782), tumor necrosis factor alpha (TNF-α) (accession number AF171082), and IL-6 (accession number AY570509) mRNA were quantified. Primer and probe sequences for TaqMan quantification of gerbil hypoxanthine phosphoribosyltransferase, IL-4, and IFN-γ have been reported previously (10). Oligonucleotides designed for IL-6 were as follows: sense, 5′-AACACCAAAACCCTAATTCGTATCTT-3′; antisense, 5′-GCCTCGGAAGTTGGTCA-3′; and probe, 5′-AACAAGAGGTGAAGGATCCAGGTCAAATAGTCTTT-3′. Oligonucleotides designed for TNF-α were as follows: sense, 5′-TCAGAACGCCAGCGACAA-3′; antisense, 5′-CAGCTGCTCCTCCACTTGGT-3′; and probe, 5′-CTGTGGCCCATGTCGTACCCAA-3′. All probes were labeled with the reporter dye 6-carboxyfluorescein and the quencher dye 6-carboxytetramethylrhodamine.
TaqMan reactions (50 μl) were performed in duplicate using Applied Biosystems Universal PCR mix according to the manufacturer's directions. Relative standard curves were constructed from cDNA prepared from RNA isolated from cells stimulated with either concanavalin A (10 μg/ml, 24 h) (10, 22) or lipopolysaccharide (LPS) (10 μg/ml) (22). The concanavalin A-stimulated cDNA was used as a standard for IL-4 and IFN-γ, and the LPS-stimulated cDNA was used for TNF-α and IL-6. All data are presented as the mean severalfold change of the treatment group compared to the appropriate control group ± standard error.
Statistics.
All statistical analyses were performed using SigmaStat (SSPS Inc., Chicago, IL). As larval recovery data and cytokine mRNA data failed normality, analyses were conducted using Kruskal-Wallis one-way analysis of variance on ranks, with subsequent pairwise comparisons analyzed using either Dunn's or Tukey's test. Statistical significance was placed at a P value of <0.05.
RESULTS
Brugia pahangi L3 migrate rapidly from the infection site.
Gerbils were inoculated with B. pahangi L3, and larvae were recovered at various time points postinfection. At 3 h, a statistically significant proportion of recovered larvae (96.3%) was found in the tissues (leg skin and muscle) associated with the infection site (Table 1). Nonetheless, at this very early time, some L3 (3.4%) had already migrated to lymph nodes. By 24 h, the number of larvae associated with the infection site had decreased to 53.2%, although the number of L3 recovered from this site was still significantly greater than that found in other tissues. At this time, almost half of the recovered larvae had migrated away from the infection site and had become established within the lymphatics, including lymphatic vessels of the spermatic cord. Parasites were well established in the lymphatic system by 28 dpi, with the majority of larvae located in the lymphatics associated with the spermatic cord (35.7%). The locations and numbers of the recovered larvae indicate a rapid pattern of migration away from the initial larva entry site and into the lymphatic system progressively through the popliteal lymph nodes to other lymph nodes, eventually establishing in the spermatic cord lymphatics as early adults. This observation is consistent with previous studies that described the location of mature parasites (4, 5, 20, 21, 22).
TABLE 1.
Mean and percent larval recoveries from gerbils i.d. inoculated with Brugia pahangi L3
| Tissue | Larval recovery at time postinfection:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 h
|
24 h
|
3 days
|
7 days
|
28 days
|
||||||
| Mean ± SDb | % | Mean ± SD | % | Mean ± SD | % | Mean ± SD | % | Mean ± SD | % | |
| Hind limb-skin muscle | 33.8 ± 18.5a | 96.3 | 11.0 ± 7.0a | 53.2 | 4.3 ± 4.9 | 13.7 | 7.3 ± 5.3 | 28.8 | 0.7 ± 1.2 | 5.9 |
| PLN | 0.8 ± 0.9 | 1.8 | 3.2 ± 3.4 | 14.7 | 7.3 ± 4.6 | 38.4 | 3.3 ± 2.1 | 15.2 | 1.3 ± 1.0 | 9.3 |
| Subiliac lymph node | 0.2 ± 0.4 | 0.3 | 2.2 ± 2.1 | 8.4 | 3.8 ± 4.8 | 16.6 | 3.7 ± 3.2 | 13.1 | 0.8 ± 1.2 | 4.9 |
| RLN | 0.7 ± 0.8 | 1.3 | 3.5 ± 2.8 | 15.5 | 6.8 ± 7.5 | 17.8 | 7.0 ± 4.0 | 26.3 | 1.8 ± 1.2 | 10.1 |
| Spermatic cord | 0 | 0 | 0.7 ± 0.8 | 2.4 | 1.5 ± 2.5 | 3.1 | 1.8 ± 3.1 | 6.6 | 8.2 ± 7.3 | 35.7 |
| Testicle | 0 | 0 | 0.2 ± 0.4 | 0.7 | 0.3 ± 0.5 | 0.7 | 1.2 ± 1.6 | 6.2 | 2.8 ± 4.2 | 8.7 |
| Heart | 0 | 0 | 0.3 ± 0.5 | 1.0 | 0.6 ± 1.0 | 2.1 | 0.3 ± 0.5 | 1.8 | 2.7 ± 4.7 | 8.3 |
| Lung | 0 | 0 | 0.3 ± 0.8 | 1.1 | 0.3 ± 0.5 | 1.0 | 0 | 0 | 1.2 ± 1.2 | 5.3 |
| Body soak | 0.2 ± 0.4 | 0.3 | 0.7 ± 0.5 | 3.0 | 2.2 ± 2.2 | 6.5 | 0.7 ± 0.8 | 3.0 | 1.5 ± 1.6 | 10.6 |
| Total no. of larvae | 35.7 ± 20.2 | 22.0 ± 10.8 | 27.3 ± 17.7 | 25.2 ± 6.7 | 21.7 ± 15.2 | |||||
Mean larval recoveries in the hind limb are statistically significant from recoveries in remaining tissues at 3 h and 24 h (P < 0.05).
Values represent the means ± standard deviations and percent total larvae recovered by tissue type.
Migrating larvae elicit an early inflammatory response in the dermis.
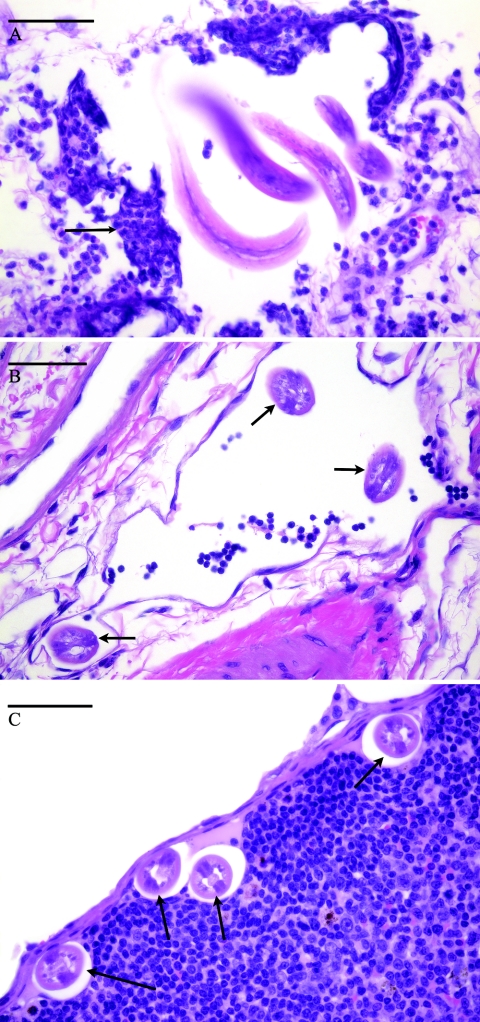
Immediately after larval inoculation (0 h), larvae were histologically visualized in the skin around the injection site. At later times, 3 h through 7 days, larvae were found in the skin and muscle near the injection site. Larvae were first found histologically in the popliteal lymph nodes at 3 h postinfection. The lymph nodes more distal to the injection site, the renal and subiliac lymph nodes, contained histologically identifiable larvae at 24 h postinfection. Larvae were found in all of these tissues after this time point. Multifocal, moderate dermatitis and rare, mild myositis were noted at 3 h and 24 h. The inflammatory infiltrate was comprised of neutrophils with mild to moderate numbers of eosinophils, occasional basophils, and rare mononuclear cells. Larvae visualized within the dermis were often surrounded by the inflammatory infiltrate (Fig. 1A), but not all areas of inflammation contained identifiable parasite material. Occasional larvae without associated inflammation at this site were also noted. At 3 and 7 dpi, inflammatory foci were occasionally noted, but most larvae had no associated inflammation. When present, inflammation at 3 and 7 dpi consisted primarily of macrophages, lymphocytes, and occasional plasma cells with lesser numbers of neutrophils and eosinophils. Larvae identified within the vascular system (lymphatics and veins) were not associated with an intravascular inflammatory response (Fig. 1B), but occasional perilymphatic inflammation was seen. Within lymph nodes, larvae were most frequently located in perinodal lymphatics and capsular sinuses, with occasional larvae noted within nodal parenchyma (Fig. 1C). Compared with control lymph nodes, no significant lymphadentitis was observed. All identified larvae appeared intact and nondegenerate. No significant inflammation was noted in any tissues removed from control gerbils injected i.d. with the final larval wash.
FIG. 1.
Histologic sections of Brugia pahangi L3 in various tissues during early migration. The bars shown indicate a size of 50 μm. (A) Larvae in the dermis of the left hind limb of a gerbil 3 h after i.d. infection with 100 B. pahangi L3. The arrow indicates inflammatory infiltrate surrounding the larvae that consists primarily of neutrophils with lesser numbers of eosinophils, basophils, and mononuclear cells. (B) Brugia pahangi larvae within a deep dermal lymphatic vessel of a gerbil 3 days after i.d. infection with 100 B. pahangi L3. Arrows indicate larvae within the lymphatic vessel. (C) Larvae in the subcapsular sinus of the left popliteal lymph node of a gerbil 7 days after i.d. infection with 100 B. pahangi L3. Arrows indicate larvae within the nodal sinus. No significant inflammation was apparent within the lymph node.
Gerbils show an early mixed cytokine response to migrating L3.
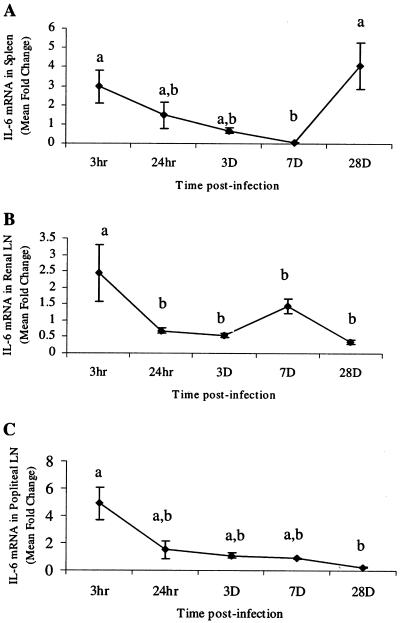
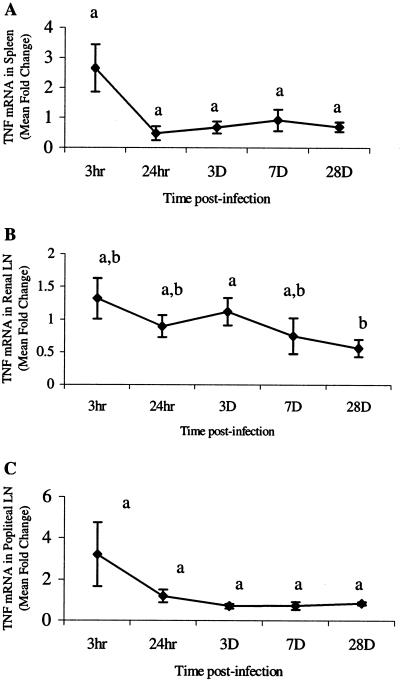
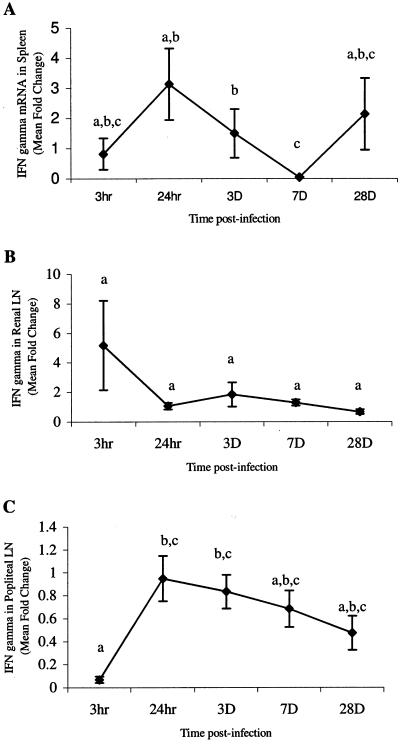
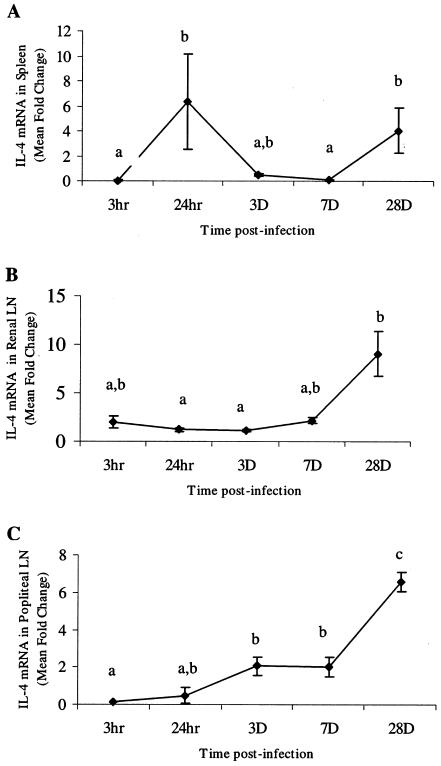
Cytokines that characterize proinflammatory events (IFN-γ, TNF-α, and IL-6) and Th2 mediator induction (IL-4) were chosen to measure early responses to parasite migration. Cytokine mRNA levels were quantified in the spleens, RLN, and PLN from gerbils at various time points after i.d. infection. In all tissues, IL-6 (Fig. 2) and TNF-α (Fig. 3) mRNA levels were elevated at 3 h, which was followed by a rapid decline. The IL-6 levels at 3 h were significantly higher (P < 0.05) than levels at later time points in the spleen (7 dpi), RLN (3 and 28 dpi), and PLN (28 dpi) (Fig. 2). Levels stayed low through 28 dpi in both PLN and RLN but rose in the spleen at 28 dpi. However, this value was not significantly different from the mRNA levels at 3 h, 24 h, or 3 dpi (Fig. 2A). Levels of IFN-γ mRNA were significantly elevated for only short periods of time (3 h and 24 h) in the spleen and PLN (Fig. 4). Levels of IL-4 mRNA were low through 7 dpi and then peaked at 28 dpi in all tissues (Fig. 5). An early peak in IL-4 levels was also seen in the spleen (24 h), which was significant compared to levels at 3 h and 7 dpi but not compared to levels at 3 dpi or 28 dpi (P < 0.05) (Fig. 5A).
FIG. 2.
Quantitation of IL-6 mRNA in spleens (A), renal lymph nodes (LN) (B), and popliteal lymph nodes (C). Gerbils were necropsied at 3 h, 24 h, 3 days, 7 days, and 28 days after i.d. inoculation with Brugia pahangi L3. mRNA levels were measured by reverse transcription (RT)-PCR, and values are expressed as mean severalfold changes compared with values from control animals. Superscript letters indicate statistical significance (P < 0.05). Values with the same letter are not statistically different.
FIG. 3.
Quantitation of TNF-α mRNA in spleens (A), renal lymph nodes (LN) (B), and popliteal lymph nodes (C). Gerbils were necropsied at 3 h, 24 h, 3 days, 7 days, and 28 days after i.d. inoculation with Brugia pahangi L3. mRNA levels were measured by RT-PCR, and values are expressed as the mean severalfold changes compared with values from control animals. Superscript letters indicate statistical significance (P < 0.05). Values with the same letter are not statistically different. No statistically significant differences were found in the spleen (P = 0.065) or popliteal lymph node (P = 0.2).
FIG. 4.
Quantitation of IFN-γ mRNA in spleens (A), renal lymph nodes (LN) (B), and popliteal lymph nodes (C). Gerbils were necropsied at 3 h, 24 h, 3 days, 7 days, and 28 days after i.d. inoculation with B. pahangi L3. mRNA levels were measured by RT-PCR, and values are expressed as mean severalfold changes compared with values from control animals. Superscript letters indicate statistical significance (P < 0.05). Values with the same letter are not statistically different. No statistically significant differences were found in the renal lymph nodes (P = 0.094).
FIG. 5.
Quantitation of IL-4 mRNA in spleens (A), renal lymph nodes (LN) (B), and popliteal lymph nodes (C). Gerbils were necropsied at 3 h, 24 h, 3 days, 7 days, and 28 days after i.d. inoculation with B. pahangi L3. mRNA levels were measured by RT-PCR, and values are expressed as the mean severalfold changes compared with values from control animals. Superscript letters indicate statistical significance (P < 0.05). Values with the same letter are not statistically different.
DISCUSSION
The events that occur during the first hours and days following filarial L3 infection are poorly understood. Likewise, the significance of these early events with regard to parasite establishment and disease progression remains to be determined. Laboratory animal models are uniquely suited to examining these questions. Previous studies have examined early filarial infection events, including parasite transfer from vector to host (16) and early larval recoveries prior to adult establishment in the lymphatics (1, 3, 5). However, these studies did not establish a quantitative, controllable in vivo model to study the events of early larval migration within mammalian hosts. Brugia species infection in the permissive Mongolian gerbil is well characterized, and the chronic state of infection resembles the microfilaremic asymptomatic state described in many human infections (19, 20, 25). Here, we utilize this model to further define inflammatory events provoked by L3 in naïve individuals.
The variety of locations in which larvae were recovered in our study supports the previous observations that B. pahangi L3 can migrate through various connective tissues (1). Furthermore, our studies indicate that filarial larval migration through the skin to the lymphatic system occurs rapidly, with large numbers of B. pahangi larvae located significant distances away from the infection site within 24 h. Locations where larvae were found included both peripheral (PLN and subinguinal lymph nodes) and central (RLN) lymph nodes. These findings are compatible with previous reports of Brugia L3 in regional lymphatics as early as 3 to 6 h after inoculation in cats (15) or 2.5 days in gerbils (3). Our subsequent studies have also demonstrated that developing L3, L4, and immature adult B. pahangi also possess the ability to migrate through these tissues (9).
In addition to parasite migration, we recorded important host events that occur soon after infection. Serial histological examination of tissues around the injection site and in distal lymph nodes demonstrated the induction of an inflammatory response consistent with an innate inflammatory event. This response was not seen in controls, which received media from the L3 collection process. These observations correlate with the observed initial increases in proinflammatory cytokines. A systemic inflammatory response, as determined by measurement of cytokine levels in the spleen and lymph nodes, was noted in gerbils within 3 h of L3 infection. This response consisted of elevated levels of mRNA from the acute proinflammatory cytokines TNF-α and IL-6 in all tissues. Recent work in vitro with live Brugia malayi L3 and human monocytes has shown that naïve T-cell activation occurs within 24 h (6). Our in vivo studies suggest that significant intrahost parasite migration has occurred within this time period. In addition, the human monocytes produced a predominantly Th1 cytokine response during this time, consisting of elevated levels of IFN-γ, TNF, granulocyte-macrophage colony-stimulating factor, IL-1α, and IL-8 (6). It is interesting that only exposure to live L3, not L3 antigen, induced this proinflammatory cytokine profile, suggesting that physical contact of the larvae with host cells may play an important role in the immune response. Similarly, we suggest that larval migration through dermal tissues in vivo results in the induction of the early, short-lived inflammatory response seen in i.d. infected gerbils.
The cellular source of the proinflammatory cytokines present early in filarid infection has not yet been determined, although monocytes have been strongly implicated (6, 28, 33). In addition, Semnani et al. (31) found that human Langerhans cells exposed to live L3 had decreased expression of IL-8 and antigen presentation genes but increased expression of IL-18, a cytokine involved in Langerhans cell migration. These findings suggest that resident antigen-presenting cells in the skin may play a role in establishing the cytokine environment during early filarid migration. Histologic examination of tissues from infected gerbils in our study showed prominent foci of neutrophils intradermally in infected animals within 3 h of infection. These foci were rarely noted at 3 dpi and were completely absent in control animals injected with larval wash medium. Interestingly, marked monocyte infiltration, as might be suggested by the in vivo studies noted above, was not observed in these lesions at any time postinfection.
It has been suggested that the inflammatory lesions seen in filariasis are mediated by LPS-like activity from Wolbachia, the endosymbiotic bacterium present in most filarid nematodes. Extracts of B. malayi have been found to elicit production of IL-1β, TNF, and nitric oxide from murine macrophages due to an LPS-like component of the parasite extract (34). Interestingly, live parasites do not stimulate inflammatory responses in murine macrophages (34), suggesting that Wolbachia must be released, probably upon the death of the filarid parasite in the host, before a macrophage response is mounted. Work with Onchocerca volvulus has also shown that adult worm extracts and live O. volvulus microfilariae injected into murine corneas elicit an innate inflammatory response to Wolbachia antigens mediated through Toll-like receptor 4 (30). Neutrophils are a major component of this inflammatory response in both human infections and the mouse model. In vivo and in vitro experiments have shown that Wolbachia induces human and murine neutrophil activation (7, 8, 18), and activated murine neutrophils can ingest Wolbachia following its release from O. volvulus (18). In the present study, we recovered only 22% of the larvae injected by 3 h postinfection, suggesting almost 80% attrition. Notably, almost all of the L3 that were recovered at this time were still associated with the area around the injection site. Histologic examination also showed the largest number of neutrophil-rich inflammatory foci and L3 in the hind limb skin and muscle at 3 h. It is possible that the early innate host response mounted against migrating L3 results in larval death, Wolbachia release, and subsequent neutrophil activation and accumulation. Alternatively, the neutrophil infiltration may be a response to Wolbachia released prior to larval death. Intact, live adult O. volvulus microfilariae in nodules of infected patients are often found surrounded by neutrophil infiltrates. These neutrophil numbers diminish in patients following treatment with doxycycline and Wolbachia reduction or elimination (27). These data suggest that intact adult O. volvulus may release sufficient Wolbachia to induce neutrophil migration and activation. However, it must be noted that Wolbachia is found concentrated in the reproductive tract of adult female filariae and may be released during the release of microfilariae. In addition, filarial L3 contain the lowest numbers of Wolbachia compared to other life cycle stages found in the vertebrate host (22). Whether these levels are sufficient to induce the observed neutrophil infiltration is yet to be determined.
Even though migrating L3 induce a rapid initial inflammatory response, some larvae do survive, migrate into lymphatics, and successfully establish infection, as indicated by worm recoveries at 28 dpi, which are comparable to those seen in gerbils following single or multiple infections (11, 12, 20, 22). Furthermore, protective immunity does not develop in this system as noted above (11, 21, 23), and multiple infections do not promote the induction of Th1 responses (11). While it is possible that the neutrophil-dominated initial inflammatory response seen in gerbils i.d. inoculated with L3 is involved in the innate killing of incoming larvae, it may also be a response mounted by the host to larvae, which die from non-host-mediated events. Regardless of the mechanism, the marked reduction in L3 seen indicates that the majority of larvae die early after infection.
Individuals in regions where filariasis is endemic are constantly exposed to bites from infected vector mosquitoes, and so the average human in these areas is likely to have multiple parasite exposures. In addition, the Th1/Th2 polarization issue is further complicated by the fact that individuals in areas of endemicity have likely been exposed to multiple life cycle stages simultaneously. We have found that a marked Th2 polarization, as determined by increased IL-4 cytokine levels, occurs later in infection (between 7 and 28 dpi) and that the proinflammatory response dominates the early period after infection. While this response was only measured in naïve animals, previous studies have shown that gerbils with multiple B. pahangi infections, and therefore carrying multiple life cycle stages, show cytokine profiles similar to those of gerbils with primary B. pahangi infections (11, 12). In both cases, the highest levels of IL-4 mRNA were observed in chronically infected animals (>130 days postinfection).
In conclusion, filarid larval migration occurs rapidly and stimulates a host systemic inflammatory response within 3 h of exposure to migrating larvae. This response is characterized by elevated proinflammatory cytokines (TNF and IL-6) and dermal neutrophilic foci. The classically described Th2-polarized immune response occurs after 7 days postinfection. These results emphasize the importance of the first few days of infection in the progression of, and response to, filariasis. The factors that determine the character of the cytokine environment and regulation of the resulting inflammatory response are still unknown. Likewise, the role of vector components in modulating host response and the potential for differing host responses to different parasite life cycle stages provide interesting paths for future research in this system. In all cases, it must be emphasized that these observations were made in parasite-naïve individuals and that similar responses in animals with existing infections are yet to be described.
Acknowledgments
We thank William Henk for his assistance in preparing the photographic figures.
This work was supported in part by NIH grant AI-19199.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Ah, H. S., T. R. Klei, J. W. McCall, and P. E. Thompson. 1974. Brugia pahangi infections in Mongolian jirds and dogs following the ocular inoculation of infective larvae. J. Parasitol. 60:643-648. [PubMed] [Google Scholar]
- 2.Reference deleted.
- 3.Ah, H. S., and P. E. Thompson. 1973. Brugia pahangi: infections and their effect on the lymphatic system of Mongolian jirds (Meriones unguiculatus). Exp. Parasitol. 34:393-411. [DOI] [PubMed] [Google Scholar]
- 4.Ash, L. R. 1973. Chronic Brugia pahangi and Brugia malayi infections in Meriones unguiculatus. J. Parasitol. 59:442-447. [PubMed] [Google Scholar]
- 5.Ash, L. R., and J. M. Riley. 1970. Development of subperiodic Brugia malayi in the jird, Meriones unguiculatus, with notes on infections in other rodents. J. Parasitol. 56:969-973. [PubMed] [Google Scholar]
- 6.Babu, S., and T. B. Nutman. 2003. Proinflammatory cytokines dominate the early immune response to filarial parasites. J. Immunol. 171:6723-6732. [DOI] [PubMed] [Google Scholar]
- 7.Brattig, N. W., C. Bazzocchi, C. J. Kirschning, N. Reiling, D. W. Buttner, F. Ceciliani, F. Geisinger, H. Hochrein, M. Ernst, H. Wagner, C. Bandi, and A. Hoerauf. 2004. The major surface protein of Wolbachia endosymbionts in filarial nematodes elicits immune responses through TLR2 and TLR4. J. Immunol. 173:437-445. [DOI] [PubMed] [Google Scholar]
- 8.Brattig, N. W., D. W. Buttner, and A. Hoerauf. 2001. Neutrophil accumulation around Onchocerca worms and chemotaxis of neutrophils are dependent on Wolbachia endobacteria. Microbes Infect. 3:439-446. [DOI] [PubMed] [Google Scholar]
- 9.Chirgwin, S. R., S. Coleman, K. H. Porthouse, and T. R. Klei. 2006. Tissue migration capability of larval and adult Brugia pahangi. J. Parasitol. 92:46-51. [DOI] [PubMed] [Google Scholar]
- 10.Chirgwin, S. R., P. H. Elzer, S. U. Coleman, J. M. Nowling, S. D. Hagius, M. D. Edmonds, and T. R. Klei. 2002. Infection outcome and cytokine gene expression in Brugia pahangi-infected gerbils (Meriones unguiculatus) sensitized with Brucella abortus. Infect. Immun. 70:5938-5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chirgwin, S. R., U. R. Rao, S. U. Coleman, J. M. Nowling, and T. R. Klei. 2005. Profiling the cellular immune response to multiple Brugia pahangi infections in a susceptible host. J. Parasitol. 91:822-829. [DOI] [PubMed] [Google Scholar]
- 12.Chirgwin, S. R., U. R. Rao, Z. Mai, S. U. Coleman, J. M. Nowling, and T. R. Klei. 2005. Kinetics of T cell cytokine gene expression in gerbils after a primary subcutaneous Brugia pahangi infection. J. Parasitol. 91:264-268. [DOI] [PubMed] [Google Scholar]
- 13.Denham, D. A., and P. B. McGreevy. 1977. Brugian filariasis: epidemiological and experimental studies. Adv. Parasitol. 15:243-309. [DOI] [PubMed] [Google Scholar]
- 14.Ewert, A. 1971. Distribution of developing and mature Brugia malayi in cats at various times after a single inoculation. J. Parasitol. 57:1039-1042. [PubMed] [Google Scholar]
- 15.Ewert, A., and S. el-Bihari. 1971. Rapid recovery of Brugia malayi larvae following experimental infection of cats. Trans. R. Soc. Trop. Med. Hyg. 65:364-368. [DOI] [PubMed] [Google Scholar]
- 16.Ewert, A., and B. C. Ho. 1967. The fate of Brugia pahangi larvae immediately after feeding by infective vector mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 61:659-662. [DOI] [PubMed] [Google Scholar]
- 17.Ewert, A., C. C. Wu, and P. C. Fan. 1987. Laboratory transmission of lymphatic filariasis by vector mosquitoes. Southeast Asian J. Trop. Med. Public Health 18:73-78. [PubMed] [Google Scholar]
- 18.Gillette-Ferguson, I., A. G. Hise, H. F. McGarry, J. Turner, A. Esposito, Y. Sun, E. Diaconu, M. J. Taylor, and E. Pearlman. 2004. Wolbachia-induced neutrophil activation in a mouse model of ocular onchocerciasis (river blindness). Infect. Immun. 72:5687-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeffers, G. W., T. R. Klei, F. M. Enright, and W. G. Henk. 1987. The granulomatous inflammatory response in jirds, Meriones unguiculatus, to Brugia pahangi: an ultrastructural and histochemical comparison of the reaction in the lymphatics and peritoneal cavity. J. Parasitol. 73:1220-1233. [PubMed] [Google Scholar]
- 20.Klei, T. R., F. M. Enright, K. C. McDonough, and S. U. Coleman. 1988. Brugia pahangi: granulomatous lesion development in jirds following single and multiple infections. Exp. Parasitol. 66:132-139. [DOI] [PubMed] [Google Scholar]
- 21.Klei, T. R., K. C. McDonough, S. U. Coleman, and F. M. Enright. 1987. Induction of lymphatic lesions by Brugia pahangi in jirds with large and small preexisting homologous intraperitoneal infections. J. Parasitol. 73:290-294. [PubMed] [Google Scholar]
- 22.Klei, T. R., C. S. McVay, V. A. Dennis, S. U. Coleman, F. M. Enright, and H. W. Casey. 1990. Brugia pahangi: effects of duration of infection and parasite burden on lymphatic lesion severity, granulomatous hypersensitivity, and immune responses in jirds (Meriones unguiculatus). Exp. Parasitol. 71:393-405. [DOI] [PubMed] [Google Scholar]
- 23.Lin, D. S., S. U. Coleman, U. R. Rao, and T. R. Klei. 1995. Absence of protective resistance to homologous challenge infections in jirds with chronic, amicrofilaremic infections of Brugia pahangi. J. Parasitol. 81:643-646. [PubMed] [Google Scholar]
- 24.McGarry, H. F., G. L. Egerton, and M. J. Taylor. 2004. Population dynamics of Wolbachia bacterial symbionts in Brugia malayi. Mol. Biochem. Parasitol. 135:57-67. [DOI] [PubMed] [Google Scholar]
- 25.McVay, C. S., T. R. Klei, S. U. Coleman, and S. C. Bosshardt. 1990. A comparison of host responses of the Mongolian jird to infections of Brugia malayi and B. pahangi. Am. J. Trop. Med. Hyg. 43:266-273. [DOI] [PubMed] [Google Scholar]
- 26.Osborne, J., and E. Devany. 1998. The L3 of Brugia induces a Th2-polarized response following activation of an IL-4-producing CD4−CD8− αβ T cell population. Int. Immunol. 10:1583-1590. [DOI] [PubMed] [Google Scholar]
- 27.Pearlman, E., L. R. Hall, A. W. Higgins, D. S. Bardenstein, E. Diaconu, F. E. Hazlett, J. Albright, J. W. Kazura, and J. H. Lass. 1998. The role of eosinophils and neutrophils in helminth-induced keratitis. Investig. Ophthalmol. Vis. Sci. 39:1176-1182. [PubMed] [Google Scholar]
- 28.Raman, U., D. Eswaran, R. B. Narayanan, K. Jayaraman, and P. Kaliraj. 1999. Proinflammatory cytokines secreted by monocytes of filarial patients. Microbiol. Immunol. 43:279-283. [DOI] [PubMed] [Google Scholar]
- 29.Reference deleted.
- 30.Saint, A. A., N. M. Blackwell, L. R. Hall, A. Hoerauf, N. W. Brattig, L. Volkmann, M. J. Taylor, L. Ford, A. G. Hise, J. H. Lass, E. Diaconu, and E. Pearlman. 2002. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science 295:1892-1895. [DOI] [PubMed] [Google Scholar]
- 31.Semnani, R. T., M. Law, J. Kubofcik, and T. B. Nutman. 2004. Filaria-induced immune evasion: suppression by the infective stage of Brugia malayi at the earliest host-parasite interface. J. Immunol. 172:6229-6238. [DOI] [PubMed] [Google Scholar]
- 32.Semnani, R. T., and T. B. Nutman. 2004. Toward an understanding of the interaction between filarial parasites and host antigen-presenting cells. Immunol. Rev. 201:127-138. [DOI] [PubMed] [Google Scholar]
- 33.Semnani, R. T., H. Sabzevari, R. Iyer, and T. B. Nutman. 2001. Filarial antigens impair the function of human dendritic cells during differentiation. Infect. Immun. 69:5813-5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor, M. J., H. F. Cross, and K. Bilo. 2000. Inflammatory responses induced by the filarial nematode Brugia malayi are mediated by lipopolysaccharide-like activity from endosymbiotic Wolbachia bacteria. J. Exp. Med. 191:1429-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]