Abstract
Cimetidine is a powerful H2 receptor antagonist that eliminates histamine's effects on chemotaxis, phagocytosis, and superoxide anion production by phagocytes. The purpose of this study was to analyze the clinical and histopathological changes associated with experimental periodontitis in rabbits in response to topically applied cimetidine. Experimental periodontitis was induced in 21 New Zealand White rabbits using Porphyromonas gingivalis (109 CFU) topically applied three times a week for a 6-week period to previously ligatured teeth. Topical application of cimetidine in a liposome carrier for the prevention of periodontitis was evaluated in four groups of four animals each: 1, 10, and 100 mg/ml and no treatment (positive control). In addition, there was a vehicle group (n = 3) that received liposome preparation (carrier) only, and two animals with ligature application alone served as negative controls. Periodontal disease was quantified by direct visualization and radiographical evaluation of bone loss on defleshed skulls and by histological analyses of sections stained with hematoxylin-eosin and tartrate-resistant acid phosphatase. In the no-treatment (positive control) and liposome (vehicle) groups, direct visualization and radiological measurements revealed statistically significant bone loss compared to the negative control. Application of cimetidine at all concentrations tested inhibited inflammation and bone loss by >90%. Histological findings revealed that ligated sites of the positive control and vehicle groups showed significant reduction in bone level (P < 0.05) compared to the three cimetidine groups, with a marked decrease in inflammation. The findings of this study provide morphological and histological evidence that topically active cimetidine is a potent inhibitor of P. gingivalis-elicited periodontal inflammation, arresting and/or preventing tissue destruction and influencing cell populations present in the inflammatory cell infiltrate.
Periodontitis is an inflammatory disease initiated by bacterial-biofilm accumulation on teeth that leads to the loss of connective tissue attachment to teeth and resorption of alveolar bone. Different types of periodontal disease affect 15 to 35% of the U.S. population, which translates into tens of millions of patients (1). The most common form of periodontal disease is observed in adults and shows chronic progression (9). The progression of periodontal disease relies on persistence and chronicity of the host response. Out of the hundreds of bacterial species present in the oral cavity, only a small number have been implicated in the etiology of periodontal disease (34). Porphyromonas gingivalis, Tannerella forsythensus, and Treponema denticola are strikingly associated with clinical measures of periodontal disease, particularly with pocket depth and bleeding on probing (35). When the virulent bacteria begin to flourish in the periodontal region, toxic and pathogenic products are released and induce an inflammatory response. Some of the pathogenic organisms can further invade the periodontal tissues, dentinal tubules, and other areas that are difficult to debride, and as a result, mechanical treatment alone may not be successful. Research over the last few decades has also shown that the host plays an important role in the initiation and progression of periodontal diseases, similar to other inflammatory diseases elsewhere in the body. Thus, a modern approach to the treatment of periodontal diseases should target both the elimination of the pathogenic bacteria and modification of the host response in an attempt to address both the etiology and pathogenesis of periodontal disease (28, 29, 38).
In response to specific stimuli, inflammatory cells, including polymorphonuclear leukocytes, monocytes, lymphocytes, macrophages, mast cells, and plasma cells, are recruited to infiltrate the periodontium and clear the area of the pathogenic organisms (16). Although extensive research has been focused on the cellular components of inflammation, early, critical cell-mediated events remain poorly understood. Mast cells play an important role in the early propagation of the inflammatory response due to the cytoplasmic granules that contain critical substances, such as histamine, slowly reacting substances of anaphylaxis, heparin, eosinophil chemotactic factor of anaphylaxis, and bradykinin, all of which are released into gingival tissues (22). One of the most important mast cell-derived mediators of inflammation, histamine, exerts its biologic actions by binding to specific cellular receptors located on the cell surface. Four different histamine receptors have been characterized and designated H1, H2, H3, and H4 (20). The H1 and H2 receptors belong to the superfamily of G-protein-coupled receptors, and H2 receptors are linked to the stimulation of adenylyl cyclase and thus to the activation of cyclic-AMP-dependent protein kinases in the target cell (10). Histamine alters a variety of neutrophil, macrophage, and monocyte functions, mediated through the binding of H2 receptors on the cell surface (18, 25).
Cimetidine is a specific competitive H2 receptor antagonist that is used for the treatment of peptic ulcers (2, 40). In AIDS patients, cimetidine administration has been shown to have a significant effect, improving clinical symptoms of disease (8). It eliminates histamine's effects on chemotaxis, phagocytosis, superoxide anion production, and the secretion of tumor necrosis factor alpha (TNF-α) and interleukin-12 (IL-12) by macrophages via the H2 receptor (19, 20, 37). Cimetidine completely reverses the histamine-mediated increase in IL-1α-induced IL-6 synthesis (23, 39). The proposed mechanism of the immunomodulative effects of H2 receptor antagonists has been suggested to be mediated through inhibition of suppressor T-lymphocyte activity, an increase in IL-2 production, and an enhancement of natural killer cell activity (12, 17). Administration of 800 mg cimetidine daily for a period of 7 days to healthy volunteers showed a decrease in CD8 (cytotoxic/suppressor) lymphocytes, along with a corresponding increase in CD4 (helper/inducer) lymphocytes (6, 7).
Previous work by our group has shown that a predictable and reproducible periodontitis can be generated in rabbits by using silk ligatures accompanied by the topical application of the periodontitis-specific microorganism P. gingivalis (19). P. gingivalis, a gram-negative black-pigmented microorganism, has been implicated as the major pathogen in the development of periodontitis in this model (19, 32). Thus, in this study, we sought to evaluate the effects of topical cimetidine application on P. gingivalis- and ligature-induced periodontitis in the rabbit model using morphometric, radiologic, histopathologic, and histomorphometric analyses.
MATERIALS AND METHODS
Animal model.
The study protocol and experimental design were reviewed and approved by the Boston University Medical Center Institutional Animal Care and Use Committee (BUMC IACUC) prior to study initiation (IACUC protocol AN-13948). In addition, the BUMC Institutional Biohazard Committee approved the use of P. gingivalis in this animal model to induce periodontal disease (Institutional Biohazard Committee protocol A-269). In total, 21 New Zealand White rabbits (male; 3.5 to 4.0 kg each) were used in the experiment. Three different doses of cimetidine (1, 10, and 100 mg/ml) were prepared in a liposome carrier by IGI, Inc. (Buena, NJ). Cimetidine-liposome formulations were prepared by encapsulating three different doses of cimetidine within novasomes (liposome vesicles) using purified water, caprylic/capric triglyceride, glyceryl stearate, ethoxydiglycol, polyglyceril-6 distearate, polysorbate 80K, and glyceryl distearate glycine soy (soybean stearol, oleic acid, and potassium sorbate). The levels of cimetidine within the liposomal vesicles used in groups D, E, and F were 1 mg/ml, 10 mg/ml, and 100 mg/ml, respectively. Each application site (the buccal and lingual sides of each tooth) received 0.1 ml of compound. The animals were divided into treatment groups as follows: group A, ligature alone (two rabbits); group B, ligature plus P. gingivalis (four rabbits; positive control); group C, ligature plus P. gingivalis plus vehicle (liposome) (three rabbits); group D, ligature plus P. gingivalis plus cimetidine 0.1 mg/site (four rabbits); group E, ligature plus P. gingivalis plus cimetidine 1 mg/site (four rabbits); and group F, ligature plus P. gingivalis plus cimetidine 10 mg/site (four rabbits). All animals were purchased from Pine Acre Farms (Norton, MA). The weights of the animals were monitored, and all animals weighed between 3.5 and 4.0 kg at the initiation of the experiment. The animals were kept in individual cages, received water ad libitum, and were fed Purina Rabbit Chow for at least 5 days before the experiment for acclimatization. The animals were cared for by experienced and licensed laboratory technicians at the Laboratory Animal Science Center at BUMC.
Experimental periodontitis.
Ligature placement was performed under general anesthesia using 40-mg ketamine/kg of body weight (Ketaset; Fort Dodge Animal Health, Fort Dodge, Iowa) and 5-mg/kg xylazine (AnaSed; Ben Venue Laboratories, Bedford, OH) injections. A 3-0 silk suture was placed around the second premolar of both mandibular quadrants. Group A received only ligatures, while groups B, C, D, E, and F received P. gingivalis in addition to ligature placement. P. gingivalis (strain A7436) was grown as previously described (19). Briefly, bacteria were cultured on agar plates containing trypticase soy agar (Gibco Industries, Inc., Los Angeles, CA) supplemented with 0.5% (wt/vol) yeast extract, 5% defibrinated sheep red blood cells (Sigma-Aldrich Co., St. Louis, MO), 5 μg hemin (Sigma-Aldrich Co., St. Louis, MO), and 1 μg/ml vitamin K (Sigma-Aldrich Co., St. Louis, MO). The plates were incubated for 3 days at 37°C in jars anaerobically maintained through palladium-catalyzed hydrogen-carbon dioxide envelopes (GasPak Plus; BD Microbiology Systems, Sparks, MD). Colonies were randomly selected and anaerobically cultured overnight at 37°C in Schaedler's broth (Gibco Industries, Inc., Los Angeles, CA) supplemented with vitamin K and hemin. Bacterial numbers were spectrophotometrically determined at 600 nm, adjusted to 109 CFU (optical density at 600 nm) and mixed with carboxymethylcellulose (Sigma-Aldrich Co., St. Louis, MO) to form a thick slurry, which was applied topically to the ligated teeth. The sutures were checked at every application, and lost or loose sutures were replaced.
Topical application of cimetidine.
Topical application of the cimetidine/liposome preparation was performed in groups D, E, and F every other day for 6 weeks at the same time as P. gingivalis application under inhalation anesthesia using isoflurane (4% induction and then 2% maintenance). Animals in group C received liposomes without cimetidine in addition to ligature placement and P. gingivalis. At the end of the study, the animals were euthanized using an overdose of pentobarbital (Euthanasia-5 Solution; Veterinary Laboratories, Inc., Lenexa, Kansas) (120 mg/kg) according to the protocol approved by the IACUC. No adverse events were observed during experimental procedures throughout the study with regard to animal care, and no animals were prematurely lost during the study.
Morphometric analysis.
After the animals were sacrificed, the mandible was dissected free of the muscles and the soft tissue, keeping the attached gingiva intact with the bone. Then, the mandible was split into halves from the midline between the central incisors. Half was taken for morphometric analysis by direct visualization, and the other half was used for histological evaluation. For direct visualization, the mandible was defleshed by immersion in 10% hydrogen peroxide (3 to 4 days at room temperature). The soft tissue was removed carefully, and then the mandible was stained with methylene blue for good visual distinction between the tooth and the bone. Next, the bone level around the second premolar was measured directly using a 0.5-mm calibrated periodontal probe. Measurements were made at three points on both the buccal and lingual sides to quantify the crestal bone level. A mean crestal bone level around the tooth was calculated. Similarly, for the proximal bone levels, measurements were made on the mesial and distal aspects of the tooth. The measurements were taken from both the buccal and lingual sides on both proximal aspects of the second premolar, and the mean proximal bone level was calculated. The bone level was also quantified by image analysis (Image-Pro Plus 4.0; Media Cybernetics, Silver Spring, MD). The sectioned mandible was mounted and photographed using an inverted microscope at ×10 magnification. The captured image was also analyzed as described above, and the mean crestal bone level around the tooth was calculated in millimeters.
Radiographic analysis.
The percentage of the tooth within the bone was calculated radiographically using a modification of the Bjorn technique (5, 19). The radiographs were taken with a digital X-ray machine (Schick Technologies Inc., Long Island, NY). To quantify bone loss, the length of the tooth from the cusp tip to the apex of the root was measured, as was the length of the tooth structure outside the bone, measured from the cusp tip to the coronal extent of the proximal bone. From this, the percentage of the tooth within the bone was calculated. Bone values are expressed as the percentage of the tooth in the bone (length of tooth in bone × 100/total length of tooth).
Histological analysis.
For histological analysis, the other half of the mandible was immersed in a volume of Immunocal (Decal Corporation, Tallman, NY) at least 10 times the size of the specimen; the solution was replaced every 24 h for 2 weeks. Decalcification was confirmed by serial radiographs, which were taken every other day. After decalcification, the tissues were rinsed for 1 to 3 min in running water, placed in Cal-Arrest (Decal Corporation, Tallman, NY) in order to neutralize the pH of the tissue, to enhance embedding and staining characteristics, and to stop further decalcification. The tissue was kept in this solution for 2 to 3 min, rinsed again in flowing deionized water for at least 3 min, and kept in formalin for at least 24 h before being embedded in paraffin. Thin sections (5 μm) were cut and stained either with hematoxylin-eosin (HE) to identify the cellular composition of the inflammatory infiltrates or with tartrate-resistant acid phosphatase (TRAP) to detect osteoclastic activity. For each analysis, 5 slides were used per sample at 20 intervals, and the averages of these measurements were calculated.
Superoxide generation.
Superoxide release was monitored spectrophotometrically at 37°C by measuring superoxide dismutase-inhibitable reduction of ferricytochrome c at 550 nm (11). Assays were carried out in 96-well microtiter plates with flat-bottom wells (Linbro type; Flow laboratories, McLean, VA). Control wells contained all of the components of the assay mixture plus superoxide dismutase (20 U/ml) to assess ferricytochrome c reduction by agents other than O2−. Human neutrophils (∼1.5 × 105 cells) were suspended in phosphate-buffered saline (200 μl/well) and stimulated by the addition of N-formyl-methionyl-leucyl-phenyalanine (fMLP; 10−6 M), and the absorbance at 550 nm was recorded in a Vmax kinetic microplate reader (Molecular Devices). Superoxide generation was monitored as a linear rate with respect to both time and cell number and is expressed as nmol O2−/min/105 neutrophils.
Statistical analysis.
Mean values for linear and area measurements were utilized to determine the changes in bone level. For histomorphometric analysis, mean counts obtained from five slides per sample were used to represent the sample. As shown previously by our group (32), ligature placement does not lead to the induction of periodontal disease in rabbits. Therefore, in this study, this group (ligature alone) was intentionally small to serve as an internal control and was not used for comparison between groups. Ratio calculations were used, and multiple comparisons between all other groups were made using analysis of variance (ANOVA) with Bonferroni correction.
RESULTS
Macroscopic analysis.
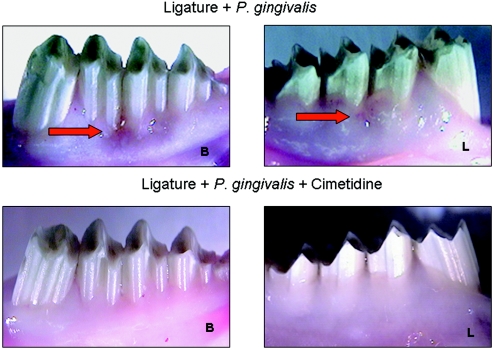
Figures 1 and 2 show the soft and hard tissue changes in the various treatment groups. Figure 1 demonstrates the gingival tissue from the buccal and lingual aspects. In the upper image, the clinical features of localized gingival inflammation, including redness, gingival recession, irregularities of the gingival margin, and edema associated with ligatures and P. gingivalis application, are shown. However, application of cimetidine (Fig. 1, bottom) prevented the soft tissue changes associated with periodontitis. Figure 2 demonstrates the hard tissue changes on the defleshed bone specimens from the buccal and lingual aspects. Similarly, in the upper image, the placebo group exhibited localized bone loss. Overall, ligature placement without additional P. gingivalis application did not lead to any significant soft or hard tissue changes in rabbit mandibles (group A; not shown). Topical delivery of three different doses of cimetidine prevented both gingival inflammation and bone destruction in significant and comparable ways, with no apparent dose-dependent effect (Fig. 1 and 2).
FIG. 1.
Visual evaluation of soft tissue changes in the areas where a ligature and P. gingivalis were applied to induce periodontitis. Ligature placement with additional P. gingivalis application led to significant soft tissue changes in rabbit mandibles (top) (groups B and C). The red arrows depict the gingival inflammation on the buccal (B) and lingual (L) aspects of the teeth, where the ligature and P. gingivalis were applied. Topical delivery of three different doses of cimetidine before P. gingivalis application prevented gingival inflammation in significant and comparable ways, with no apparent dose-dependent effect (bottom) (groups D, E, and F).
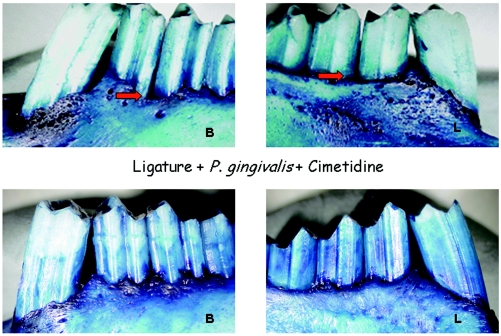
FIG. 2.
Visual evaluation of hard tissue changes on defleshed bone specimens. Defleshed bone specimens were stained with methylene blue to indicate the changes in bone level in the areas where periodontitis was induced. Similar to soft tissue changes, the red arrows depict significant bone loss on both aspects (buccal [B] and lingual [L]) of the teeth in groups B and C. Conversely, topical application of cimetidine before P. gingivalis application prevented bone destruction in a significant way with no apparent dose-dependent effect (bottom) (groups D, E, and F).
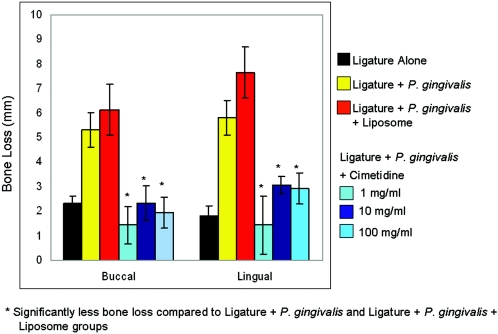
The quantitative analyses of defleshed bone specimens appear in Fig. 3. Cimetidine application prevented periodontitis in rabbits (P < 0.05; ANOVA). Again, the prevention by cimetidine occurred at all three doses with no apparent difference between doses.
FIG. 3.
Quantitative analyses of defleshed bone specimens. Preventive effects of cimetidine on P. gingivalis and ligature-induced experimental periodontitis in rabbits are statistically significant compared to animals that received liposome (vehicle) as a placebo, where the bone loss was significantly higher (P < 0.05; ANOVA).
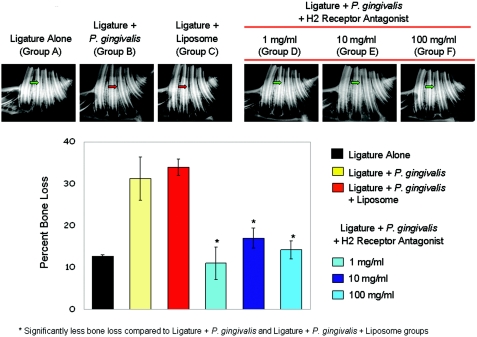
Radiographic analysis.
A radiographic analysis of bone and other hard tissue components is seen in Fig. 4. On top is shown the bone loss in animals that received ligature alone (A, negative control) or ligature plus P. gingivalis (B, positive control) and in animals that received ligature plus P. gingivalis and liposome (C, placebo). Bone loss is visible and significantly different compared to animals that received ligature alone (A). Topical application of cimetidine (all three doses) prevented bone loss, and the radiographs of alveolar bone revealed bone levels the same as those of animals that received the ligature application alone (D, E, and F). The graph shows the percentage of bone loss as calculated by the Bjorn technique. This measurement further confirms that cimetidine application quantitatively prevents the destructive effects of P. gingivalis-induced periodontitis (P < 0.05). No significant difference was found between the three doses of cimetidine used.
FIG. 4.
Radiographic analyses of bone and other hard tissue components. The upper panel demonstrates the bone loss in animals that had received ligature placement plus P. gingivalis and in animals that had received ligature placement plus P. gingivalis and vehicle (liposome) (groups B and C). The green arrow depicts the normal bone level, while bone loss (red arrow) is visible and significantly different than in animals that had received ligature alone (group A, green arrow). The lower panel depicts the percentage of bone loss as calculated by the Bjorn technique. Significant differences were found with cimetidine compared to liposome plus ligature or ligature alone (*, P < 0.05).
Histological analysis.
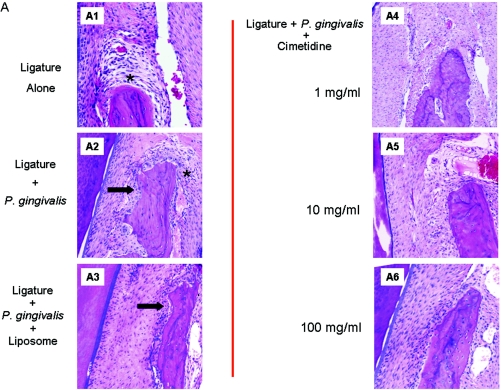
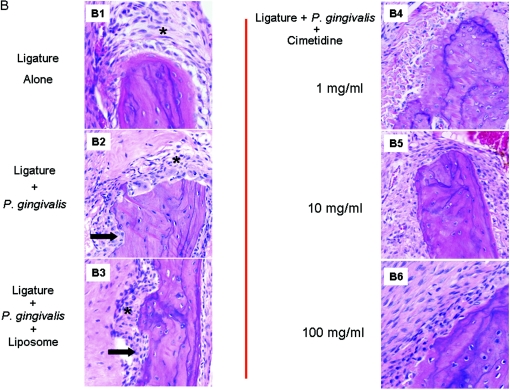
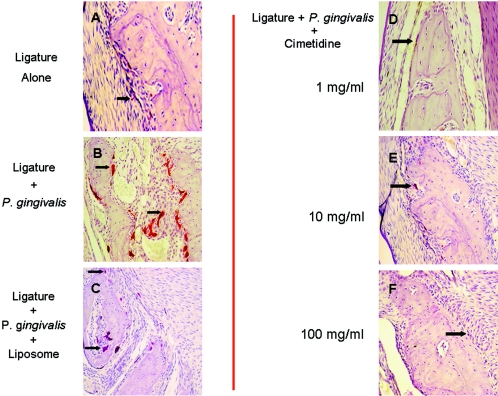
Figure 5A and B shows the histological changes in response to different treatments. Ligature placement alone around the second premolars of rabbit mandibles led to increased numbers of inflammatory cells, while neither bone loss nor any osteoclastic activity was visible (A1). Local P. gingivalis administration in addition to ligature placement (A2) led to significant bone resorption and increased inflammation. Hematoxylin- and eosin-stained sections of the ligated and diseased sites showed disrupted connective-tissue layers with irregular fiber arrangement. Numerous blood vessels and inflammatory cells were localized adjacent to the basal layer in the connective tissue. Dense inflammatory infiltration spread to the lamina dura of the alveolar bone, leading to bone destruction, and the alveolar borders were extremely ragged. The nonligated sites showed no evidence of bone loss. Liposomes alone did not have any preventive or aggravating effect on the development of periodontitis (A3). Higher magnification (×200) of the samples demonstrates the extent of inflammatory infiltrate (B1 to B3).
FIG. 5.
Histological changes in response to different treatments. (A) HE-stained sections of the ligated sites. Ligature placement alone around the second premolars of rabbit mandibles led to increased numbers of inflammatory cells (*), while neither bone loss nor any osteoclastic activity was visible (A1). Local P. gingivalis administration in addition to ligature placement (A2) led to significant bone resorption, as depicted by the black arrows, and increased inflammation. Liposome alone did not have any preventive or aggravating effect on the development of periodontitis (A3). All three doses of topical cimetidine applications (1, 10, and 100 mg/ml) prevented both bone loss and inflammatory changes in rabbits that received P. gingivalis and ligature placement (A4 to A6). (B) Higher magnification (×200) of histological response; inflammatory infiltration is clearly observed adjacent to the bone resorption areas (B2 and B3), and topical cimetidine groups present no evidence of cellular infiltrate (B4 to B6).
All three doses of topical cimetidine (1, 10, and 100 mg/ml) prevented both bone loss and inflammatory changes in rabbits that received P. gingivalis and ligature placement (5A4 to A6). In these groups, where cimetidine was applied at a dose of 1 to 100 mg/ml, the HE-stained sections showed intact epithelium; dense, well-defined connective-tissue fibers; fewer blood vessels; and markedly reduced inflammatory cells (A4 to A6). Alveolar bone borders were regular in most areas, and a few signs of alveolar bone resorption or resorptive lacunae were seen. The deposition of secondary bone on borders was also observed. No significant differences were observed between different doses of cimetidine. However, the higher-magnification images illustrate that all doses of cimetidine resulted in minimal infiltration of inflammatory cells adjacent to bone (B4 to B6) (P < 0.05).
Histomorphometrical analysis.
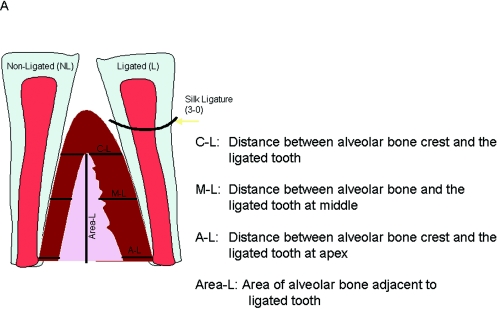
In order to quantify periodontal disease progression, the mean value (±standard deviation) of the linear distance and the area of bone loss were calculated for each group. Figure 6A illustrates the technique that was used to calculate the bone changes at three different sections of the root using ProImage software. The linear measurements were made at three levels, each corresponding to one-third of the root and alveolar bone interface: crestal, middle, and apical. Linear distance is reported as the distance from the base of the epithelium to the alveolar-crest border at the three chosen levels, the apical, the middle, and the coronal third of the root, and was expressed as the difference between ligated and nonligated sites. Likewise, area measurements were presented as the difference between the ligated and nonligated total areas.
FIG. 6.
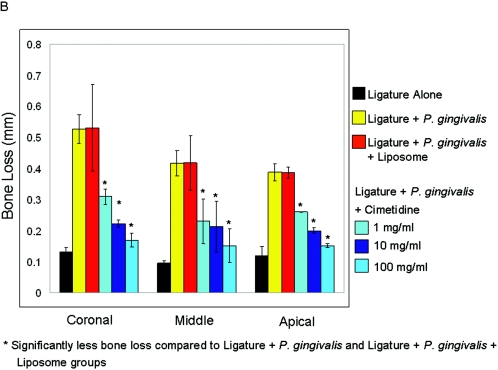
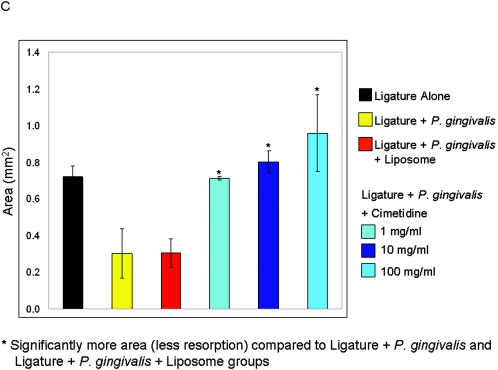
Schematic illustration of histometrical measurements of histologic sections. The histomorphometric measurements were performed at the ligated site of each tooth. The linear measurements were made at three points: crestal, middle, and apical third of the alveolar bone. The area measurements were also done on the ligated site of the alveolar bone (A). The ligated sites in the ligature plus P. gingivalis and ligature plus P. gingivalis plus liposome groups showed significantly increased (P < 0.05) distances compared to the cimetidine-treated groups (B). The total area, as well as the area of the ligated side, of the alveolar crest was significantly reduced in the control and vehicle groups (P < 0.05) (C).
The ligature plus P. gingivalis (positive control) and ligature plus P. gingivalis plus liposome (placebo) groups showed significantly increased (P < 0.05) distances compared to the cimetidine-treated groups, which indicates the destruction of the alveolar bone crest due to disease activity (Fig. 6B). The total area, as well as the area of the ligated side, of the alveolar crest was significantly reduced in the experimental-periodontitis and vehicle groups (P < 0.05) (Fig. 6C).
Osteoclastic cell activity.
The TRAP-stained sections of the ligated and diseased sites of the positive control group showed disrupted connective tissue and increased inflammatory cell infiltrate, especially at the alveolar bone borders. Ligation alone did not lead to any increase in osteoclast numbers (Fig. 7A). In the positive control (Fig. 7B), however, the alveolar bone borders were extremely ruffled, with increased numbers of irregularly shaped Howship's resorptive lacunae presenting osteoclast activity. Many multinucleated osteoclasts were seen in the resorptive areas (Fig. 7B). In the vehicle group, liposome alone, in addition to the experimental periodontitis, did not prevent osteoclastic activity (Fig. 7C). The TRAP-stained sections of this group showed the same descriptive histology as the experimental-periodontitis group. The disrupted connective tissue and increased inflammatory cell infiltrate were obvious, especially at the alveolar bone borders. The alveolar bone borders were extremely ruffled, with increased numbers of irregularly shaped Howship's resorptive lacunae presenting multinucleated osteoclastic activity.
FIG. 7.
TRAP-stained sections of ligature alone, ligature plus P. gingivalis, ligature plus P. gingivalis plus liposome, and three different applications of cimetidine. Ligation alone did not lead to any increase in osteoclast numbers (A). The alveolar bone borders were extremely ruffled, with increased numbers of irregularly shaped Howship's resorptive lacunae presenting osteoclastic activity (B). In the vehicle group, liposome alone in addition to the experimental periodontitis did not prevent osteoclastic activity (C). Osteoclastic cells were either unidentifiable or few in number in cimetidine groups (D to F).
In the cimetidine groups, osteoclasts were either unidentifiable or few in number (Fig. 7D and F). All three doses had a significant (P < 0.05) preventive effect on bone resorptive activity induced by periodontitis. Overall, the TRAP-stained sections showed intact epithelium and dense connective-tissue layers with few blood vessels. Intact, regular, and well-defined alveolar bone borders were seen in most areas, except for a few TRAP-positive cells. The deposition of secondary bone on the borders was seen. No signs of multinucleated osteoclastic activity were seen.
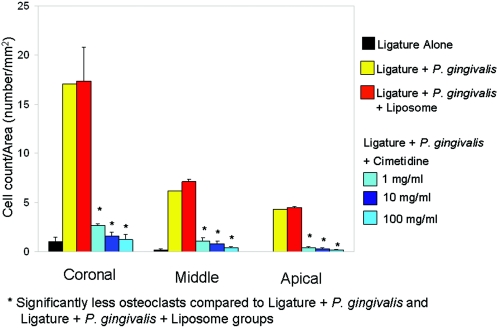
The numbers of osteoclasts at the apical, middle, and coronal thirds of the root were compared between the groups. The positive control and placebo groups presented markedly increased numbers of osteoclasts at all three levels (P < 0.05), whereas all cimetidine groups showed significant prevention of increase in osteoclast numbers at the apical, middle, and coronal thirds of the root (P < 0.05) (Fig. 8). There was no significant difference between cimetidine doses in preventing the osteoclastic activity (P > 0.05).
FIG. 8.
The ligature plus P. gingivalis and ligature plus P. gingivalis plus liposome groups presented markedly increased numbers of osteoclasts at all three levels with statistically significant values (P < 0.05), whereas the cimetidine groups showed comparable, nonsignificant values at the tip, middle, and base of the crest (P < 0.05).
Impacts of different concentrations of cimetidine on neutrophil superoxide generation.
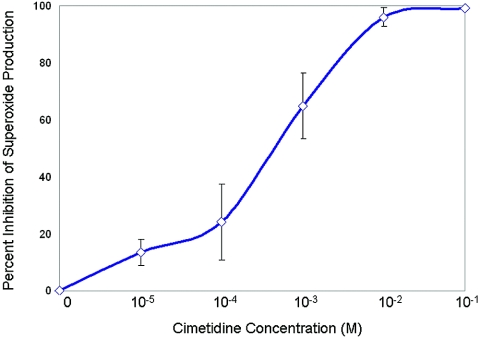
In order to determine the dose-response effects of cimetidine, neutrophils were incubated with various concentrations of cimetidine (10−1 to 10−5 M) and later challenged with fMLP (Fig. 9). The superoxide response to fMLP stimulation was blocked by cimetidine up to 97% in healthy neutrophils in a dose-dependent manner.
FIG. 9.
The data represent the means ± standard errors for neutrophils isolated from the peripheral blood of three healthy individuals without evidence of periodontal disease. Superoxide generation of neutrophils incubated with various concentrations of cimetidine (10−1 to 10−5 M) and challenged with fMLP was blocked by cimetidine up to 97%.
DISCUSSION
Periodontal diseases affect tens of millions of people, and tooth loss, as well as potential systemic effects of infection and local inflammation, as a result of periodontitis continues to be a public health problem. Although bacteria appear to cause periodontitis, the progression of the disease is dependent on the host response to pathogens that colonize the tooth surface. While the removal of bacteria reduces tissue destruction, not all individuals respond predictably to elimination of bacteria alone (9, 28). Therefore, in addition to bacterial control, adjunctive host modulation therapy may aid in the prevention of the disease or enhance clinical therapeutic responses in the susceptible host. Recent work has demonstrated that, in addition to bacterial control, modulation of the host's immunoinflammatory response is also capable of controlling periodontitis (17, 32, 33). The current study demonstrates that blocking H2 receptors prevents periodontitis at the clinical, histopathological, and histomorphometric levels.
In this study, we demonstrated that local administration of cimetidine in three different concentrations prevents tissue destruction and affects cell populations present in the inflammatory cell infiltrate associated with experimentally induced periodontitis in rabbits. The resulting histopathological and morphological observations showed that periodontitis was induced by topical application of P. gingivalis and ligature placement. These changes were prevented by topical application of an H2 receptor antagonist (cimetidine), while simultaneous topical administration of P. gingivalis was continued. There were statistically significant (P < 0.05) histomorphometric differences between the control (periodontitis), vehicle (liposome), and cimetidine (treatment) groups. The ligated sites of the positive control and vehicle groups showed significant differences in the linear distances from the epithelium to the alveolar-crest border at three levels—the apical, middle, and coronal thirds (P < 0.05)—compared to the other three groups. The mean ratio of the linear distances of the ligated sites to those of the nonligated sites of the vehicle group was significantly higher than for the other three groups (P < 0.05).
Overall, the results support the concept that histamine, which has an immunomodulatory action, may be involved in the regulation of the local acute inflammatory responses in periodontal disease. Furthermore, the findings of this study showed histological evidence that treatment of periodontally diseased teeth with topically active cimetidine was a potent inhibitor of P. gingivalis-elicited leukocyte migration toward the site of infection and therefore that it arrests or prevents tissue destruction and affects cell populations present in the inflammatory cell infiltrate.
Histamine's effect on inflammation may be due to direct or indirect effects on cells at early stages of inflammation and seems to be receptor regulated. While enhancing helper T-cell type 1 (TH1)-type responses via the H1 receptor, both TH1- and TH2-type responses are negatively regulated by H2 receptor activation (17). Histamine's effect on neutrophil granulocytes has been well documented and linked to inflammatory events. Histamine inhibits T-lymphocyte- and natural killer cell-mediated cytotoxicity (31). Histamine also altered chemotaxis of neutrophils and the production of superoxide anion, hydrogen peroxide formation, and degranulation of B-glucuronidase and lysozyme and stimulated changes in membrane potential (31). The effects of histamine on neutrophil motility were associated with increased levels of intracellular cyclic AMP. In a series of in vitro experiments, it was demonstrated that histamine in a range of 10 nM to 1 mM exerted a progressive and profound inhibition of neutrophil chemotaxis, an effect that could be eliminated by an H2 receptor antagonist (3). These data suggest that H2 receptors may play a pivotal role in regulating histamine-mediated inflammatory reactions and multiple physiological events extending from gastric acid secretion to tissue inflammation (25). Indeed, treatment with H2 receptor antagonists has been shown to increase neutrophil chemotaxis (3, 31). Cimetidine alters superoxide (O2−) and hydrogen peroxide (H2O2) production of neutrophils in a dose-dependent manner (24). We further tested different doses of cimetidine on superoxide anion production by human peripheral neutrophils in vitro. Cimetidine showed a clear and profound inhibition of superoxide produced by fMLP-stimulated neutrophils in a range of 10−1 M to 10−5 M.
Histamine and H2 receptor antagonists are also recognized as modulators of B-cell and T-cell functions via cell surface H2 receptor interactions. Specifically, histamine has been shown to directly inhibit B-cell production of immunoglobulin (immunoglobulin G [IgG] and IgM) (4, 15). This inhibition of B-cell antibody production by histamine can be blocked by treatment with cimetidine, which has also been shown to stimulate antibody production (12, 15, 21). In addition, cimetidine treatment appears to modulate IgG subclass (enhanced IgG1 production) expression (21). H2 receptor antagonists are known to modulate T-cell function through inhibition of suppressor T-lymphocyte activity, an increase in interleukin-2 production, and enhancement of natural killer cell activity (26). Taken together, these observations suggest that H2 receptor antagonists may enhance host defenses through both humoral and cellular pathways and result in reduced inflammation.
Different receptor profiles can explain different responses to histamine and cell-specific functions. In general, the immunoregulatory effects of histamine are mediated by H2 receptors (23). Monocytes/macrophages are among the major inflammatory cells that have many functions, such as production of cytokines and matrix metalloproteinases and phagocytosis. Histamine is known to regulate the expression of various cytokines by inflammatory cells (30). TNF-α is an inflammatory cytokine expressed during the progression to periodontal inflammation. Endotoxin-induced TNF-α expression is upregulated by histamine via the H2 receptor in peripheral blood macrophages (30). Histamine inhibits lysosomal enzyme release, respiratory burst, adhesion, chemotaxis, and calcium influx in agonist-stimulated human neutrophils. All of these inhibitory effects of histamine on human polymorphonuclear leukocytes are the consequences of H2 receptor activation, which causes the elevation of intracellular cyclic AMP concentrations (13, 31). Periodontal diseases are initiated by bacterial plaque on tooth surfaces, inducing inflammation in gingival and periodontal tissues. An increase in T suppressor cells has been reported in the gingival tissues during periodontitis, and a direct correlation between the histamine level in gingival tissue and the degree of periodontal disease has been demonstrated (36). It has also been hypothesized that increased immune function is produced by blocking the effects of histamine on H2 receptors and that H2 receptor antagonists may reduce inflammation by inhibiting the formation of reactive oxygen inflammatory products. In addition, dental plaque has been reported to activate C5 in serum, activating basophils (27). Increased histamine levels in gingival crevicular fluid, saliva, and gingival tissues have been found to correlate with increasing severity of periodontal disease and tissue inflammation (37). In contrast, studies examining H2 receptor antagonists in vivo have demonstrated that treatment with H2 receptor antagonists increased neutrophil chemotaxis, overriding the suppressive effects of histamine (18, 25).
Both cimetidine and metiamide, another H2 receptor antagonist, markedly influence the primary humoral antibody response of immunized normal cells in vitro (14). Optimum enhancement occurs at a lower dosage (10 μg) on the first day (14). Cimetidine influences certain IgG subclasses (enhanced IgG1 production) and IgM expression in vitro; however, the route, timing, and dosage of cimetidine administration are critical in modulating these effects (4, 21). The variations in their effects might be due to their structural differences. Among all the H2 receptor antagonists (cimetidine, ranitidine, and famotidine) cimetidine has the strongest immunomodulating effect, and only cimetidine augments the cytotoxicity and proliferative responses of lymphocytes to mitogen (17).
Here, we quantitatively analyzed periodontal disease progression in rabbits treated with cimetidine, using clinical, histopathological, and histomorphometric analyses. The histomorphometric analysis of the histological sections showed the preventive role of cimetidine against periodontal disease. In fact, the results presented in this work showed significant alveolar bone loss within 6 weeks of the induction of experimental periodontitis. Increased multinucleated osteoclastic cells with resorptive lacunae and inflammatory infiltrate dominated the pathological sections of the control and vehicle groups. Furthermore, numerous blood vessels and inflammatory cells were localized adjacent to the basal layer in the connective tissue. On the other hand, the cimetidine-treated groups at three different dosages showed intact epithelium; dense, well-defined connective-tissue fibers and scarce blood vessels; and few inflammatory cells, with very regular alveolar bone borders. No signs of alveolar bone resorption and borders of secondary bone deposition were seen. The doses of cimetidine for this study were chosen empirically, and the three cimetidine groups showed comparable results. Thus, it appears that future studies will have to lower the dose to determine the minimal effective dose in animals, or current doses could be used in developing efficient medications in human disease models.
Therapeutic agents that are directed at modulation of various host mediators have shown significant promise for the management of adult periodontitis and may be most appropriately indicated for individuals with substantially increased risk for periodontitis. This paper provides histological evidence confirming the role of a therapeutic host modulator agent via the topical application of an H2 receptor antagonist (cimetidine) in the prevention of inflammatory cell infiltration, connective-tissue destruction, and bone loss in the rabbit periodontitis model. In conclusion, we have provided prospective data suggesting that local cimetidine application can arrest the periodontal inflammation induced by P. gingivalis. Further, the evidence suggests that topical cimetidine may be used as a preventive agent in those subjects who have susceptibility to periodontal disease. The findings of this study suggest that the clinical therapeutic effect of local cimetidine application in chronic periodontal conditions may be positive in humans, which may lead us to discover new, effective, and safer therapeutic applications to modulate host defenses in response to resistant biofilms.
Acknowledgments
We thank Martha Warbington for her laboratory assistance during P. gingivalis preparation. We also thank Franklin Richardson and Andrew Clary for their technical support during histological preparations.
This project was supported in part by a grant from Procter and Gamble, Inc.
Editor: J. D. Clements
REFERENCES
- 1.Albandar, J. M., J. A. Brunelle, and A. Kingman. 1999. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988-1994. J. Periodontol. 70:13-29. [DOI] [PubMed] [Google Scholar]
- 2.Alekseenko, S. A., and S. S. Timoshin. 1999. The effect of histamine H2 receptor blockers on reparative processes in the gastric mucosa of patients with gastric peptic ulcer. Ter. Arkh. 71:23-26. [PubMed] [Google Scholar]
- 3.Anderson, R., A. Glover, and A. R. Rabson. 1977. The in vitro effects of histamine and metiamide on neutrophil motility and their relationship to intracellular cyclic nucleotide levels. J. Immunol. 118:1690-1696. [PubMed] [Google Scholar]
- 4.Badger, A. M., A. E. Brown, and G. Poste. 1983. The effect of cimetidine on antibody synthesis in vitro and in vivo. Immunology 48:151-155. [PMC free article] [PubMed] [Google Scholar]
- 5.Bjorn, H., A. Halling, and H. Thyberg. 1969. Radiographic assessment of marginal bone loss. Odontol. Revy. 20:165-179. [PubMed] [Google Scholar]
- 6.Brockmeyer, N. H., E. Kreuzfelder, C. Bluhm, G. Shen, E. Scheiermann, H. O. Keinecke, and E. E. Ohnhaus. 1989. Immunomodulation of cimetidine in healthy volunteers. Klin. Wochenschr. 67:26-30. [DOI] [PubMed] [Google Scholar]
- 7.Brockmeyer, N. H., E. Kreuzfelder, W. Guttmann, L. Mertins, M. Goos, and E. E. Ohnhaus. 1989. Cimetidine and the immuno-response in healthy volunteers. J. Investig. Dermatol. 93:757-761. [DOI] [PubMed] [Google Scholar]
- 8.Brockmeyer, N. H., E. Kreuzfelder, L. Mertins, N. Chalabi, W. Kirch, N. Scheiermann, M. Goos, and E. E. Ohnhaus. 1988. Immunomodulatory properties of cimetidine in ARC patients. Clin. Immunol. Immunopathol. 48:50-60. [DOI] [PubMed] [Google Scholar]
- 9.Brook, I. 2003. Microbiology and management of periodontal infections. Gen. Dent. 51:424-428. [PubMed] [Google Scholar]
- 10.Brown, N. J., and L. J. Robetrs II. 2001. Histamine, bradykinin, and their antagonists, p. 645-668. In J. G. Hardman, L. E. Limbird, and A. G. Gilman (ed.), Goodman and Gilman’s the pharmacological basis of therapeutics, 10th ed. McGraw-Hill, New York, N.Y.
- 11.Cohen, H. J., and M. E. Chovaniec. 1978. Superoxide production by digitonin-stimulated guinea pig granulocytes. The effects of N-ethyl maleimide, divalent cations, and glycolytic and mitochondrial inhibitors on the activation of the superoxide generating system. J. Clin. Investig. 61:1088-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ershler, W. B., M. P. Hacker, B. J. Burroughs, A. L. Moore, and C. F. Myers. 1983. Cimetidine and the immune response. I. In vivo augmentation of nonspecific and specific immune response. Clin. Immunol. Immunopathol. 26:10-17. [DOI] [PubMed] [Google Scholar]
- 13.Flamand, N., H. Plante, S. Picard, M. Laviolette, and P. Borgeat. 2004. Histamine-induced inhibition of leukotriene biosynthesis in human neutrophils: involvement of the H2 receptor and cAMP. Br. J. Pharmacol. 141:552-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman, H., I. Lee, and D. T. Walz. 1982. Effects of histamine receptor antagonists metiamide and cimetidine on antibody formation in vitro by murine cells. Proc. Soc. Exp. Biol. Med. 169:222-225. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto, M., and H. Kimata. 1994. Histamine inhibits immunoglobulin production via histamine H2 receptors without affecting cell growth in human B cells. Clin. Immunol. Immunopathol. 73:96-102. [DOI] [PubMed] [Google Scholar]
- 16.Graham, L. 2003. An emerging new standard of care: initial and continued treatment for patients with signs and symptoms of active periodontal disease. Gen. Dent. 51:570-577. [PubMed] [Google Scholar]
- 17.Hahm, K. B., W. H. Kim, S. I. Lee, J. K. Kang, and I. S. Park. 1995. Comparison of immunomodulative effects of the histamine-2 receptor antagonists cimetidine, ranitidine, and famotidine on peripheral blood mononuclear cells in gastric cancer patients. Scand. J. Gastroenterol. 30:265-271. [DOI] [PubMed] [Google Scholar]
- 18.Hirasawa, N., M. Watanabe, S. Mue, S. Tsurufuji, and K. Ohuchi. 1991. Downward regulation of neutrophil infiltration by endogenous histamine without affecting vascular permeability responses in air-pouch-type carrageenin inflammation in rats. Inflammation 15:117-126. [DOI] [PubMed] [Google Scholar]
- 19.Jain, A., E. L. Batista, Jr., C. Serhan, G. L. Stahl, and T. E. Van Dyke. 2003. Role for periodontitis in the progression of lipid deposition in an animal model. Infect. Immun. 71:6012-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katzung, B. G., and D. J. Julius. 2000. Histamine, serotonin, and the ergot alkaloids, p. 265-291. In B. G. Katzung (ed.), Basic and clinical pharmacology, 8th ed.
- 21.Kumar, A., R. E. Pantarotto, and D. B. Kaufman. 1990. IgG subclasses and IgM in rats: immunoregulatory effects of cimetidine on serum levels. Comp. Immunol. Microbiol. Infect. Dis. 13:147-153. [DOI] [PubMed] [Google Scholar]
- 22.Lagunoff, D., M. T. Phillips, O. A. Iseri, and E. P. Benditt. 1964. Isolation and preliminary characterization of rat mast cell granules. Lab. Investig. 13:1331-1344. [PubMed] [Google Scholar]
- 23.MacGlashan, D., Jr. 2003. Histamine: a mediator of inflammation. J. Allergy Clin. Immunol. 112:S53-S59. [DOI] [PubMed] [Google Scholar]
- 24.Mikawa, K., H. Akamatsu, K. Nishina, M. Shiga, N. Maekawa, H. Obara, and Y. Niwa. 1999. The effects of cimetidine, ranitidine, and famotidine on human neutrophil functions. Anesth. Analg. 89:218-224. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen, H. J., H. Nielsen, S. Jensen, and F. Moesgaard. 1994. Ranitidine improves postoperative monocyte and neutrophil function. Arch. Surg. 129:309-315. [DOI] [PubMed] [Google Scholar]
- 26.Nishibori, M., H. Kohka-Takahashi, and S. Mori. 2001. Regulation of cytokine production by histamine through H2-receptor stimulation. Nippon Yakurigaku Zasshi 118:29-35. [DOI] [PubMed] [Google Scholar]
- 27.Olsson-Wennstrom, A. W. J., S. E. Mergenhagen, and R. P. Siraganian. 1978. The mechanism of basophil histamine release in patinets with perioodntal disease. Clin. Exp. Immunol. 33:166-173. [PMC free article] [PubMed] [Google Scholar]
- 28.Page, R. C. 1991. The role of inflammatory mediators in the pathogenesis of periodontal disease. J. Periodontal Res. 26:230-242. [DOI] [PubMed] [Google Scholar]
- 29.Reddy, M. S., N. C. Geurs, and J. C. Gunsolley. 2003. Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents. A systematic review. Ann. Periodontol. 8:12-37. [DOI] [PubMed] [Google Scholar]
- 30.Sasaguri, Y., and A. Tanimoto. 2004. Role of macrophage-derived histamine in atherosclerosis—chronic participation in the inflammatory response. J. Atheroscler. Thromb. 11:122-130. [DOI] [PubMed] [Google Scholar]
- 31.Seligmann, B. E., M. P. Fletcher, and J. I. Gallin. 1983. Histamine modulation of human neutrophil oxidative metabolism, locomotion, degranulation, and membrane potential changes. J. Immunol. 130:1902-1909. [PubMed] [Google Scholar]
- 32.Serhan, C. N., A. Jain, S. Marleau, C. Clish, A. Kantarci, B. Behbehani, S. P. Colgan, G. L. Stahl, A. Merched, N. A. Petasis, L. Chan, and T. E. Van Dyke. 2003. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J. Immunol. 171:6856-6865. [DOI] [PubMed] [Google Scholar]
- 33.Seymour, G. J. 1991. Importance of the host response in the periodontium. J. Clin. Periodontol. 18:421-426. [DOI] [PubMed] [Google Scholar]
- 34.Socransky, S. S., and A. D. Haffajee. 2002. Dental biofilms: difficult therapeutic targets. Periodontol. 2000 28:12-55. [DOI] [PubMed] [Google Scholar]
- 35.Socransky, S. S., A. D. Haffajee, M. A. Cugini, C. Smith, and R. L. Kent, Jr. 1998. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25:134-144. [DOI] [PubMed] [Google Scholar]
- 36.Steinsvoll, S., K. Helgeland, and K. Schenck. 2004. Mast cells—a role in periodontal diseases? J. Clin. Periodontol. 31:413-419. [DOI] [PubMed] [Google Scholar]
- 37.Van Dyke, T. E., C. W. Cutler, M. Kowolik, R. S. Singer, W. Buchanan, and A. R. Biesbrock. 2005. Effect of topical cimetidine rinse on gingival crevicular neutrophil leukocyte function. J. Periodontol. 76:998-1005. [DOI] [PubMed] [Google Scholar]
- 38.Van Dyke, T. E., and C. N. Serhan. 2003. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J. Dent. Res. 82:82-90. [DOI] [PubMed] [Google Scholar]
- 39.Vannier, E., and C. A. Dinarello. 1993. Histamine enhances interleukin (IL)-1-induced IL-1 gene expression and protein synthesis via H2 receptors in peripheral blood mononuclear cells. Comparison with IL-1 receptor antagonist. J. Clin. Investig. 92:281-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, K., H. J. Lin, C. L. Perng, G. Y. Tseng, K. W. Yu, F. Y. Chang, and S. D. Lee. 2004. The effect of H2-receptor antagonist and proton pump inhibitor on microbial proliferation in the stomach. Hepatogastroenterology 51:1540-1543. [PubMed] [Google Scholar]