Abstract
The rlrA pathogenicity islet in Streptococcus pneumoniae TIGR4 encodes three surface proteins, RrgA, RrgB, and RrgC, and three sortase enzymes. Using transmission electron microscopy, cell fractionation, cell wall sorting signal domain swapping, and Western blotting, we show that RrgA and RrgB are incorporated into a multisubunit pilus in S. pneumoniae.
Gram-positive bacteria have evolved a number of mechanisms to localize proteins to their surfaces. One mechanism of surface display is through the covalent linkage of proteins to cell wall stem peptides, a process mediated by sortases. Sortases are membrane-anchored transpeptidases that recognize a C-terminal cell wall sorting signal (CWSS) characterized by the presence of an LPXTG motif. Sortase covalently links the threonine of this motif to cell wall precursors, which are then incorporated into the cell wall (19). Most gram-positive bacteria contain at least one sortase orthologue, SrtA, which has recently been shown to be required for colonization in Streptococcus pneumoniae (3, 10). Additionally, SrtA has been shown to be required for virulence in several other pathogens (2, 4, 5, 11, 26); however, multiple sortase paralogues have also been found in the genomes of a subset of these species (17). In many cases, the additional sortase paralogues are genetically linked to cell wall-anchored proteins with variant CWSSs that serve as substrates for the linked sortase, including the rlrA pathogenicity islet in S. pneumoniae TIGR4 (1, 8, 14, 16, 19, 22).
We sought to characterize the rlrA pathogenicity islet further for the following reasons: RrgA is a virulence factor in a murine lung infection model (8), yet the islet has a varied distribution among serotypes (18), and the RrgA, RrgB, and RrgC proteins exhibit homology to ones that were recently described to form pili in other gram-positive organisms (20-22). The most well-studied model of gram-positive pilus formation is Corynebacterium diphtheriae, where there are four motifs within the major pilin subunit, SpaA, that are necessary for pilus assembly. These motifs are an N-terminal signal peptide, a CWSS, a pilin motif, and an E-box motif (20-22). The identification and characterization of these motifs have led to a model of assembly wherein SpaA is polymerized into a pilus structure by covalent linkage between subunits catalyzed by the linked sortase, and the base of the pilus is covalently attached to the cell wall (20, 22). In addition, there appear to be minor subunit proteins that are incorporated along the pilus shaft and at the tip, one of which contains a variant CWSS. Incorporation of these minor subunits has been shown to require additional sortases that are also genetically linked; however, the mechanisms of their incorporation into the pilus are poorly understood. All four of the required motifs found in SpaA are present in RrgB, suggesting that the latter is the major subunit of a pilus in S. pneumoniae and that the other surface proteins and sortases in the islet are involved in pilus formation. Although a pilus has never been identified in S. pneumoniae, it has been previously reported in the literature that other streptococcal species, including S. parasanguis, S. salivarius, and S. sanguis, produce adhesive organelles such as fimbriae (6, 7, 13, 23-25), and it has recently been shown that S. agalactiae and S. pyogenes produce pili (12, 15).
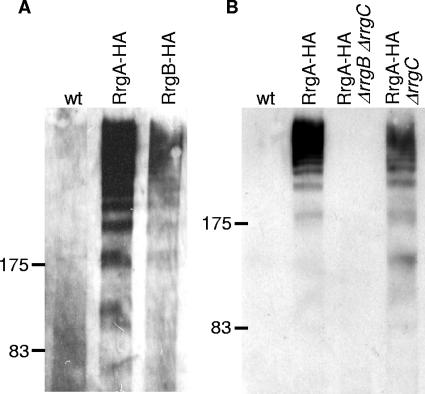
To determine if RrgB forms a pilus structure analogous to that of SpaA, cell wall-anchored proteins were isolated from bacteria from the mid-exponential phase expressing a hemagglutinin (HA)-tagged RrgB (RrgB-HA) (inserted at residue 133) after cell wall digestion of whole cells with 10 mM Tris-HCl (pH 8.0), 30% raffinose, 100 U mutanolysin, 1 mg lysozyme, and EDTA-free protease inhibitor cocktail (Roche). The soluble molecules from this digest were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and RrgB-HA was detected by Western blotting using a polyclonal anti-HA antibody (Santa Cruz Biotech). This analysis revealed multiple high-molecular-weight species of RrgB-HA, the majority of which are present in the stack of the gel (Fig. 1A), similar to that seen for SpaA in C. diphtheriae cell wall extracts. This ladder of bands is hypothesized to represent pili of various lengths that are composed of covalently linked major and minor pilin subunits (22). A similar banding pattern was observed for RrgA-HA (HA tag inserted at residue 224 to replace the nine amino acids at residues 224 to 232) (Fig. 1A) as well as for native RrgA immunoblotted with anti-RrgA polyclonal antiserum (Covance Research Products Inc.) (data not shown). The reduction in RrgB-HA signal relative to that for RrgA-HA was consistently observed by Western blotting, suggesting partial obscuring of HA epitopes or, alternatively, reduced assembly of this mutant protein into pili. These results suggest that the rlrA pathogenicity islet encodes a pilus similar to that described for C. diphtheriae. Furthermore, the similar banding patterns exhibited by RrgA and RrgB suggested two models: RrgA and RrgB are incorporated into a single multimeric pilus, or RrgB and RrgA form two independent pili. The absence of a consensus pilin motif and E box in RrgA favors the former hypothesis. To test these possibilities, we analyzed the migration of RrgA-HA by SDS-PAGE and Western blotting using cell wall preparations from double- and single-gene in-frame deletion strains, ΔrrgB ΔrrgC and ΔrrgC. We were unable to construct a deletion of rrgB by itself. RrgA-HA is undetectable in the cell wall fraction by Western blotting in the ΔrrgB ΔrrgC strain background, but the wild-type-like high-molecular-weight banding pattern is maintained in the ΔrrgC strain background (Fig. 1B). These data demonstrate a dependence of RrgA pilus formation on RrgB.
FIG. 1.
RrgA and RrgB are polymerized into pili. (A) Cell wall fractions of RrgA-HA and RrgB-HA S. pneumoniae TIGR4 form high-molecular-weight complexes when immunoblotted with anti-HA antisera. Wild-type (wt) TIGR4 (lane 1), RrgA-HA (lane 2), and RrgB-HA (lane 3) are shown. (B) The high-molecular-weight RrgA-HA complexes are not present in a strain with rrgB deleted and rrgC but are present when only rrgC is deleted. Wild-type TIGR4 (lane 1), RrgA-HA (lane 2), RrgA-HA ΔrrgB ΔrrgC (lane 3), and RrgA-HA ΔrrgC (lane 4) strains are shown.
Visualization of a pilus structure on the surface of S. pneumoniae.
The conserved motifs found in RrgB, in addition to the Western blot data, led us to hypothesize that RrgB is the major pilus subunit. We sought to visualize a pilus structure by specifically looking at the RrgB protein via immunogold transmission electron microscopy. To this end, mouse antiserum raised against two peptides comprising the central portion of RrgB between the signal sequence and the C-terminal CWSS (N-terminal peptide at residues 44 to 341; C-terminal peptide at residues 343 to 624) was used. This antiserum was specific for RrgB, since it detected RrgB in cell wall extracts via Western analysis and did not recognize cell wall extract from an ΔrrgB ΔrrgC strain (data not shown). Finally, the antiserum but not the preimmune serum recognized the RrgB peptides that were used to raise the antiserum (Fig. 2).
FIG. 2.
RrgB antiserum is specific to RrgB. (A) Two RrgB peptides were separated by SDS-PAGE, transferred onto a nitrocellulose membrane, and probed separately with mouse preimmune serum (lane 1) or mouse anti-RrgB serum (lane 2). The two RrgB peptides (filled arrow) and a breakdown product (open arrow) were detected by the antiserum.
Wild-type S. pneumoniae strain TIGR4 was incubated with mouse anti-RrgB serum, and bound antibody was visualized with 18-nm Colloidal Gold-AffiniPure anti-mouse immunoglobulin G (IgG) and negatively stained with uranyl acetate. The presence of numerous pili was detected on the surface of the bacteria (Fig. 3A and B). No labeling was observed with the mouse preimmune serum (Fig. 3H) or when the primary antibody was not used (data not shown). To test the hypothesis that RrgA is also incorporated into pili, wild-type S. pneumoniae and a strain expressing a second HA-tagged RrgA (HA inserted at residue 820 to replace the nine amino acids at residues 820 to 828) were incubated with rabbit anti-HA antibody and visualized with 12-nm Colloidal Gold-AffiniPure anti-rabbit IgG. RrgA-HA was detected within “patches” that were equally spaced along fibers, which are presumably pili (Fig. 3C). In the RrgA-HA background, if rrgB and rrgC are deleted, the pilus structure is not observed and the gold particles are seen close to the surface of the cell, indicating that RrgA-HA cannot be assembled into pili in the absence of RrgB and/or RrgC (Fig. 3D). To determine which of these two proteins is required for incorporation of RrgA-HA into pili, an RrgA-HA ΔrrgC strain was examined. RrgA-HA labeling in pili was observed (Fig. 3E), suggesting that RrgB is the pilin subunit, which is consistent with the Western blot results and the presence of pilin motifs in RrgB. The presence of both RrgA and RrgB in the same pilus structure was detected by double labeling with two different-sized gold particles (Fig. 3F and G).
FIG. 3.
Immunogold transmission electron microscopy visualization of RrgB and RrgA-HA incorporated into pilus-like structures on the surface of S. pneumoniae TIGR4. (A and B) Representative micrographs of TIGR4 labeled with mouse anti-RrgB antiserum and visualized with anti-mouse IgG Colloidal Gold 18-nm particles. (C to E) Representative micrographs of TIGR4 labeled with rabbit polyclonal anti-HA antibody and visualized with anti-rabbit IgG Colloidal Gold 12-nm particles. (C) RrgA-HA; (D) RrgA-HA ΔrrgB ΔrrgC; (E) RrgA-HA ΔrrgC. (F and G) S. pneumoniae RrgA-HA labeled with both mouse anti-RrgB antiserum and anti-mouse IgG Colloidal Gold 18-nm particles as well as rabbit polyclonal anti-HA antibody and anti-rabbit IgG Colloidal Gold 12-nm particles. RrgA, labeled with 12-nm gold particles (arrows), is found periodically within the pilus. (H) TIGR4 labeled with mouse preimmune serum. Magnifications: (A) ×10,500 (scale bar, 1 μm); (B, G, and H) ×19,000 (scale bar, 1 μm); (C to F) ×25,000 (scale bar, 0.5 μm).
This is the first report where an external structure of this nature has been visualized on the surface of S. pneumoniae. These data, together with the Western blot and homology data, suggest that pili are assembled on the surface of S. pneumoniae TIGR4. RrgB is the major pilin subunit that forms the length of the pilus into which RrgA is incorporated as a minor subunit at periodic intervals. The precise locations of RrgA, RrgB, and possibly RrgC in the pilus are an interesting area of study, and the mechanism of their incorporation will require further investigation.
Incorporation of RrgA into pili requires srtD.
Due to the genetic linkage of rrgA, rrgB, and rrgC with srtB, srtC, and srtD, and the variant LPXTG motifs in each Rrg protein, we previously hypothesized that each sortase would direct the cell wall anchoring of one Rrg protein (8). To test this and to begin to analyze the mechanism of incorporation of RrgA into pili, the cell wall localization of RrgA was examined in previously described strains in which each sortase was mutated (8). Cell wall extracts were prepared from identical amounts of mid-exponential-phase bacteria and analyzed by Western blotting using rabbit anti-RrgA antiserum. The high-molecular-weight ladder of RrgA was absent in the cell wall fraction of the rrgA and srtD strains, whereas a wild-type banding pattern was observed in each of the other mutants (Fig. 4A). The srtD mutation did not affect the expression of RrgA, since RrgA was detected in large amounts in the protoplast and secreted fractions (data not shown). This result suggests that SrtD is required for the incorporation of RrgA into pili.
FIG. 4.
Incorporation of RrgA into pili requires SrtD. (A) Cell wall fractions of wild-type and sortase mutant strains were examined by Western blotting with polyclonal anti-RrgA antiserum. Fractions were from wild-type TIGR4 (lane 1) and strains carrying mutations in rrgA (lane 2), srtA (lane 3), srtB (lane 4), srtC (lane 5), and srtD (lane 6). (B) Schematic of the RrgA chimeras examined by Western blotting. RrgA (black arrow and rectangles) containing the C-terminal CWSS of each gene listed (arrowheads) is shown. The RrgA-RrgBCWSS chimera was generated by a suicide plasmid insertion-duplication mutation as illustrated. The integrated plasmid is a derivative of pAC1000 (9) containing rrgA′ (bases 1 to 2586) joined to ′rrgB (bases 1902 to 1999). The RrgA-RrgCCWSS and RrgA-SP0082CWSS chimeras were generated by double-crossover allelic exchange using pAC1000 derivatives, resulting in the replacement of the 3′ end of rrgA (bases 2586 to 2683) with ′rrgC (bases 1084 to 1150) and ′SP0082 (bases 2461 to 2574), respectively. (C) Western blots of the cell wall fractions of chimeric strains of AC1441, AC1442, and AC1443 in an otherwise-wild-type background (lane 1 in each panel) or in the sortase mutant backgrounds indicated above each lane. The cell wall fractions were immunoblotted with anti-RrgA polyclonal antibody. The intense band at approximately 120 kDa consists of two poorly resolved bands.
To confirm the specificity of SrtD for incorporating RrgA into pili, we replaced the CWSS of RrgA with that of RrgB and RrgC and examined the RrgA chimeras in strains deleted for individual sortases by Western blotting as described above. The chimeras contained the entire sorting signal of the indicated protein (from the first residue of the LPXTG motif to the end of the protein) in place of the analogous segment of RrgA (Fig. 4B). In addition, we created an RrgA chimera using a canonical (SrtA-recognized) LPXTG CWSS from an unrelated protein (SP0082). All three chimeras (RrgA-RrgBCWSS, RrgA-RrgCCWSS, and RrgA-SP0082CWSS) were present in the cell wall fraction in the wild-type strain background (Fig. 4C); however, they were no longer present in the high-molecular-weight ladder exhibited by the wild type (compare Fig. 4A and C). Thus, although the chimeras are localized to the cell wall fraction, the wild-type RrgA CWSS is required for incorporation of the protein into pili in an SrtD-dependent manner.
The above-described results suggest that RrgB and RrgC require other sortases (presumably SrtB and SrtC) for their incorporation into the cell wall compartment. To examine this, the sortase mutations used as described above were introduced into the RrgA chimeric strains by natural transformation, and cell wall extracts were analyzed by Western blotting as described above. Cell wall localization of RrgA-RrgBCWSS was greatly reduced in the srtB strain, whereas that of RrgA-RrgCCWSS was only moderately reduced in the srtC strain (Fig. 4C). Consistent with a previous report of canonical LPXTG-anchored proteins in S. pneumoniae (10), cell wall localization of the RrgA-SP0082CWSS chimera required srtA (Fig. 4C). Note that the absence of a high-molecular-weight banding pattern in these experiments is due to use of RrgA in these chimeras, which apparently is unable to be incorporated into pili by SrtA, SrtB, or SrtC. This is consistent with RrgA lacking the consensus pilin and E-box motifs. Cumulatively, these data indicate that there is a one-to-one sortase-to-surface-protein correspondence for gene products in the rlrA pathogenicity islet and further suggest that this relationship is essential for the formation of multisubunit pili on the cell surface.
To date, pili have been shown to be present in several gram-positive organisms. In this study, we show evidence of similar pili in S. pneumoniae TIGR4 encoded by the rlrA pathogenicity islet and additionally show that RrgA is incorporated into pili as a minor subunit in an SrtD-dependent manner.
Acknowledgments
This work was funded by the Howard Hughes Medical Institute and by NIH grant AI52374 to A.C., by Fondation pour la Recherche Médicale for A.B., and by the Center for Gastroenterology Research on Absorptive and Secretory Processes, NEMC (P30 DK34928).
Editor: V. J. DiRita
REFERENCES
- 1.Barnett, T. C., A. R. Patel, and J. R. Scott. 2004. A novel sortase, SrtC2, from Streptococcus pyogenes anchors a surface protein containing a QVPTGV motif to the cell wall. J. Bacteriol. 186:5865-5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bierne, H., S. K. Mazmanian, M. Trost, M. G. Pucciarelli, G. Liu, P. Dehoux, L. Jansch, F. Garcia-del Portillo, O. Schneewind, and P. Cossart. 2002. Inactivation of the srtA gene in Listeria monocytogenes inhibits anchoring of surface proteins and affects virulence. Mol. Microbiol. 43:869-881. [DOI] [PubMed] [Google Scholar]
- 3.Chen, S., G. K. Paterson, H. H. Tong, T. J. Mitchell, and T. F. DeMaria. 2005. Sortase A contributes to pneumococcal nasopharyngeal colonization in the chinchilla model. FEMS Microbiol. Lett. 253:151-154. [DOI] [PubMed] [Google Scholar]
- 4.Garandeau, C., H. Reglier-Poupet, I. Dubail, J. L. Beretti, P. Berche, and A. Charbit. 2002. The sortase SrtA of Listeria monocytogenes is involved in processing of internalin and in virulence. Infect. Immun. 70:1382-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaspar, A. H., L. A. Marraffini, E. M. Glass, K. L. Debord, H. Ton-That, and O. Schneewind. 2005. Bacillus anthracis sortase A (SrtA) anchors LPXTG motif-containing surface proteins to the cell wall envelope. J. Bacteriol. 187:4646-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Handley, P. S., P. L. Carter, and J. Fielding. 1984. Streptococcus salivarius strains carry either fibrils or fimbriae on the cell surface. J. Bacteriol. 157:64-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handley, P. S., P. L. Carter, J. E. Wyatt, and L. M. Hesketh. 1985. Surface structures (peritrichous fibrils and tufts of fibrils) found on Streptococcus sanguis strains may be related to their ability to coaggregate with other oral genera. Infect. Immun. 47:217-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hava, D. L., and A. Camilli. 2002. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 45:1389-1406. [PMC free article] [PubMed] [Google Scholar]
- 9.Hava, D. L., C. J. Hemsley, and A. Camilli. 2003. Transcriptional regulation in the Streptococcus pneumoniae rlrA pathogenicity islet by RlrA. J. Bacteriol. 185:413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kharat, A. S., and A. Tomasz. 2003. Inactivation of the srtA gene affects localization of surface proteins and decreases adhesion of Streptococcus pneumoniae to human pharyngeal cells in vitro. Infect. Immun. 71:2758-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lalioui, L., E. Pellegrini, S. Dramsi, M. Baptista, N. Bourgeois, F. Doucet-Populaire, C. Rusniok, M. Zouine, P. Glaser, F. Kunst, C. Poyart, and P. Trieu-Cuot. 2005. The SrtA sortase of Streptococcus agalactiae is required for cell wall anchoring of proteins containing the LPXTG motif, for adhesion to epithelial cells, and for colonization of the mouse intestine. Infect. Immun. 73:3342-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauer, P., C. D. Rinaudo, M. Soriani, I. Margarit, D. Maione, R. Rosini, A. R. Taddei, M. Mora, R. Rappuoli, G. Grandi, and J. L. Telford. 2005. Genome analysis reveals pili in group B Streptococcus. Science 309:105. [DOI] [PubMed] [Google Scholar]
- 13.Levesque, C., C. Vadeboncoeur, and M. Frenette. 2004. The csp operon of Streptococcus salivarius encodes two predicted cell-surface proteins, one of which, CspB, is associated with the fimbriae. Microbiology 150:189-198. [DOI] [PubMed] [Google Scholar]
- 14.Mazmanian, S. K., H. Ton-That, K. Su, and O. Schneewind. 2002. An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc. Natl. Acad. Sci. USA 99:2293-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mora, M., G. Bensi, S. Capo, F. Falugi, C. Zingaretti, A. G. O. Manetti, T. Maggi, A. R. Taddei, G. Grandi, and J. L. Telford. 2005. Group A Streptococcus produce pilus-like structures containing protective antigens and Lancefield T antigens. Proc. Natl. Acad. Sci. USA 102:15641-15646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newton, S. M., P. E. Klebba, C. Raynaud, Y. Shao, X. Jiang, I. Dubail, C. Archer, C. Frehel, and A. Charbit. 2005. The svpA-srtB locus of Listeria monocytogenes: Fur-mediated iron regulation and effect on virulence. Mol. Microbiol. 55:927-940. [DOI] [PubMed] [Google Scholar]
- 17.Pallen, M. J., A. C. Lam, M. Antonio, and K. Dunbar. 2001. An embarrassment of sortases—a richness of substrates? Trends Microbiol. 9:97-102. [DOI] [PubMed] [Google Scholar]
- 18.Paterson, G. K., and T. J. Mitchell. 2005. The role of Streptococcus pneumoniae sortase A in colonisation and pathogenesis. Microbes Infect. 8:145-153. [DOI] [PubMed] [Google Scholar]
- 19.Ton-That, H., L. A. Marraffini, and O. Schneewind. 2004. Protein sorting to the cell wall envelope of gram-positive bacteria. Biochim. Biophys. Acta 1694:269-278. [DOI] [PubMed] [Google Scholar]
- 20.Ton-That, H., L. A. Marraffini, and O. Schneewind. 2004. Sortases and pilin elements involved in pilus assembly of Corynebacterium diphtheriae. Mol. Microbiol. 53:251-261. [DOI] [PubMed] [Google Scholar]
- 21.Ton-That, H., and O. Schneewind. 2004. Assembly of pili in gram-positive bacteria. Trends Microbiol. 12:228-234. [DOI] [PubMed] [Google Scholar]
- 22.Ton-That, H., and O. Schneewind. 2003. Assembly of pili on the surface of Corynebacterium diphtheriae. Mol. Microbiol. 50:1429-1438. [DOI] [PubMed] [Google Scholar]
- 23.Wu, H., and P. M. Fives-Taylor. 1999. Identification of dipeptide repeats and a cell wall sorting signal in the fimbriae-associated adhesin, Fap1, of Streptococcus parasanguis. Mol. Microbiol. 34:1070-1081. [DOI] [PubMed] [Google Scholar]
- 24.Wu, H., and P. M. Fives-Taylor. 2001. Molecular strategies for fimbrial expression and assembly. Crit. Rev. Oral Biol. Med. 12:101-115. [DOI] [PubMed] [Google Scholar]
- 25.Wu, H., K. P. Mintz, M. Ladha, and P. M. Fives-Taylor. 1998. Isolation and characterization of Fap1, a fimbriae-associated adhesin of Streptococcus parasanguis FW213. Mol. Microbiol. 28:487-500. [DOI] [PubMed] [Google Scholar]
- 26.Zink, S. D., and D. L. Burns. 2005. Importance of srtA and srtB for growth of Bacillus anthracis in macrophages. Infect. Immun. 73:5222-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]