Abstract
The spore-forming, gram-positive bacterium Bacillus anthracis, the causative agent of anthrax, has achieved notoriety due to its use as a bioterror agent. In the environment, B. anthracis exists as a dormant endospore. Upon infection, germination of endospores occurs during their internalization within the phagocyte, and the ability to survive exposure to antibacterial killing mechanisms, such as O2·−, NO·, and H2O2, is a key initial event in the infective process. Macrophages generate NO· from the oxidative metabolism of l-arginine, using an isoform of nitric oxide synthase (NOS 2). Exposure of murine macrophages (RAW264.7 cells) to B. anthracis endospores up-regulated the expression of NOS 2 12 h after exposure, and production of NO· was comparable to that achieved following other bacterial infections. Spore-killing assays demonstrated a NO·-dependent bactericidal response that was significantly decreased in the presence of the NOS 2 inhibitor l-N6-(1-iminoethyl)lysine and in l-arginine-depleted media. Interestingly, we also found that B. anthracis bacilli and endospores exhibited arginase activity, possibly competing with host NOS 2 for its substrate, l-arginine. As macrophage-generated NO· is an important pathway in microbial killing, the ability of endospores of B. anthracis to regulate production of this free radical has important implications in the control of B. anthracis-mediated infection.
Endospores of the gram-positive bacterium Bacillus anthracis, the causative agent of anthrax, when inhaled and deposited in the lung, are phagocytosed by resident macrophages, where the spore evades host defenses and germinates into the vegetative bacterium (6, 13, 14). Germination is a prerequisite to replication and dissemination of B. anthracis (2). Unfortunately, mechanisms by which the spore enters the macrophage, germinates, survives therein, and escapes from this phagocytic cell are poorly understood. To study spore-specific interactions, the ΔgerH mutant of the B. anthracis Sterne 34F2 strain, which is a germination-deficient variant incapable of conversion from spores to bacilli in macrophages, is an appropriate model (34). The use of this strain focuses on distinct spore-macrophage interactions independent of contamination of B. anthracis vegetative bacilli.
The virulence of B. anthracis and the efficiency with which it can infect humans via inhalation are reasons the organism and the disease are currently being investigated. B. anthracis spores are comprised of an endospore, containing the infectious portions of the bacterium, and a surrounding exosporium, the outermost layer of the spore, composed of polysaccharides, lipids, and approximately 20 different proteins (27). The exosporium is the primary site of contact for spore interactions with host defenses. The process of sonication has been shown to selectively remove the exosporium while keeping the endospore intact (18). We postulate that spore-macrophage interaction at the exosporium is pivotal to the anthrax disease process.
Once inside the macrophage, survival of a bacterium depends upon several mechanisms (17, 34). Evidence suggests that through capsule formation and anthrax toxin release, B. anthracis spores survive by programmed resistance to the normal antimicrobial response (13). These normal antimicrobial responses to intracellular pathogens include but are not restricted to (i) production of superoxide (O2·−) by NADPH oxidase and subsequent formation of hydrogen peroxide (H2O2) by self-dismutation of O2·−; (ii) generation of nitric oxide (NO·), O2·−, peroxynitrite (ONOO−), and H2O2 by inducible nitric oxide synthase (NOS 2) (32); (iii) activation of cationic proteins (25); and (iv) syntheses of defensins (10).
The molecular events that trigger B. anthracis spore-host interaction are not well understood. Identification of the B. anthracis genome has suggested insights into possible molecular regulation by the bacterium, and the complete sequence of B. anthracis has proven to be a valuable step toward comprehending anthrax pathogenesis (26). Of importance to our laboratory was the identification of a B. anthracis spore surface-located superoxide dismutase (SOD) (29), the enzyme that regulates cellular levels of O2·−. In a recent publication, we reported on two important observations pertinent to spore-macrophage interaction: (i) endospores of B. anthracis scavenged O2·−, which may enhance the ability of the bacterium to survive within the hostile environment of the phagolysosome, and (ii) a significant flux of O2·− that was not scavenged by the spore surface-located SOD stimulated endospore germination (1).
Another protein characterized in the B. anthracis genome is arginase. This enzyme is encoded by a gene called rocF and shows 20 to 27% amino acid identity with other bacterial arginases, with the greatest homology (27.1%) to Bacillus subtilis RocF (22). Arginase is responsible for the metabolism of l-arginine to l-ornithine and urea. As such, arginase competes with NOS 2 for l-arginine, thereby controlling the biosynthesis of NO· (11). Independent of regulating NO· production by limiting the availability of l-arginine, B. anthracis arginase also participates in the formation of important metabolites, such as glutamic acid, which is a component in bacterium capsule formation (28). These findings indicate that arginase may have a dual function in B. anthracis growth and survival.
Herein, we examine spore-macrophage interaction using the murine macrophage-like cell line RAW264.7 and peritoneal macrophages. Exposure to B. anthracis endospores induced phagocytosis and up-regulated expression of active NOS 2. The initial interaction of the spore and macrophage occurs at the B. anthracis exosporium, the outermost layer of the spores containing highly immunogenic glycoproteins (29, 30). Therefore, we sought to determine whether the exosporium served to protect B. anthracis spores from the normal antimicrobial macrophage response. We demonstrated that removal of the exosporium from the endospore by sonication increased the production of NO·, identifying a function for the B. anthracis exosporium in regulating NO· generation. Bacterial killing assays demonstrated a NO·-dependent bactericidal response that was decreased 50% in the presence of the NOS 2 inhibitor l-N6-(1-iminoethyl)lysine (L-NIL). Finally, primary macrophages grown in l-arginine-depleted medium, in which NO· production is markedly depressed, decreased bactericidal response in comparison to macrophages grown in l-arginine-containing medium. As macrophage-generated NO· is an important pathway in microbial killing, the ability of endospores of B. anthracis to regulate the production of this free radical has important implications in the control of B. anthracis-mediated infection.
MATERIALS AND METHODS
Reagents.
RPMI 1640 was purchased from Gibco BRL (Frederick, MD). Fetal bovine serum (FBS) was obtained from Atlanta Biologicals (Lawrenceville, GA). Gentamicin and phosphate-buffered saline (PBS) were purchased from Biosource International (Rockville, MD). Brewer modified thioglycolate medium was from Becton Dickinson (Cockeysville, MD). Sulfanilamide, N-1-naphthylethylenediamine dihydrochloride, lipopolysaccharide (LPS), cytochalasin D, bovine arginase, and L-NIL were purchased from Sigma Co. (St. Louis, MO). Gamma interferon (IFN-γ) was obtained from PBL Biomedical Laboratories (Piscataway, NJ). Staphylococcus aureus ATCC 29213 and Escherichia coli (O18:H7:K1) ATCC 35150 were purchased from American Type Culture Collection (Rockville, MD). The spin trap 5-tert-butoxycarbonyl-5-methyl-1-pyrroline N-oxide (BMPO) was synthesized as described previously (31). NOS 2 was provided by Linda Roman of the Department of Biochemistry at the University of Texas Health Science Center, San Antonio, TX.
RAW264.7 cell culture preparation.
Cell culture media and supplements were obtained from Life Technologies (Invitrogen, Carlsbad, CA). The murine macrophage cell line RAW264.7 was obtained from American Type Culture Collection (catalog no. TIB-71; Manassas, VA). These cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum at 37°C in a 5% CO2, 95% air-humidified incubator. At the time of experimentation, cells were cultured in fresh medium. Cultured cells were washed three times with cold PBS containing CaCl2 (0.9 mM) and glucose (5.5 mM). Cells were then harvested in tissue lysis buffer (1% Triton X-100, NaCl [150 mM], and Tris buffer [10 mM] containing EDTA [1 mM], EGTA [1 mM], sodium orthovanadate [0.2 mM], phenylmethylsulfonyl fluoride [0.2 mM], and 0.5% Nonidet P-40 at a final pH of 7.4) using a rubber scraper. Cells were lysed and centrifuged at 15,000 rpm to remove insoluble material. For some experiments, B. anthracis-infected macrophages were treated with the actin microfilament disrupter cytochalasin D (10 μg/ml) for 10 min at 37°C prior to infection.
B. anthracis strain.
The Sterne 34F2 strain of B. anthracis (Colorado Serum Company, Denver, CO) (34), an attenuated variant employed extensively as an animal vaccine, and the ΔgerH strain constructed from the Sterne 34F2 (pXO1+, pXO2−) strain of B. anthracis (34), a germination-deficient variant which allows for examination of spore interactions independent of vegetative bacilli, were employed in this study. The organisms were stored in 10% glycerol L broth at −20°C. Both Difco L agar and Difco L broth were obtained from Becton Dickinson and Company (Franklin Lakes, NJ) and prepared as suggested by the manufacturer. Isolation agar was formulated as follows. Oxoid nutrient broth no. 2 (6 g; Oxoid Ltd.), Oxoid agar no. 3 (12 g), MnSO4 (0.3 g; J.T.Baker, Phillipsburg, NJ), and NaH2PO4 (0.25 g; OmniPur EM Science) were added to 1 liter sterile distilled water. The pH was adjusted to 6.7, and the agar was sterilized by autoclaving at 121°C for 15 min. Brain heart infusion broth (Difco) was used as a general growth medium.
Spore production.
A single colony harvested from an overnight culture grown on L agar at 37°C was used to inoculate 100 ml of L broth in a 250-ml conical flask. The culture was incubated at 37°C on an orbital shaker (200 rpm) for 6 h. At the end of this period, 3 ml of culture was transferred to a 225-ml vented tissue flask (Corning, Inc.) containing 175 ml isolation agar. Following inoculation, flasks were incubated at 37°C until 99 to 100% of the bacilli had formed endospores (phase-contrast microscopic examination). The percent yield of spores was determined by comparing colony counts of heated (70°C for 20 min) and unheated samples. Endospores were harvested by adding 20 ml of sterile PBS to the flask. The resuspended endospores from 20 flasks were pooled and centrifuged at 4,200 rpm for 10 min at 4°C. The resulting pellet was resuspended in 200 ml of sterile PBS and centrifuged again. To determine the effect of repeated washings on spore activity, endospores were washed a total of 10 times, with the endospores resuspended in a final volume of 50 ml, which was refrigerated at 4°C until used. The final endospore concentration was determined to be approximately 1 × 109 endospores/ml. Endospores used for experimentation were heat shocked at 56°C for 30 min to kill off any remaining bacilli.
Sonication of spores.
The exosporium is the primary site of contact for spore interactions with the environment; therefore, we sought to examine the susceptibility of B. anthracis spores in the absence of the exosporium. The exosporium was removed by disruption of the spores with sonication, as previously described (26). Spores were centrifuged at 4,200 rpm for 10 min at 4°C. Pellets were resuspended to approximately 3 × 107 spores/ml in Tris-HCl (50 mM) and EDTA buffer (0.5 mM, pH 7.5). All subsequent manipulations were at 4°C. Spores were sonicated (Branson Sonifier 150; Branson Ultrasonics Co., Danbury, CT) with maximum power (amplitude, 12 μm, 50 W/10 min) for 10 1-min bursts, each separated by 2 min of cooling on ice. Exosporium fragments were separated from spores by centrifugation at 4,000 rpm for 15 min. The spore pellets were washed once in PBS (pH 7.2), and the exosporium-containing supernatants were pooled and then centrifuged again to remove the remaining spores. Any residual endospores in the exosporium-containing supernatant were removed by filtration through 0.45- and/or 0.2-μm low-protein-binding filters (Acrodisc syringe filter; Pall Co., Timonium, MD).
B. anthracis spore induction of NOS 2 in RAW264.7 cells.
The mouse macrophage cell line RAW264.7 grown on tissue culture plates was infected for up to 18 h with nonsonicated ΔgerH spores (1 × 106 spores/ml). Cell viability was estimated using trypan blue exclusion. After infection, extracellular cell culture medium was subjected to a nitrite assay, and lysed cells were prepared for immunoblot analysis.
RAW264.7 cell phagocytosis of ΔgerH spores.
To determine phagocytic uptake, media from control and cytochalasin D-treated macrophages were examined for cultured bacterial colony growth. Samples of medium were taken from control and cytochalasin D (10 μg/ml)-pretreated macrophages infected with ΔgerH spores (multiplicity of infection [MOI], 1:1 [spore to macrophage]) for 18 h. Samples were then streaked onto L agar plates and incubated at 37°C overnight. For the cytochalasin D experiments, bacterial colonization present on L agar plates indicated that B. anthracis remained extracellular throughout the duration of the experiment.
Nitrite measurement by Griess reaction assay.
Accumulation of nitrite, an estimate of NO3 production, was determined colorimetrically after mixing 0.5 ml each of culture medium and freshly prepared Griess reagent [0.1% N-(1-naphthyl)ethylenediamine in water and 1% sulfanilamide in 5% phosphoric acid, mixed 1:1] (3, 12). Concentrations of nitrite were estimated by comparing absorbance readings at 550 nm against standard solutions of sodium nitrite prepared in the same medium.
Electron microscopic studies of B. anthracis infection of RAW264.7 macrophage cells.
Infection with the ΔgerH strain of B. anthracis spores was carried out at an MOI of 1:1 in a six-well culture plate containing RAW264.7 cells grown in DMEM plus 10% FBS. The plates were then incubated at 37°C in 5% CO2 for 18 h. At predetermined time points, the infected cells were gently scrapped and collected by centrifugation at 1,000 × g for 5 min. Cells were fixed in 4% formaldehyde with 1% glutaraldehyde in buffer. Then, they were postfixed in 1% osmium tetroxide. After the cells were stained en bloc with aqueous 2% uranyl acetate, they were dehydrated in a graded series of ethanol washes. Cell pellets were embedded in Spurr's resin and were polymerized. Ultrathin sections were stained with uranyl acetate and lead citrate and examined on a Zeiss 10 CA electron microscope at 80-kV accelerating voltage.
Killing of B. anthracis by murine peritoneal macrophages.
Peritoneal macrophages were cultured according to the method of Fortier et al. as previously described with some minor modifications (9). Primary peritoneal macrophages were obtained from Crl:CD-1 (ICR)BR mice (Jackson Laboratories, Bar Harbor, ME) 4 days after intraperitoneal inoculation with 3 ml of 3% thioglycolate. Peritoneal fluid was drawn through the abdominal wall with a 23-gauge needle. Fluid from mice was pooled and washed, total cell counts were determined using a hemacytometer, and the remaining fluid was centrifuged at 250 × g for 10 min at 4°C. Washed cell suspensions were adjusted to 106 macrophages/ml in RPMI 1640 with 10% FBS and sodium pyruvate (1 mM), l-glutamine (2 mM), penicillin (50 U/ml), and streptomycin (50 μg/ml) and then incubated in polypropylene tubes (Elkay Products, Inc., Shrewsbury, MA) in 5% CO2 at 37°C overnight before exposure to spores. Sterne 34F2 spores were added to the macrophages at an MOI of 1:10, with or without L-NIL (1 mM), in l-arginine-containing and -depleted media and incubated at 37°C in 5% CO2 for 30 min to allow phagocytosis. When the MOI was 1:10, spore killing increased. In contrast, there was no killing of ΔgerH spores even at an MOI of 1:20. We also demonstrated that sonicated Sterne 34F2 spores were more efficiently killed by macrophages than nonsonicated Sterne 34F2 spores. Therefore, experiments were carried out at an MOI of 1:10 with Sterne 34F2 sonicated spores. Infected macrophages were washed once in RPMI 1640 and incubated for 30 min in medium containing gentamicin (at a final concentration of 50 μg/ml) to remove extracellular bacilli. Macrophages were then resuspended to the original volume in fresh medium, and samples, diluted in PBS for colony counts, were obtained at 1, 3, 5, and 24 h following initial infection. Aliquots from each sample were incubated at 65°C for 30 min to assess the presence of vegetative cells. Samples were plated on L agar plates for colony counts. Log kill was calculated utilizing the following equation: (log CFU at 1 h) − (log CFU at 3, 5, or 24 h). Culture supernatants were collected for nitrite measurement and stored at −70°C until analyses.
Immunoblot analysis of NOS 2 protein.
Cell protein lysates were diluted with an equal volume of 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer and heated to 100°C for 2 min. Total protein (20 μg) was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. The immobilized proteins were then blocked in Tris-buffered saline (TBS-Tween; Tris [50 mM, pH 7.5], NaCl [150 mM], and Tween 20 [0.1%]) and 5% nonfat dry milk. Following an overnight incubation at 4°C with primary antibodies NOS 2, ERK1, and α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA), the membranes were washed several times with TBS-Tween. The horseradish peroxidase-conjugated secondary antibody was diluted 1:10,000 in TBS-Tween and incubated with the membrane for 45 min at room temperature. Following washes with TBS-Tween, proteins were detected using enhanced chemiluminescence (PerkinElmer Life Sciences).
Measurement of arginase activity.
Arginase is a manganese metalloenzyme responsible for the hydrolysis of l-arginine to l-ornithine and urea. Arginase activity was estimated by following the metabolism of l-arginine to urea using a modification of a previously developed method (5). Before an enzymatic activity assay, glycine (0.1 M) and MnCl2 (10 mM) were added to B. anthracis vegetative bacilli and spore suspensions at predetermined concentrations in PBS (pH 7.4). After preactivation, initiation of enzymatic activity proceeded by the addition of l-arginine at 37°C. Data were collected after 3 h of incubation. The reaction was stopped with 10% perchloric acid solution. A urea colorimetric assay was then performed on samples brought to a 2.0-ml volume and incubated with 1 ml of concentrated phosphoric acid and sulfuric acid at a ratio of 3:1 and 2-isonitrosopropiophenone (4%) in a boiling water bath for 1 h. Absorbance readings were measured at 540 nm against standard solutions of urea. B. anthracis arginase activity was estimated utilizing a concentration curve for purified bovine arginase with an activity of 19.3 U/mg, where 1 U of enzyme catalyzes the hydrolysis of 1.0 μmol of l-arginine/min at 37°C (pH 9.5).
Spin trapping O2·−.
Spin trapping of O2·− from purified NOS 2 was conducted in the following manner. NADPH (150 μM) was added to 50 mM (pH 7.4) potassium phosphate buffer (chelexed; 1 mM EGTA, 1 mM DTPA [diethylenetriaminepentaacetic acid]) at 23°C that contained NOS 2 (6.5 μg), BMPO (50 mM), and l-arginine (either 0 or 100 μM) in the absence and presence of L-NIL (100 μM) to a final volume of 0.3 ml. The reaction mixture was then transferred to a flat quartz cell and placed into the cavity of an electron paramagnetic resonance (EPR) spectrometer (model E-109; Varian Associates, Inc., Palo Alto, CA). The EPR quartz cell was open to the air, allowing O2 to enter. EPR spectra were recorded at room temperature. The instrument settings were as follows: microwave power, 20 mW; modulation frequency, 100 kHz; modulation amplitude, 0.5 G; sweep time, 12.5 G/min; and response time, 0.5 s.
Densitometry and statistical analysis.
Immunoblot images were quantified by densitometer analysis using NIH ImageJ software for Windows. The results were expressed as the means ± standard deviations from at least three independent experiments. The means were compared using Student's t test, with P values of <0.05 considered statistically significant.
RESULTS
B. anthracis spores induce macrophage phagocytosis.
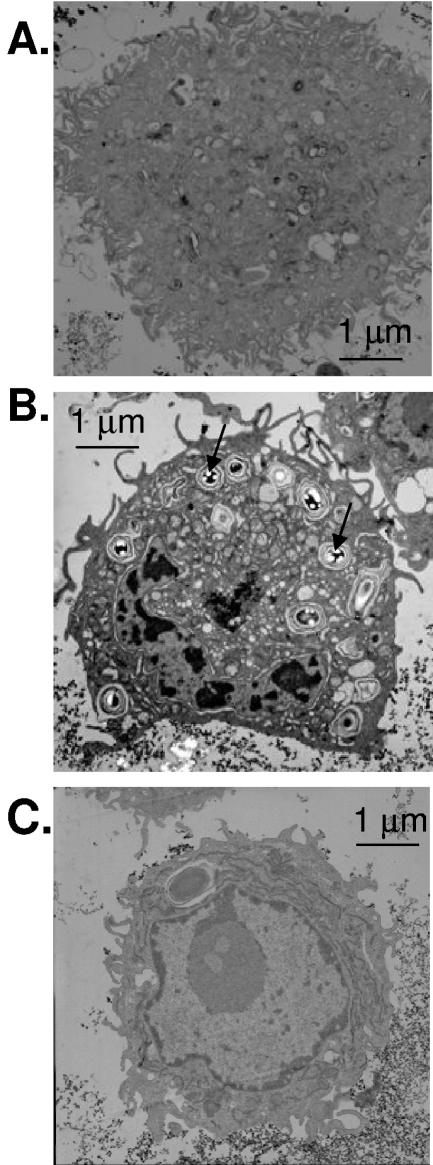
It has been reported that macrophages rapidly phagocytose B. anthracis spores, which then germinate to vegetative bacteria (7). We, however, were interested in whether the engulfment of spores, per se, would up-regulate NOS 2, as has been observed with other bacteria (21, 24). Thus, for the initial series of experiments, the ΔgerH strain of B. anthracis spores, which is germination deficient in macrophages (34), was chosen as the model. The ΔgerH mutant is a useful model for determining spore-host interactions with the macrophage in the absence of germination. In these studies, the murine macrophage cell line RAW264.7 was grown in normal DMEM, and prior to experimentation, fresh medium was added. The ΔgerH strain of B. anthracis spores was then incubated with RAW264.7 cells, as described in Materials and Methods. Visualization by transmission electron microscopy (TEM) showed that the B. anthracis ΔgerH spores were engulfed by the macrophages and taken up into the phagolysosome. Data shown in Fig. 1B were from after an 18-h infection. In contrast, RAW264.7 cells that had not been exposed to the B. anthracis ΔgerH spores showed normal morphology (Fig. 1A).
FIG. 1.
B. anthracis induces phagocytosis in RAW264.7 macrophage cells. RAW264.7 cells were infected with the ΔgerH strain of B. anthracis (MOI, 1:1) and then washed and fixed for TEM. (A) TEM of control RAW264.7 cells without infection. (B) TEM of RAW264.7 cells infected with B. anthracis ΔgerH spores for 18 h. Arrows indicate spores (light intense images) inside of phagolysosomes. (C) TEM of RAW264.7 cells pretreated for 10 min with cytochalasin D (10 μg/ml) to inhibit phagocytosis, infected with B. anthracis ΔgerH spores for 18 h, and then washed. No spores were visualized in the presence of cytochalasin D; the vacuole present in the cell is of unknown origin, but size and absence of light fragmentation suggest it is not a B. anthracis spore. The general morphology of cell organelles was not altered by exposure to B. anthracis. Bars, 1 μm.
We next sought to determine whether phagocytosis of B. anthracis ΔgerH spores was actin dependent, as is the case for other bacteria (36). Therefore, macrophages were incubated for 10 min with cytochalasin D (10 μg/ml), and then these cells were infected with B. anthracis ΔgerH spores. Data shown in Fig. 1C were from after an 18-h infection. The medium was then removed, and cells were washed and visualized for the presence of spores by TEM. Results shown in Fig. 1C confirmed that B. anthracis ΔgerH spores were not taken up by the macrophages treated with cytochalasin D. To further demonstrate that B. anthracis spores did not undergo phagocytic uptake in the presence of cytochalasin D, media from the macrophage-spore experiments were examined for the presence of bacteria. Samples of medium of RAW264.7 cells that were either treated or not treated with cytochalasin D prior to infection with B. anthracis spores revealed the presence of bacterial colonies on L agar plates. In the case of cytochalasin D-treated macrophages, the number of bacterial colonies was found to be considerably greater than that observed for untreated macrophages. Thus, these findings in accordance with TEM data suggest that the RAW264.7 macrophage uptake of B. anthracis spores is by an actin-dependent phagocytic process.
B. anthracis spores induce expression of NOS 2 and nitrite generation in the macrophage cell line RAW264.7.
Upon confirmation that RAW264.7 cells phagocytosed B. anthracis spores, we next sought to identify whether de novo synthesis of NOS 2 would occur, as has been reported for other bacteria (19). RAW264.7 cells were cultured as previously described and infected with spores at a concentration of 1.0 × 106 spores/ml (MOI, 1:1) for a time course of 2 to 18 h. At each 2-h time point, macrophages from six-well plates (n = 12) were scraped, lysed, and prepared for immunoblot analyses, using the specific 130-kDa NOS 2 antibody as a marker of the protein. NOS 2 expression as a function of time was estimated (Fig. 2A). Detectable levels of NOS 2 were first observed at 12 h, and this level of protein remained constant over the next 6 h, when the experiment was terminated. The time course for up-regulation of NOS 2 paralleled that found for LPS induction of NOS 2 in RAW264.7 cells, where protein expression was sustained for 18 h after treatment (Fig. 2B). Similarly, NOS 2 protein expression in RAW264.7 cells was obtained after an 18-h treatment with IFN-γ and LPS, as well as after infection with S. aureus and E. coli (Fig. 2C).
FIG. 2.
B. anthracis induces NOS 2 activity. (A) RAW264.7 cells infected with B. anthracis ΔgerH spores (MOI, 1:1) at 37°C for 2 to 18 h were lysed, protein run on polyacrylamide gel, and subjected to immunoblot analysis for NOS 2 expression. Sample lysates were probed for α-tubulin as a protein loading control. (B) RAW264.7 cells infected with LPS (100 ng/ml) for 10 to 18 h were lysed, protein run on polyacrylamide gel, and subjected to immunoblot analysis for NOS 2 expression. Sample lysates were probed for α-tubulin as a protein loading control. (C) RAW264.7 cells infected with B. anthracis ΔgerH spores (MOI, 1:1), Staphyloccus aureus (MOI, 1:10), E. coli (MOI, 1:10), LPS (100 ng/ml), and LPS (100 ng/ml) plus IFN-γ (10 μM) for 18 h. Samples then underwent immunoblot analysis for NOS 2 expression. Lysates were probed for α-tubulin as a protein loading control. Data are representative of three independent experiments.
To determine whether NOS 2 up-regulation was dependent upon phagocytosis of B. anthracis spores, macrophages were incubated for 10 min with cytochalasin D (10 μg/ml) and then infected with B. anthracis ΔgerH spores for 18 h at 37°C and subjected to immunoblot analysis. The data revealed no detectable NOS 2 protein expression (Fig. 3) in lysates with inhibited phagocytosis, suggesting that NOS 2 is synthesized after spore phagocytosis.
FIG. 3.
Phagocytosis-mediated NOS 2 expression. RAW264.7 cells pretreated for 10 min with cytochalasin D (10 μg/ml) and infected with B. anthracis ΔgerH spores for 18 h were lysed, protein run on polyacrylamide gel, and subjected to immunoblot analysis for NOS 2 expression. Sample lysates were probed for α-tubulin as a protein loading control. Data are representative of three independent experiments.
It has been reported that macrophage-derived NO· is generated from NOS 2 (24). We determined whether NOS 2 protein that was expressed in RAW264.7 cells after exposure to B. anthracis spores was functionally active, e.g., generating NO·. We also sought to examine whether the removal of the exosporium by sonication would alter NO· production in infected macrophages. Therefore, RAW264.7 cells cultured in DMEM were infected with sonicated and nonsonicated B. anthracis ΔgerH spores at a concentration of 1.0 × 106 spores/ml for up to 24 h, with the extracellular medium being removed at defined times to estimate nitrite concentration using the Griess assay (Fig. 4). The Griess assay estimates NO· production by measuring one of its oxidation products, nitrite, in the growth medium and has a sensitivity limit of ∼1.0 μM. Data presented in Fig. 4 demonstrate that significant levels of nitrite were detected at 6 h in macrophages infected with sonicated spores and at 10 h in those infected with nonsonicated spores. Nitrite levels then increased for the next 10 h. These results suggest that NOS 2 generated NO· in response to sonicated and nonsonicated B. anthracis spore infection (Fig. 4). As also shown in Fig. 4, RAW264.7 cells infected with sonicated B. anthracis spores generated twice as much NO· as did RAW264.7 cells infected with nonsonicated spores. These findings in conjunction with TEM data shown in Fig. 1 suggest that during phagocytosis of B. anthracis spores, NO· was generated by the newly transcribed NOS 2. This finding suggests that the B. anthracis exosporium, which is removed by the process of sonication (18), is important in regulating NO· production by NOS 2 in RAW264.7 cells.
FIG. 4.
Differential nitrite production by RAW264.7 macrophage cells infected with sonicated and nonsonicated B. anthracis. The Griess reaction assay was used to demonstrate the generation of μM concentrations of nitrite, the oxidation product of NO·, up to 24 h after sonicated (without exosporium) and nonsonicated (normal, with exosporium) B. anthracis ΔgerH spore (MOI, 1:1) infection of RAW264.7 macrophage cells. Black bars represent RAW264.7 cells infected with sonicated B. anthracis ΔgerH spores. White bars represent RAW264.7 cells infected with nonsonicated B. anthracis ΔgerH spores. Data are representative of three independent experiments. *, significance at P < 0.05 compared to control.
Arginase activity in B. anthracis.
Given that B. anthracis contains an arginase (28), we designed experiments to determine whether this enzyme is active and, if it is, whether it can regulate NO· production by diminishing the concentration of l-arginine available to be oxidized by NOS 2. Bacilli and spores of B. anthracis, at 1.0 × 107 spores/ml, were suspended in PBS at pH 7.4 and preactivated in a solution containing the enzyme cofactor Mn2+ (10 mM). Initiation of arginase activity began with the addition of l-arginine, and the reaction was allowed to proceed for 3 h before being terminated with perchloric acid. Urea production was measured thereafter as an estimate of arginase activity, as described in Material and Methods. Arginase activities for bacilli and spores were found to be 1.91 ± 0.54 U/1.0 × 107 bacilli and 0.17 ± 0.07 U/1.0 × 107 spores, respectively. Thus, arginase activity is 10-fold greater in B. anthracis bacilli than in B. anthracis spores. These findings would suggest that competition for available l-arginine between NOS 2 in the macrophage and arginase in B. anthracis is possible, since both enzymes demonstrate significant activity.
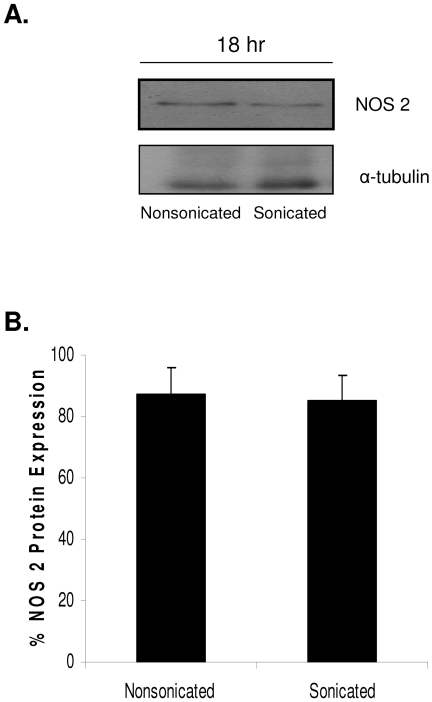
Given that spores of B. anthracis are phagocytosed by macrophages and presumably still retain arginase activity, experiments were conducted to determine the impact of an active arginase on NO· production by macrophages that express NOS 2. RAW264.7 cells were infected with either sonicated or nonsonicated spores of B. anthracis at a concentration of 1.0 × 106 spores/ml (MOI, 1:1) for 18 h. Cells were then lysed and prepared for immunoblot analysis. Expression of NOS 2 was demonstrated in sonicated and nonsonicated spores (Fig. 5A). Quantification of protein expression by densitometry showed that there was no significant difference in the levels of NOS 2 protein after macrophage infection with B. anthracis spores, whether they were sonicated or nonsonicated (Fig. 5B), at 18 h. Equivalent protein expression levels would suggest that increased NO· generation by RAW264.7 cells infected with sonicated B. anthracis would not be the result of changes in transcriptional activity but rather substrate availability. The results presented in Fig. 4 and 5 imply that the B. anthracis exosporium contains an active arginase that can effectively compete with NOS 2 for l-arginine.
FIG. 5.
Differential NOS 2 induction in sonicated and nonsonicated B. anthracis. The removal of the B. anthracis exosporium by the process of sonication allowed examination of the differences in spore-host interactions mediated by the exosporium. (A) RAW264.7 cells infected with sonicated and nonsonicated B. anthracis ΔgerH spores (MOI, 1:1) at 37°C for 18 h were lysed, protein run on polyacrylamide gel, and subjected to immunoblot analysis for NOS 2 expression. Sample lysates were probed for α-tubulin as a protein loading control. (B) Quantitative analyses through immunoblot densitometry were performed utilizing the NIH ImageJ program. Data are representative of three independent experiments.
B. anthracis spores induce NO· production in primary murine peritoneal macrophages.
Primary murine peritoneal macrophages have been shown to mediate killing of the Sterne 34F2 spore, which can germinate in the macrophage (18). We were interested, therefore, in determining what role, if any, NO· played in mediating this microbicidal activity. Before arriving at that point, however, we first designed experiments to determine whether these murine macrophages infected with this strain of spores produced NO·. As shown in Fig. 6A and B, primary murine peritoneal macrophages infected with Sterne 34F2 spores generated NO·, and the levels of this free radical at 24 h from macrophages infected with spores which lacked the exosporium were significantly higher than those found for macrophages infected with nonsonicated spores under identical experimental conditions. Moreover, the pretreatment with IFN-γ increased the release of NO· from primary macrophages infected by Sterne 34F2 spores (Fig. 6A and B), which is expected because of IFN-γ's known ability to increase NO· production through the priming of NOS 2. The increased NO· production in macrophages infected with sonicated Sterne 34F2 spores paralleled data found with the RAW264.7 macrophage cell line, as shown in Fig. 4. Finally, when L-NIL (1 mM), a NOS 2 competitive inhibitor, was included in the culture medium in which primary murine peritoneal macrophages were infected with Sterne 34F2 spores, no detectable levels of nitrite, an estimate of NO· generation, were measured using the Griess assay (data not shown).
FIG. 6.
Nitrite production in B. anthracis spore-infected macrophages. Primary murine peritoneal macrophages (1 × 106cells/ml) were pretreated with (black bars) or without (white bars) IFN-γ (100 U/ml) overnight and infected with B. anthracis spores (1 × 106 spores/ml) prepared from nonsonicated B. anthracis Sterne 34F2 (A) and sonicated B. anthracis Sterne 34F2 (B). Cell culture supernatants were collected, and nitrite production was determined at 1, 3, 5, and 24 h postinfection utilizing the Griess reaction assay. *, significance at P < 0.01 compared to 1 h postinfection. Differences in nitrite production between results shown in panels A and B are in agreement with data presented in Fig. 4.
NOS 2-mediated B. anthracis killing in macrophages.
We earlier demonstrated that while primary murine peritoneal macrophages are unable to kill the spore form of B. anthracis, these phagocytic cells do kill the vegetative form emerging from the spore (18). We therefore studied whether these macrophages could mediate the killing of B. anthracis Sterne 34F2 and whether NO· was required for killing of B. anthracis by the activated macrophages. We used a bacterial killing assay as an end point.
Primary murine peritoneal macrophages infected with Sterne 34F2 spores exhibited a 0.35 and 0.5 log kill at 5 and 24 h, respectively (Fig. 7A). Macrophages treated with L-NIL (1 mM) and then infected with spores showed reduced killing: 0.11 and 0.26 log kill at the same time points, respectively (Fig. 7A). To further define the importance of NO· in macrophage-mediating killing of Sterne 34F2 bacilli, macrophages were cultured for 24 h in medium that did not contain l-arginine, at which point the macrophages were infected with spores. Even after 24 h, there were no measurable levels of nitrite in the growth medium (data not shown). Next, resting and IFN-γ-activated macrophages were cultured in medium with or without l-arginine and infected with spores. In the absence of IFN-γ pretreatment, the addition of spores to macrophages cultured with l-arginine enhanced killing of B. anthracis by macrophages to 0.29 and 0.58 log kill at 5 and 24 h postinfection, respectively, while the addition of spores to macrophages cultured in medium without l-arginine resulted in growth (−0.14 log kill) at 5 h and modest killing (0.35 log kill) at 24 h (Fig. 7B). IFN-γ-primed macrophages increased the killing of administered spores to 0.56 and 0.71 logs at 5 and 24 h, respectively, in medium with l-arginine (data not shown in Fig. 7). However, following IFN-γ treatment in medium without l-arginine, macrophages infected with spores had reduced killing of spores (0.09 and 0.40 log kill at 5 and 24 h, respectively) (data not shown in Fig. 7). These findings demonstrate that l-arginine availability is critical to NOS 2-mediated killing of Sterne 34F2 spores in primary murine peritoneal macrophages.
FIG. 7.
l-Arginine-dependent killing of B. anthracis in spore-infected macrophages. (A) Primary murine peritoneal macrophages (1 × 106 cells/ml) were infected with spores (1 × 105 spores/ml) prepared from sonicated B. anthracis strain Sterne 34F2 and treated with (▪) or without (□) L-NIL (1 mM). The infected macrophages were incubated for 1, 3, 5, and 24 h in 5% CO2 at 37°C, washed, and lysed for viable count plating, and numbers of CFU were determined. The data are expressed as log values. Log kill values were as defined in Materials and Methods. (B) Macrophages (1 × 106 cells/ml) were infected with spores (1 × 105 spores/ml) prepared from sonicated B. anthracis strain Sterne 34F2 in culture medium with (□) or without (▪) l-arginine. The infected macrophages were incubated for 1, 3, 5, and 24 h in 5% CO2 at 37°C, washed, and lysed for viable count plating, and numbers of CFU were determined. The data are expressed as differences in log values. Data are shown as means ± standard deviations of values obtained from two independent experiments, each conducted in duplicate.
DISCUSSION
Upon encountering a microorganism, resident macrophages phagocytose the microbe, resulting in the assembly of the NADPH oxidase with the secretion of O2·−. Similarly, this phagocytosis event leads to the up-regulation of NOS 2, which metabolizes l-arginine to l-citrulline and NO·. These free radicals and the products derived from them, e.g., H2O2 and ONOO−, are part of the microbicidal arsenal that is aimed at killing the microorganism. Like other microbes, endospores of B. anthracis were phagocytosed by macrophages (Fig. 1), and under physiological conditions, germinate into the vegetative bacteria. With a compromised phagocyte, these bacilli are able to exit and infect the host (15). The question remains as to the mechanisms by which the endospores of B. anthracis evade the normal host immune responses of the macrophage. One clue comes from a previous study in our laboratory in which we demonstrated that the endospores of B. anthracis scavenge O2·−, which we surmised was catalyzed by the SODs that are expressed in the endospore. Further, whatever amount of O2·− that somehow avoids the SODs is then used to promote germination (1).
The importance of NO· in host immune response to microbes is pathogen dependent (8, 16, 33). For example, nitrite, a NO· by-product, appears to inhibit Bacillus cereus spore germination (4, 23), whereas in the case of Helicobacter pylori, this free radical is essential for bacterial killing by the macrophage. A microbial arginase in H. pylori evades NO· flux by limiting the availability of l-arginine (11, 20). In our initial series of experiments, the endospore-macrophage interaction resulted in the phagocytosis of the spore concomitant with expression of NOS 2, as was also seen for S. aureus and E. coli (Fig. 2). Given the induction of NOS 2 by the endospores, why was NOS 2-generated NO· not sufficient to mediate complete macrophage killing of B. anthracis? The answer may lie in the following observations. We first identified a NOS 2 transcription-independent twofold increase in NO· generation in macrophages infected with sonicated spores lacking an intact exosporium. We also confirmed the presence of an active arginase present in B. anthracis spores and vegetative bacilli, which catalyzes the conversion of l-arginine to urea and l-ornithine. As in the case of H. pylori, one may suggest that there is competition with NOS 2 for l-arginine, resulting in diminished production of NO· by NOS 2.
One may speculate from data presented herein that O2·− may play an important role in promoting the survival of B. anthracis, once localized in macrophages. First, the observed microbicidal activity shown in Fig. 7A was decreased by inclusion of the specific NOS 2 antagonist L-NIL. From spin trapping experiments conducted with purified NOS 2, we observed that L-NIL inhibited NO· production, whereas this compound did not decrease O2·− generation (data not shown). Second, when these NOS 2-induced macrophages were cultured in medium depleted of l-arginine, essentially reducing NO· production while promoting generation of O2·− (35), there was no killing of B. anthracis present at 5 h and significantly reduced (<25%) killing at 24 h. Thus, the decrease in B. anthracis killing seen in the presence of L-NIL and in l-arginine-depleted medium could be mediated through differential free radical production by NOS 2: diminished NO· and increased O2·−.
In conclusion, this research suggests a possible functional role of the B. anthracis exosporium in NO·-mediated killing. Previously published work has identified several novel proteins of the B. anthracis exosporium, noting their possible contributions to the in vivo activities of the exosporium (27). Data herein indicate that B. anthracis may regulate NO·-mediated killing in host cells through a mechanism involving decreased production of NO·, as production of arginase effectively competes with NOS 2 for l-arginine. Understanding spore survival in the macrophage is important because the subsequent germination, multiplication, and evacuation from the macrophage lead to the bacterium's virulence. It should be noted that NOS 2 is capable of generating NO·, O2·−, H2O2, and ONOO−, and this current study is limited in determining the role of NO· in B. anthracis killing.
Acknowledgments
We are grateful to Richard Coleman of the University of Maryland School of Medicine, Department of Physiology, who performed the transmission electron microscopy experiments.
This research was supported in part by grants from the National Institutes of Health, EB-2034, the Mid-Atlantic Regional Center for Biodefense and Emerging Infectious Diseases, and NIAID U54 AI-057168 (A.S.C. and G.M.R.).
Editor: A. D. O'Brien
REFERENCES
- 1.Baillie, L., S. Hibbs, P. Tsai, G. L. Cao, and G. M. Rosen. 2005. Role of superoxide in the germination of Bacillus anthracis endospores. FEMS Microbiol. Lett. 245:33-38. [DOI] [PubMed] [Google Scholar]
- 2.Baillie, L., and T. D. Read. 2001. Bacillus anthracis, a bug with attitude! Curr. Opin. Microbiol. 4:78-81. [DOI] [PubMed] [Google Scholar]
- 3.Beckman, J. S., R. L. Minor, Jr., C. W. White, J. E. Repine, G. M. Rosen, and B. A. Freeman. 1988. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J. Biol. Chem. 263:6884-6892. [PubMed] [Google Scholar]
- 4.Buchman, G. W., III, and J. N. Hansen. 1987. Modification of membrane sulfhydryl groups in bacteriostatic action of nitrite. Appl. Environ. Microbiol. 53:79-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis, R. H., and J. Mora. 1968. Mutants of Neurospora crassa deficient in ornithine-δ-transaminase. J. Bacteriol. 96:383-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon, T. C., A. A. Fadl, T. M. Koehler, J. A. Swanson, and P. C. Hanna. 2000. Early Bacillus anthracis-macrophage interactions: intracellular survival and escape. Cell. Microbiol. 2:453-463. [DOI] [PubMed] [Google Scholar]
- 7.Dixon, T. C., M. Meselson, J. Guillemin, and P. C. Hanna. 1999. Anthrax. N. Engl. J. Med. 341:815-826. [DOI] [PubMed] [Google Scholar]
- 8.Fang, F. C. 1997. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 99:2818-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortier, A. H., T. Polsinelli, S. J. Green, and C. A. Nacy. 1992. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect. Immun. 60:817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganz, T., and R. I. Lehrer. 1995. Defensins. Pharmacol. Ther. 66:191-205. [DOI] [PubMed] [Google Scholar]
- 11.Gobert, A. P., D. J. McGee, M. Akhtar, G. L. Mendz, J. C. Newton, Y. Cheng, H. L. Mobley, and K. T. Wilson. 2001. Helicobacter pylori arginase inhibits nitric oxide production by eukaryotic cells: a strategy for bacterial survival. Proc. Natl. Acad. Sci. USA 98:13844-13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126:131-138. [DOI] [PubMed] [Google Scholar]
- 13.Guidi-Rontani, C., M. Weber-Levy, E. Labruyere, and M. Mock. 1999. Germination of Bacillus anthracis spores within alveolar macrophages. Mol. Microbiol. 31:9-17. [DOI] [PubMed] [Google Scholar]
- 14.Guidi-Rontani, C., M. Weber-Levy, M. Mock, and V. Cabiaux. 2000. Translocation of Bacillus anthracis lethal and oedema factors across endosome membranes. Cell. Microbiol. 2:259-264. [DOI] [PubMed] [Google Scholar]
- 15.Hanna, P. C., and J. A. Ireland. 1999. Understanding Bacillus anthracis pathogenesis. Trends Microbiol. 7:180-182. [DOI] [PubMed] [Google Scholar]
- 16.Hibbs, J. B., Jr., R. R. Taintor, Z. Vavrin, and E. M. Rachlin. 1988. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem. Biophys. Res. Commun. 157:87-94. [DOI] [PubMed] [Google Scholar]
- 17.Ireland, J. A. W., and P. C. Hanna. 2002. Macrophage-enhanced germination of Bacillus anthracis endospores requires gerS. Infect. Immun. 70:5870-5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang, T. J., M. J. Fenton, M. A. Weiner, S. Hibbs, S. Basu, L. Baillie, and A. S. Cross. 2005. Murine macrophages kill the vegetative form of Bacillus anthracis. Infect. Immun. 73:7495-7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan, S. S., R. E. Basford, M. H. Jeong, and R. L. Simmons. 1996. Biomaterial-neutrophil interactions: dysregulation of oxidative functions of fresh neutrophils induced by prior neutrophil-biomaterial interaction. J.Biomed. Mater. Res. 30:67-75. [DOI] [PubMed] [Google Scholar]
- 20.Kuwahara, H., Y. Miyamoto, T. Akaike, T. Kubota, T. Sawa, S. Okamoto, and H. Maeda. 2000. Helicobacter pylori urease suppresses bactericidal activity of peroxynitrite via carbon dioxide production. Infect. Immun. 68:4378-4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyons, C. R. 1995. The role of nitric oxide in inflammation. Adv. Immunol. 60:323-371. [DOI] [PubMed] [Google Scholar]
- 22.McGee, D. J., F. J. Radcliff, G. L. Mendz, R. L. Ferrero, and H. L. T. Mobley. 1999. Helicobacter pylori rocF is required for arginase activity and acid protection in vitro but is not essential for colonization of mice or for urease activity. J. Bacteriol. 181:7314-7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris, S. L., and J. N. Hansen. 1981. Inhibition of Bacillus cereus spore outgrowth by covalent modification of a sulfhydryl group by nitrosothiol and iodoacetate. J. Bacteriol. 148:465-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathan, C., and Q. W. Xie. 1994. Regulation of biosynthesis of nitric oxide. J. Biol. Chem. 269:13725-13728. [PubMed] [Google Scholar]
- 25.Patterson-Delafield, J., R. J. Martinez, and R. I. Lehrer. 1980. Microbicidal cationic proteins in rabbit alveolar macrophages: a potential host defense mechanism. Infect. Immun. 30:180-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Read, T. D., S. N. Peterson, N. Tourasse, L. W. Baillie, I. T. Paulsen, K. E. Nelson, H. Tettelin, D. E. Fouts, J. A. Eisen, S. R. Gill, E. K. Holtzapple, O. A. Okstad, E. Helgason, J. Rilstone, M. Wu, J. F. Kolonay, M. J. Beanan, R. J. Dodson, L. M. Brinkac, M. Gwinn, R. T. DeBoy, R. Madpu, S. C. Daugherty, A. S. Durkin, D. H. Haft, W. C. Nelson, J. D. Peterson, M. Pop, H. M. Khouri, D. Radune, J. L. Benton, Y. Mahamoud, L. Jiang, I. R. Hance, J. F. Weidman, K. J. Berry, R. D. Plaut, A. M. Wolf, K. L. Watkins, W. C. Nierman, A. Hazen, R. Cline, C. Redmond, J. E. Thwaite, O. White, S. L. Salzberg, B. Thomason, A. M. Friedlander, T. M. Koehler, P. C. Hanna, A. B. Kolsto, and C. M. Fraser. 2003. The genome sequence of Bacillus anthracis Ames and comparison to closely related bacteria. Nature 423:81-86. [DOI] [PubMed] [Google Scholar]
- 27.Redmond, C., L. W. Baillie, S. Hibbs, A. J. Moir, and A. Moir. 2004. Identification of proteins in the exosporium of Bacillus anthracis. Microbiology 150:355-363. [DOI] [PubMed] [Google Scholar]
- 28.Soru, E. 1983. Chemical and immunological properties of B. anthracis arginase and its metabolic involvement. Mol. Cell. Biochem. 50:173-183. [DOI] [PubMed] [Google Scholar]
- 29.Steichen, C., P. Chen, J. F. Kearney, and C. L. Turnbough, Jr. 2003. Identification of the immunodominant protein and other proteins of the Bacillus anthracis exosporium. J. Bacteriol. 185:1903-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sylvestre, P., E. Couture-Tosi, and M. Mock. 2002. A collagen-like surface glycoprotein is a structural component of the Bacillus anthracis exosporium. Mol. Microbiol. 45:169-178. [DOI] [PubMed] [Google Scholar]
- 31.Tsai, P., K. Ichikawa, C. Mailer, S. Pou, H. J. Halpern, B. H. Robinson, R. Nielsen, and G. M. Rosen. 2003. Esters of 5-carboxyl-5-methyl-1-pyrroline N-oxide: a family of spin traps for superoxide. J. Org. Chem. 68:7811-7817. [DOI] [PubMed] [Google Scholar]
- 32.Weaver, J., S. Porasuphatana, P. Tsai, S. Pou, L. J. Roman, and G. M. Rosen. 2005. A comparative study of neuronal and inducible nitric oxide synthases: generation of nitric oxide, superoxide, and hydrogen peroxide. Biochim. Biophys. Acta 1726:302-308. [DOI] [PubMed] [Google Scholar]
- 33.Wei, X. Q., I. G. Charles, A. Smith, J. Ure, G. J. Feng, F. P. Huang, D. Xu, W. Muller, S. Moncada, and F. Y. Liew. 1995. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375:408-411. [DOI] [PubMed] [Google Scholar]
- 34.Weiner, M. A., and P. C. Hanna. 2003. Macrophage-mediated germination of Bacillus anthracis endospores requires the gerH operon. Infect. Immun. 71:3954-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia, Y., and J. L. Zweier. 1997. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc. Natl. Acad. Sci. USA 94:6954-6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, B., G. L. Cao, A. Cross, J. B. Domachowske, and G. M. Rosen. 2002. Differential antibacterial activity of nitric oxide from the immunological isozyme of nitric oxide synthase transduced into endothelial cells. Nitric Oxide 7:42-49. [DOI] [PubMed] [Google Scholar]