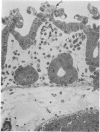Abstract
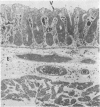
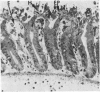
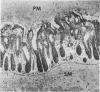
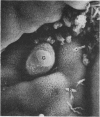
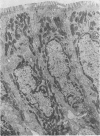
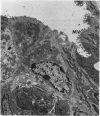
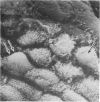
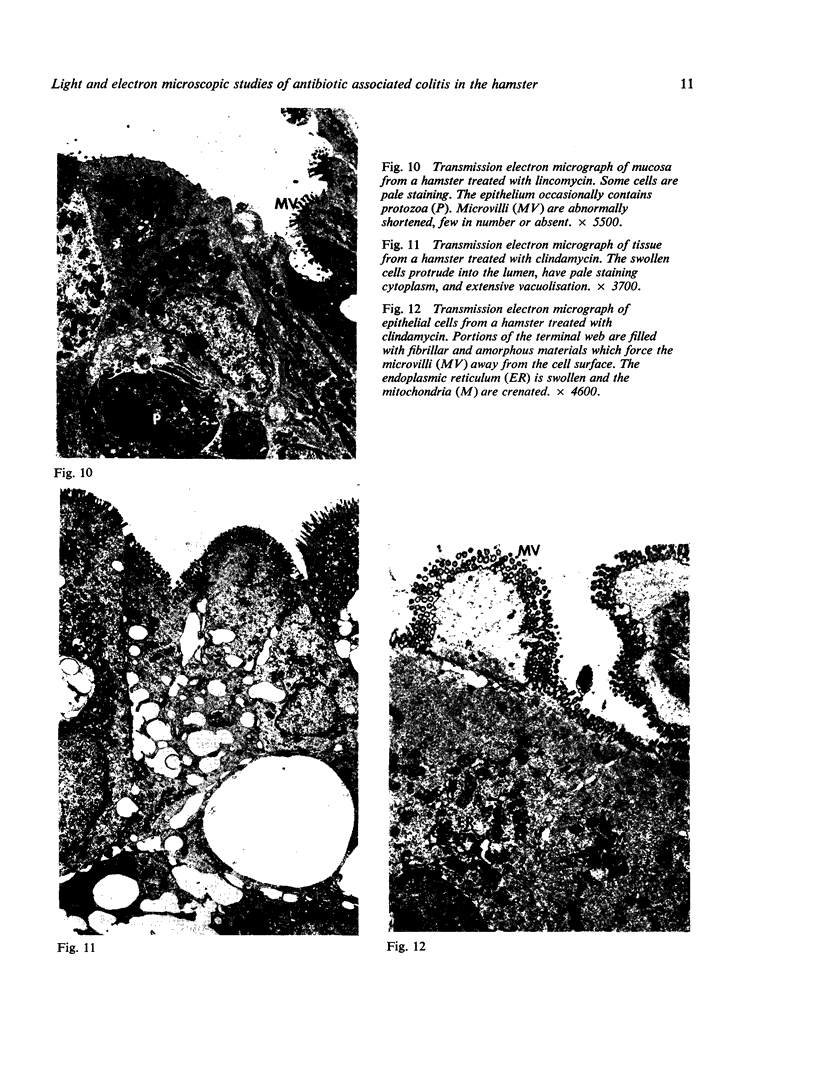
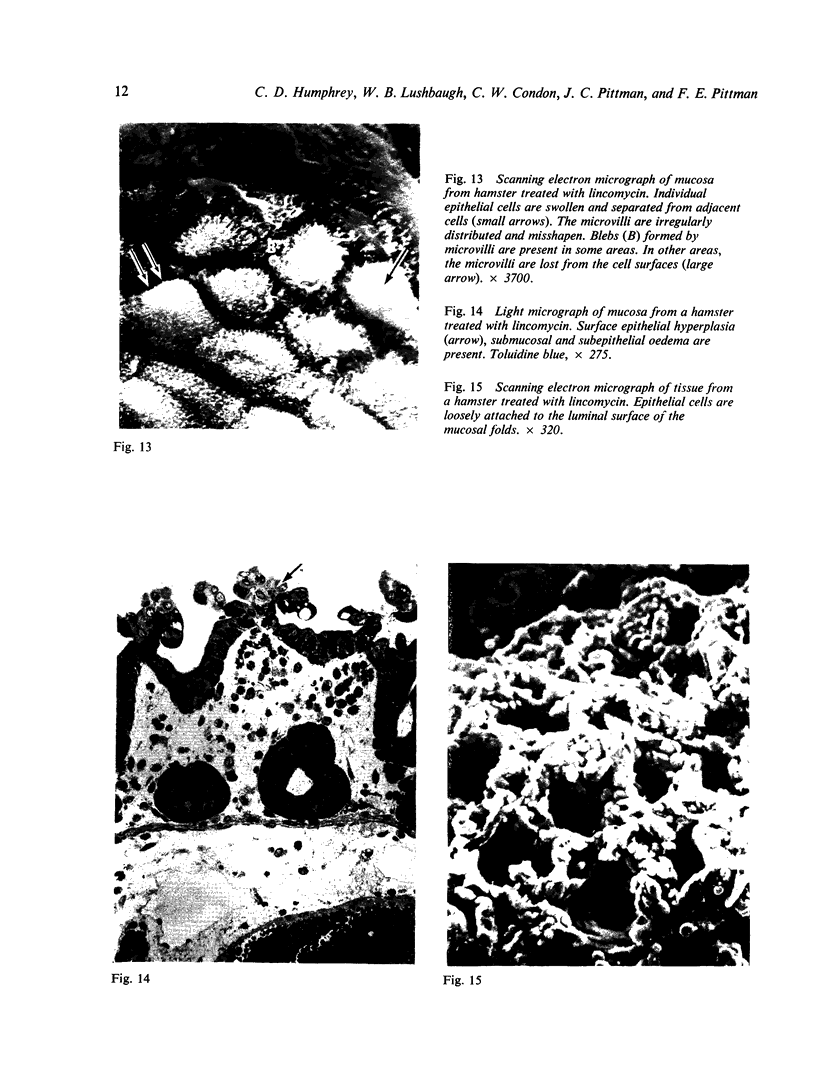
Lincomycin and its analogue, clindamycin, are capable of producing mild to severe colonic mucosal injury in humans (antibiotic associated colitis). Patients with the disorder may have severe diarrhoea, pseudomembranous plaques, confluent pseudomembranes, and/or a frank, diffuse haemorrhagic colitis. The present study was designed to assess the Golden Syrian hamster as an animal model for antibiotic associated colitis and to describe lesions seen in the animal model by light, transmission electron, and scanning electron microscopy. A colitis was produced in Golden Syrian hamsters by oral or parenteral administration of lincomycin, clindamycin, or N-demethyl clindamycin. Animals were killed at intervals and microscopic studies made of sequential morphological changes in the ileum, caecum, and colon. The microscopic lesions in the early stages of the disorder were abnormalities within the brush border, cellular oedema, and hyperaemia. Changes in the intracellular organelles were observed in more severely damaged epithelial cells. Epithelial hyperplasia resulted in the piling up of cells on the mucosal surfaces. In specimens with the most severe damage, complete loss of epithelium from the mucosal surface was observed. Pseudomembranous plaques were occasionally seen. Comparison of the clinical, gross, and histological features of the animal disease with the human disorder suggest that, although minor differences are present, the hamster model is suitable for experimental studies of antibiotic associated colitis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G., Chang T. W., Gurwith M., Gorbach S. L., Onderdonk A. B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978 Mar 9;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Onderdonk A. B., Cisneros R. L., Kasper D. L. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J Infect Dis. 1977 Nov;136(5):701–705. doi: 10.1093/infdis/136.5.701. [DOI] [PubMed] [Google Scholar]
- Chandler R. L., Bird R. G., Bland A. P. Letter: Particles associated with microvillous border of intestinal mucosa. Lancet. 1975 Nov 8;2(7941):931–932. doi: 10.1016/s0140-6736(75)92175-3. [DOI] [PubMed] [Google Scholar]
- Dougherty W. J. Microblebs of intestinal epithelial cell microvilli of Citellus tridecemlineatus. Anat Rec. 1976 May;185(1):77–83. doi: 10.1002/ar.1091850107. [DOI] [PubMed] [Google Scholar]
- Humphrey C. D., Pittman F. E. A simple methylene blue-azure II-basic fuchsin stain for epoxy-embedded tissue sections. Stain Technol. 1974 Jan;49(1):9–14. doi: 10.3109/10520297409116929. [DOI] [PubMed] [Google Scholar]
- Katz L., LaMont J. T., Trier J. S., Sonnenblick E. B., Rothman S. W., Broitman S. A., Rieth S. Experimental clindamycin-associated colitis in rabbits. Evidence of toxin-mediated mucosal damage. Gastroenterology. 1978 Feb;74(2 Pt 1):246–252. [PubMed] [Google Scholar]
- Kelley R. O., Dekker R. A., Bluemink J. G. Ligand-mediated osmium binding: its application in coating biological specimens for scanning electron microscopy. J Ultrastruct Res. 1973 Nov;45(3):254–258. doi: 10.1016/s0022-5320(73)80051-6. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson H. E., Parry J. V., Price A. B., Davies D. R., Dolby J., Tyrrell D. A. Undescribed toxin in pseudomembranous colitis. Br Med J. 1977 May 14;1(6071):1246–1248. doi: 10.1136/bmj.1.6071.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson H. E., Price A. B. Pseudomembranous colitis: Presence of clostridial toxin. Lancet. 1977 Dec 24;2(8052-8053):1312–1314. doi: 10.1016/s0140-6736(77)90363-4. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Pittman F. E., Pittman J. C. An electron microscopic study of epithelium of normal human sigmoid colonic mucosa. Gut. 1966 Dec;7(6):644–661. doi: 10.1136/gut.7.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman F. E., Pittman J. C., Humphrey C. D. Colitis following oral lincomycin therapy. Arch Intern Med. 1974 Aug;134(2):368–372. [PubMed] [Google Scholar]
- Rifkin G. D., Fekety F. R., Silva J., Jr Antibiotic-induced colitis implication of a toxin neutralised by Clostridium sordellii antitoxin. Lancet. 1977 Nov 26;2(8048):1103–1106. doi: 10.1016/s0140-6736(77)90547-5. [DOI] [PubMed] [Google Scholar]
- Rifkin G. D., Silva J., Jr, Fekety R. Gastrointestinal and systemic toxicity of fecal extracts from hamsters with clindamycin-induced colitis. Gastroenterology. 1978 Jan;74(1):52–57. [PubMed] [Google Scholar]
- Rijke R. P., Hanson W. R., Plaisier H. M., Osborne J. W. The effect of ischemic villus cell damage on crypt cell proliferation in the small intestine: evidence for a feedback control mechanism. Gastroenterology. 1976 Nov;71(5):786–792. [PubMed] [Google Scholar]
- Ruddell C. L. Embedding media for 1-2 micron sectioning. 2. Hydroxyethyl methacrylate combined with 2-butoxyethanol. Stain Technol. 1967 Sep;42(5):253–255. doi: 10.3109/10520296709115020. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer H. W. The pseudomembranous colitis associated with clindamycin therapy--a viral colitis. Gut. 1975 Sep;16(9):695–706. doi: 10.1136/gut.16.9.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroehlein J. R., Sedlack R. E., Hoffman H. N., 2nd, Newcomer A. D. Clindamycin-associated colitis. Mayo Clin Proc. 1974 Apr;49(4):240–243. [PubMed] [Google Scholar]
- Tomkins A. M., Wright S. G., Bird R. G., James W. P. Letter: Virus-like particles in jejunal mucosa. Lancet. 1975 Jul 5;2(7923):36–37. doi: 10.1016/s0140-6736(75)92983-9. [DOI] [PubMed] [Google Scholar]
- Viteri A. L., Howard P. H., Dyck W. P. The spectrum of lincomycin-clindamycin colitis. Gastroenterology. 1974 Jun;66(6):1137–1144. [PubMed] [Google Scholar]