Abstract
Cyclops of zooplankton propagates prolifically in eutrophic waterbody and it cannot be exterminated by conventional disinfection process. The mutagenicity of Mesocyclops leukarti and its extermination with oxidants in a drinking waterworks in China were studied. Among five oxidants for use in bench-scale, chlorine dioxide is the most effective and the potassium permanganate is the weakest against Mesocyclops leukarti under the same conditions. Full-scale results showed that Mesocyclops leukarti could be effectively removed from water by 1.0 mg/L chlorine dioxide preoxidation combined with conventional removal physical process. After filtration, chlorite, a by-product of prechlorine dioxide, is stable at 0.45 mg/L, which is lower than the critical value of the USEPA. GC-MS examination and Ames test further showed that the quantity of organic substance and the mutagenicity in water treated by chlorine dioxide preoxidation are obviously less than those of prechlorination.
Keywords: Oxidants, Chlorine, Chlorine dioxide, Mesocyclops leukarti, Preoxidation, Water safety
INTRODUCTION
Eutrophication of surface water is a significantly increasing worldwide problem (Ma and Liu, 2002). Mesocyclops leukarti, a kind of Cyclops, is a waterborne animal propagating prolifically in eutrophic water reservoirs or fresh water lakes in China. Some reservoirs such as Dahuofang Reservoir (Liaoning Province), East Taihu Lake (Jiangsu Province), Gaozhou, Qiyeshi and Xinfengjiang Reservoir (Guangdong Province), Miyun Reservoir (Beijing), etc. are sources of drinking water supply. The omnipresence of Mesocyclops leukarti in surface water is a currently growing problem in the production of drinking water.
In drinking water treatment, conventional process is still the main method for Cyclops removal. At present, in most waterworks, Cyclops is killed or inactivated by prechlorination and removed from water by water treatment process. For example, in Shijiazhuang waterworks located in North China, Cyclops is inactivated by prechlorination with 2.5 mg/L, there are still a few active Cyclops after filtration (Xing, 2004). Cyclops cannot be effectively inactivated by conventional disinfection due to its strong resistance to oxidation (Cui et al., 2002). In addition, the mobility of Cyclops makes it easily penetrate sand filter and move into the clean water tank in waterworks, even municipal service pipe. In recent years, there were several contamination accidents with Cyclops appearing in municipal service pipe in Shijiazhuang, Tianjin and Binxian cities in China.
Adult Cyclops with body length of one to several millimeters that can be seen by naked eye brings diseases to consumers, as it is intermediate host of some diseases. For example, Guinea worm disease still affects a large number of people in rural areas in tropical countries. Olajide and Sridhar (1987) proposed that the disease could be effectively controlled by killing the intermediate host, Cyclops. Study of the problem revealed that the present solution is limited to conventional water treatment. Lupi et al.(1994) considered that flocculation and sand filtration processes were insufficient for removing zooplankton normally present in surface water, and nematodes and water fleas might still occur in treated water. It was reported that the inactivation of zooplankton was necessary to eliminate zooplankton in water treatment process and that rotifer abundance would be reduced from 20~300 ind./L to 0~5 ind./L in drinking water by oxidation with 2.5~3 mg/L of chlorine for 20 min (Bernhardt and Lusse, 1989). Mitcham et al.(1983) found that chloramine was more effective against Copepoda (Cyclops) but less effective against Cladocera (Daphnia and Bosmina).
Prechlorination to remove Cyclops may resolve the problem of drinking water security by increasing the halomethane content in treated water. It is essential to look for a more effective and safe removal technique. Laboratory experiments on inactivation effects of oxidants such as chlorine, chlorine dioxide, ozone, chloramine and potassium permanganate on Mesocyclops leukarti were conducted at the Binxian waterworks in China. After finding the optimum oxidant, full-scale use of chlorine dioxide preoxidation combined with routine clearing process for Mesocyclops leukarti removal was implemented. GC-MS examination and Ames test further revealed the kind and amount of organic substance and the mutagenicity in the treated water.
MATERIALS AND METHODS
Raw reservoir water
In Binxian waterworks located in northeast part of China (Heilongjiang Province), an eutrophic reservoir named Erlongshan is used as drinking water source, where the amount of Cyclops has greatly increased due to eutrophication. Microscopic observation results showed that Cyclops density is 10~24 ind./L and that the species of Cyclops is Mesocyclops leukarti. The Erlongshan Reservoir (126°55′41″ E, 128°19′17″ N) has capacity of 9.4×106 m3 and highest water level of 191.5 m and control drainage area of 278.5 km2.
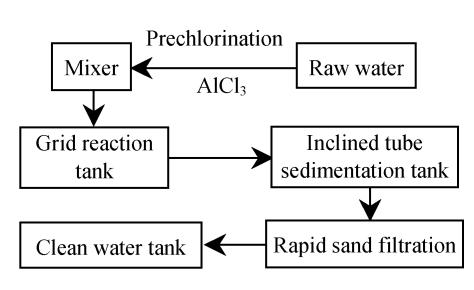
At present, Mesocyclops leukarti in the waterworks is removed by prechlorination at dosage of 3.0 mg/L, which removes 80% of Mesocyclops leukarti from the water after filtration. The water treatment process used in the waterworks is shown in Fig.1.
Fig. 1.
The chart of drinking water treatment processes in Binxian waterworks
The raw water quality is listed as follows: average water temperature 15~19 °C, turbidity 10~30 NTU, CODMn 5.68 mg/L, pH 7.3~7.5, algae density 2.1×106~2.6×106 cells/L and Mesocyclops leukarti density 20 ind./L.
Experimental equipment
Chlorine dioxide gas generator was manufactured by ourselves. Componential analysis of chlorine dioxide gas is given in Table 1. OF-3 type ozone generator was made by Shanghai Environmental Protection Company. XSB-1A inverted series biological microscope was made by Alltion Wuzhou Company. QP5050A type gas chromatograph-mass spectrometer was made by Shimadzu Instruments Factory. TA100 and TA98 strains without metabolic activation S9, were obtained from Harbin Medical University in China. pH and temperature measurements are conducted using WTW pH/Oxi 340i (made in Germany) with pH and temperature probe (pH-Electrode SenTix 4).
Table 1.
Componential analysis of chlorine dioxide gas
| Chemical composition | Chlorine dioxide | Chlorine | Chlorite | Chlorate |
| Percentage composition (%) | 82.3 | 10.4 | 5.2 | 2.1 |
Methods
All analyses were performed according to the standard methods (Chinese EPA, 1997). Bench-scale study on the removal effect on Mesocyclops leukarti by five oxidants was in local waterworks lab. Full-scale study of water treatment process was carried out at the Binxian waterworks. Mesocyclops leukarti concentration was determined by microscopic count method, and the changes of Mesocyclops leukarti number between raw water and treated water were investigated.
Chlorine dioxide was generated by mixing hydrochloric acid solution (23% weight) and sodium chlorite solution (25% weight) at weight ratio of 1:1. More than 300% stoichiometric acid is to improve the efficiency and lower Cl2, ClO2 − and ClO3 − production. Iodimetry was used for componential analysis of chlorine dioxide.
Ozone was produced in our laboratory from water cooled ordinary grade air using low frequency ozone generator (OF-3, Xingshen Environmental Facility Co. Ltd., Shanghai, China) operated with a dielectric tube outlet. Ozone output was 3 g/h and generation concentration was 18~20 mg/L. The ozone doses applied to the solutions were subsequently controlled by keeping the rate of gas flow constant and the contact time of ozone was quantified by the iodometry method.
Raw water and treated water by chlorine dioxide or chlorine preoxidation were sampled by 250 L, respectively. Organic substances in water samples were enriched with macroporous resin and then were eluted with about 60 ml of carbinol. The carbinol samples containing organic substances were concentrated to about l ml with K-D concentrator. The concentrated samples were examined in duplicate with gas chromatograph-mass spectrometer (GC-MS) and tested by Ames test procedure using TA100 and TA98 strains without metabolic activation S9 at increasing doses of concentration (corresponding to 1, 3, 5 and 7 L of equivalent volume per plate).
RESULTS AND DISCUSSION
Removal effect of five oxidants on Mesocyclops leukarti
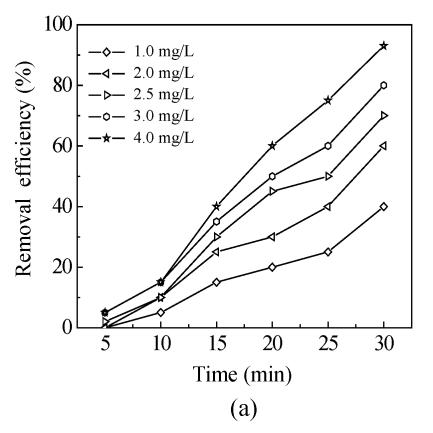
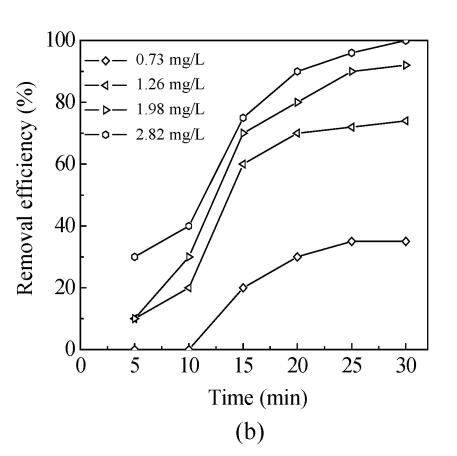
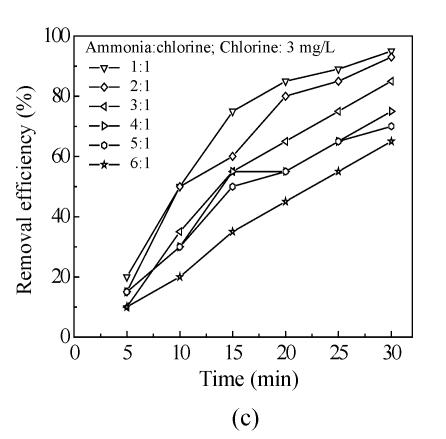
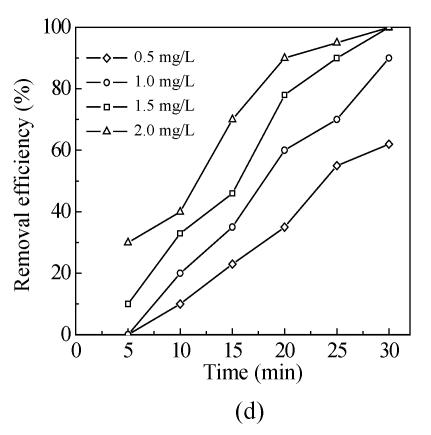
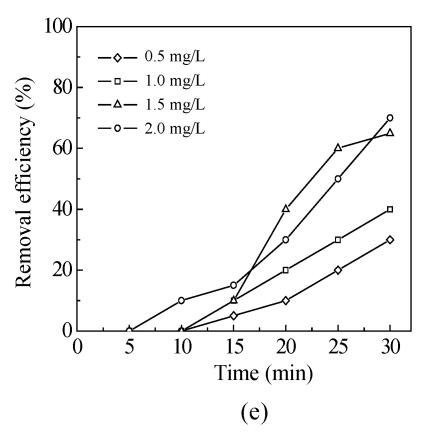
The effects of five oxidants chlorine, chlorine dioxide, ozone, chloramine and potassium permanganate on Mesocyclops leukarti were assessed and compared in laboratory in order to determine the optimum oxidant for Binxian waterworks. The results of experiment are shown in Fig.2. Various dosages of five oxidants were added into a given volume of 10 L reservoir water with Mesocyclops leukarti density of 20 ind./L, pH of 7.4, and temperature of 18 °C.
Fig. 2.
The inactivation effect of five oxidants on Mesocyclops leukarti (a) Cl2; (b) O3; (c) Chloramine; (d) ClO2; (e) KMnO4
Fig.2 shows that the inactivation effects of five oxidants on Mesocyclops leukarti strengthened gradually with increasing dosage and that there were obvious differences between the inactivation efficiency of different oxidants (Fig.2). Chlorine dioxide has the greatest inactivating effect on Mesocyclops leukarti, with the efficiency of potassium permanganate being the lowest under the same conditions.
For chlorine in Fig.2a, the inactivity efficiency could reach 80% and 90% after 30 min when adding dosage was 3.00 mg/L and 4.00 mg/L, respectively. Although good removal effect can be obtained if dosage of liquid chlorine increases to 5 mg/L or more, the negative effects resulting from the formation of by-products, like trihalomethanes and haloacetic acids, limit the use of prechlorination. Therefore, the maximal dosage of chlorine in this study was 4 mg/L due to human safety reason.
Chlorine dioxide has been reported to be more effective in inactivating pathogenic organisms including Cryptosporidium parvum (Liyanage et al., 1996). In Fig.2d of this study, 100% of Mesocyclops leukarti could be inactivated by 1.5 mg/L of chlorine dioxide contact for 30 min. Unlike chlorine, chlorine dioxide cannot react with humic substances to form trihalomethanes (THMs) (Li et al., 1996).
For ozone in Fig.2b, the results showed that the trend consisted of a rapid ozone consumption phase followed by a slower ozone decay phase. This is the same as the typical ozone decomposition trend in natural water (Park et al., 2001). Raw water quality can influence ozonation reactions with the main parameters considered to be natural organic materials (NOM) (Laplanche et al., 1995). One-hundred percent of Mesocyclops leukarti could be inactivated by 2.82 mg/L contact for 30 min. For potassium permanganate in Fig.2e, only 60% of inactivation effect was achieved by 2 mg/L contact for 30 min.
The chloramine in Fig.2c can achieve 95% effect of inactivation for 30 min with the chlorine:ammonia ratio=1:1 when the available chlorine is 3 mg/L. Some cities use chlorine for disinfection, and then later use chloramine to provide lasting disinfection as it is sent into pipes (Sung, 2002). Chloramines are weaker disinfectants than chlorine, but are more stable, thus extending disinfectant benefits throughout a water utility’s distribution system. Generally chloramines are used for maintaining a disinfectant residual in the distribution system and are not used as the primary disinfectant (Kouame and Haas, 1991).
Among five oxidants, the rank of removal efficiency is: ClO2>O3>chloramine>Cl2>KMnO4. Thus, ClO2 will be selected to further full-scale test.
It is worth noting that the rate of removing Mesocyclops leukarti was almost zero under condition of oxidant dosage of 1.0 mg/L and reaction time of 5 min. According to the raw water quality: CODMn 5.68 mg/L, algae density 2.1×106~2.6×106 cells/L, these substances consumed oxidants, showing that mature Mesocyclops leukarti could not be effectively inactivated with lower dose of oxidants and shorter reaction time. So to inactivate or weaken Mesocyclops leukarti with adequate oxidants is a key factor. It showed that the NOM such as humic acids and fluvic acids in water react first with oxidants, and then react with Mesocyclops leukarti. So at the beginning of the experiment the removal efficiency was almost zero.
Dosage for removal of ClO2 in full-scale study of Mesocyclops leukarti
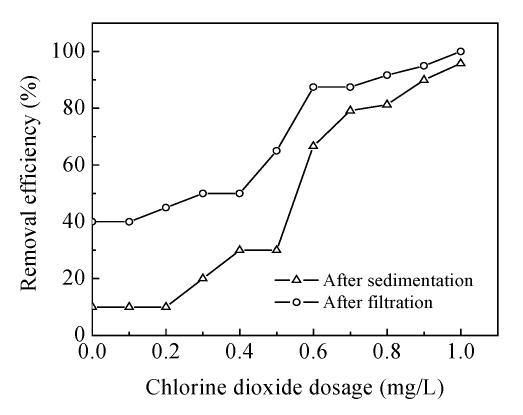
The oxidant, chlorine dioxide, was added into the grid reaction tank together with coagulant, 15 mg/L of AlCl3, and chlorine dioxide dosage ranged from 0 to 1.0 mg/L during the full-scale study. The results are shown in Fig.3 showing that the removal effects are strengthened gradually with the increase of ClO2 dose. One-hundred percent of removal effect on treated water from sand filter can be achieved by 1.0 mg/L of ClO2. In addition, 10% and 40% of removal rate may be achieved by sedimentation and filtration process under no chlorine dioxide dosing condition, respectively.
Fig. 3.
Removal effect of different chlorine dioxide dosage on Mesocyclops leukart
The removal of Mesocyclops leukarti in water treatment process is the result of oxidizing inactivation by chlorine dioxide in combination with physically clearing process. The removal rate of Mesocyclops leukarti is 10% and 40% by sedimentation and filtration with no chlorine dioxide dosing, respectively. It means that Mesocyclops leukarti could be partially removed from water by single physically clearing process.
The vitality of Mesocyclops leukarti in raw water is found to be distinct, namely, the activity of larvae and senile Mesocyclops leukarti are obviously weaker than that of mature Mesocyclops leukarti. Therefore, part of weaker Mesocyclops leukarti may deposit together with the floc formed in flocculation process or be trapped by sand filter. However, the removal effect on Mesocyclops leukarti by single physically clearing process is finite because Mesocyclops leukarti with stronger vitality still might penetrate sand filter so further removal of it requires its inactivation by preoxidation with sufficient oxidant.
The above experimental results showed that Mesocyclops leukarti can be inactivated and its vitality greatly weakened with the increase of ClO2 dosing. Similarly, larvae and senile Mesocyclops leukarti, are effectively removed from water by subsequent physical clearing processes. The results showed that the conventional clearing processes, such as sedimentation and filtration, play an important role in removing Mesocyclops leukarti, and that the inactivation of Mesocyclops leukarti by preoxidation with adequate available oxidant is essential for ensuring drinking water safety. Mesocyclops leukarti can be thoroughly removed from water by oxidant preoxidation in combination with physical clearing process, such as sedimentation and filtration.
By-products of ClO2
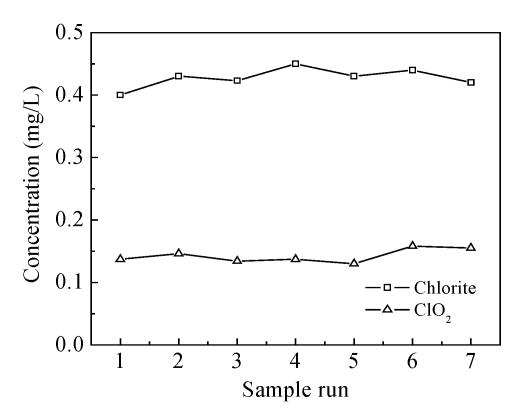
Fig.4 shows that the concentration of residual prechlorine dioxide after filtration is constant at 0.15 mg/L and chlorite is 0.45 mg/L. According to rule of USEPA, the concentration of residual chlorine dioxide and chlorite in drinking water must be controlled to less than 0.8 mg/L and 1.0 mg/L, respectively. Although chlorine dioxide volatile, it can still keep its persistent oxidation ability in water. In local waterworks, the water in the clean tank could keep residual chlorine in the range of standard with small dosage of post-chlorine. According to USEPA rules (USEPA, 1998), if the dosage of chlorine dioxide is under 1.4~2.0 mg/L, then the concentration of chlorite could keep lower than 1.0 mg/L.
Fig. 4.
The by-products in water after filtration with 1.0 mg/L prechlorine dioxide
Comparison of removal effects of two preoxidation processes
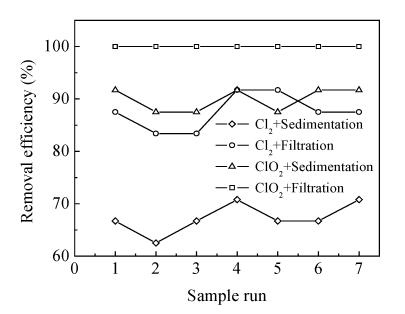
So 100% of Mesocyclops leukarti could be removed from treated water after filtration by 1.0 mg/L of ClO2. At present, in local waterworks, Mesocyclops leukarti is removed by prechlorination process with 3 mg/L of chlorine. During the full-scale study, the removal effect on Mesocyclops leukarti by chlorine dioxide preoxidation with 1.0 mg/L of ClO2 was compared with the existing prechlorination process with 3.0 mg/L of chlorine. The results are shown in Fig.5.
Fig. 5.
Comparison of removal effect on Mesocyclops leukarti by two different processes
Fig.5 shows that the removal effect of chlorine dioxide preoxidation is better than that of prechlorination. For instance, Mesocyclops leukarti is thoroughly removed from waterbody by filtration with chlorine dioxide, whereas only 80% of removal effect is achieved with chlorine.
Mature Mesocyclops leukarti is hardly removed from water by sedimentation unless it is effectively inactivated by oxidation with oxidant. The removal result of sedimentation tank shows that Mesocyclops leukarti inactivation by chlorine dioxide is superior to that by chlorine. Fig.5 shows that 90% of removal rate in treated water after sedimentation is achieved by chlorine dioxide preoxidation, but for prechlorination only about 65% is achieved. The distinct difference in Mesocyclops leukarti inactivation efficiency of the two preoxidation processes may be the results of the body structure of Mesocyclops leukarti and the different performance of oxidant in oxidizing cellular tissue.
In contrasted to common bacteria or virus, Mesocyclops leukarti has a special surface structure consisting of seven layers cell tissue such as bottom membrane, epithelium, calcific layer, etc. The body surface provides Mesocyclops leukarti stronger protection against oxidation. Mesocyclops leukarti cannot be effectively inactivated unless the oxidant destroys its surface structure by oxidation or directly penetrates through it into the body to oxidize inner protein to lose enzyme activity and not participate in the activities of the oxidation-reduction system. Therefore, higher oxidizability of oxidant is the key to thoroughly inactivate Mesocyclops leukarti. For chlorine in ClO2, oxidation number is expressed as +4. According to counting, it contains 263% of available chlorine, i.e. oxidizability of ClO2 is about 2.5 times as high as that of chlorine (Huang et al., 1997). Therefore, the ability of chlorine dioxide to oxidize Mesocyclops leukarti body structure is more effective than that of chlorine. In addition, after dissolving in water, 100% of ClO2 exists in molecular state not reacting with water molecular like chlorine, which makes ClO2 contact Mesocyclops leukarti surface easily and permeate through it into inner body to destroy inner cellular protein in order to inactivate Mesocyclops leukarti. The above advantages of chlorine dioxide not only result in a better inactivation of Mesocyclops leukarti but also lower required amount of it than chlorine.
Influence of preoxidation on organic substances and mutagenicity
GC-MS examination and Ames test were carried out with raw water sample and two water samples treated by preoxidation in order to evaluate the safety of the drinking water. The GC-MS examination results are shown in Fig.6 and Table 2. The Ames test results are shown in Table 3.
Fig. 6.
Graphic representation of total organic ions of (a) raw water, (b) water sample treated by prechlorination and (c) water sample treated by chlorine dioxide preoxidation
Table 2.
Analysis of GC-MS results of three water samples
| Organic species | Raw water |
Water treated by preoxidation |
||||
| Chlorine |
Chlorine dioxide |
|||||
| Amount | Peak area | Amount | Peak area | Amount | Peak area | |
| Paraffin | 22 | 50052134 | 19 | 83418846 | 14 | 38552821 |
| Olefin | 4 | 3703129 | 0 | 0 | 1 | 722801 |
| Halogenated hydrocarbon | 2 | 5619919 | 13 | 65706374 | 3 | 24916046 |
| Area | 2 | 6571797 | 3 | 5218775 | 3 | 5853788 |
| Heterocyclic compound | 2 | 7668507 | 1 | 1626622 | 1 | 775786 |
| Nitrogen compound | 11 | 64235860 | 4 | 26415371 | 1 | 36663305 |
| Benzene | 7 | 236040538 | 5 | 108419603 | 6 | 134638566 |
| Alcohol, aldehyde, etc. | 21 | 386596361 | 31 | 333345213 | 12 | 81050299 |
| Total | 71 | 760488245 | 76 | 624150804 | 41 | 323173412 |
Table 3.
Changes of TA98-S9 and TA100-S9 dose-response relationship
| Water sample | Dosage (L/plate) |
MR |
|
| TA98-S9 | TA100-S9 | ||
| Raw water | 1 | 0.67 | 1.01 |
| 3 | 0.66 | 1.28 | |
| 5 | 1.15 | 1.51 | |
| 7 | 1.75 | 1.66 | |
| Prechlorination | 1 | 0.87 | 0.95 |
| 3 | 1.13 | 1.55 | |
| 5 | 2.54 | 2.23 | |
| 7 | 2.81 | 2.82 | |
| Chlorine dioxide preoxidation | 1 | 0.84 | 1.33 |
| 3 | 1.06 | 1.44 | |
| 5 | 1.56 | 1.77 | |
| 7 | 2.10 | 2.07 | |
GC-MS examination
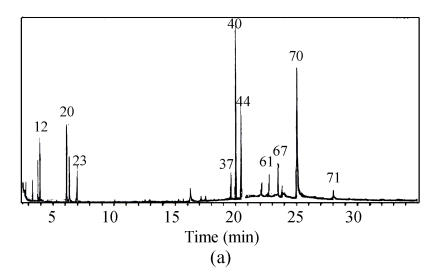
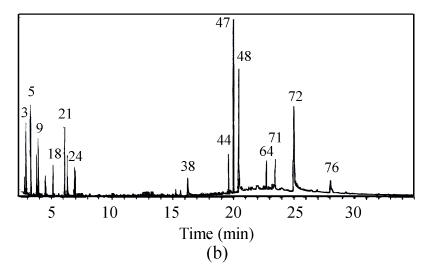
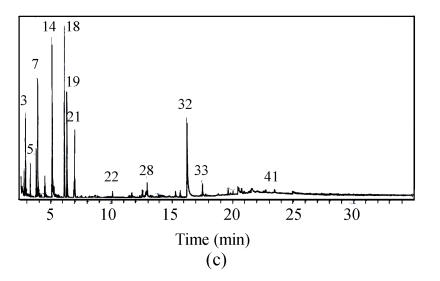
Compared with the amount of halogenated hydrocarbon in raw water (Table 2), it increases to 13 species in water treated by prechlorination and the total amount of organic substance increases to 5 species. Fig.6 shows that the amount of organic species occurring in retention time between 20 min and 30 min accounts for 44.4% of total amount. Moreover, the spectrum peak intensity is stronger and the area of the peak in the whole scanning area is larger than that of the water sample treated by chlorine dioxide preoxidation.
The amount of organic substance decreases to 41 species by chlorine dioxide preoxidation, which is equal to 57.5% of that in raw water (Fig.6). Species of 85.4% is low boiling point and micromolecule organic substances, which occurs in retention time before 20 min. In addition, the number of halogenated hydrocarbon is only 3 species in water treated by chlorine dioxide preoxidation.
The total amount of organic substance resulting from chlorine dioxide preoxidation was decreased from 71 species to 41 species in raw water, only about half that from prechlorination, 76 species. Especially, the matters harmful to people’s health, halogenated hydrocarbon, were only 3 species in water treated by chlorine dioxide preoxidation compared with 13 species from prechlorination. In addition, most matters produced by chlorine dioxide preoxidation are low boiling point and micromolecule organic substances such as paraffin, alcohol, etc., which account for almost 85.4%. But for prechlorination, about 55% of matters are macromolecule organic compounds such as halogenated hydrocarbon with high boiling point.
The different reaction mechanisms of organic substances reacting with chlorine dioxide and chlorine cause diverse results in GC-MS examination. Free radical oxidation reaction occurs when chlorine dioxide reacts with organic substances. The macromolecule organic compounds are oxidized into oxocompounds and micromolecule substances, in which some volatile compounds, even CO2 are formed (Benjamin et al., 1986). Moreover, the relatively stronger oxidizability of chlorine dioxide may easily cause the oxidation of organic substance to such products as those with low boiling point and minimal molecular weight and short retention time in the graphic result of GS-MS examination. When chlorine oxidizes organic substance, electrophilic substitution reaction results in the formation of more mutagenic halogenated hydrocarbons such as trichloromethane with high boiling point and macromolecule organic compounds with long retention time (Reckhow et al., 1990; Långvik and Holmbom, 1994). In addition, many intermediate products such as aldehyde may be produced during chlorination due to no thoroughly oxidizing organic substances with chlorine. It results in the total amount of organic substance by prechlorination being more than that treated by chlorine dioxide preoxidation. Therefore, it can be concluded that the drinking water quality by chlorine dioxide preoxidation is better than that by prechlorination.
Ames test
The mutagenicity of concentrated water samples before and after preoxidation is expressed by MR value (rate of mutagenicity). The result with MR value greater than 2 is used considered as having mutagenic activity. The results of Ames test are shown in Table 3.
The results of raw water have not behaved mutagenic activity under any dose condition and the MR value of water sample by prechlorination is higher than that by chlorine dioxide preoxidation at the same doses.
TA98 strain is used to detect frame-shift point mutations and TA100 strain is applied to detect base pair substitution mutations. Cl2-treated water shows mutagenicity with TA98 strain (MR=2.54) and TA100 strain (MR=2.23) at lower doses (5 L equivalent/plate), whereas ClO2-treated water might have lower mutagenic activity detectable only at the highest doses (7 L equivalent/plate). And the MR value with TA98 and TA100 are 2.1 and 2.07, respectively. Thirteen species of halogentated compounds are formed during prechlorination because of electrophilic substitution reaction between chlorine and organic substances. And their special chemically active group, halogen with relatively strong electrophonic effect, reinforces molecule polarity to react with biologic enzyme system, while the mutagenic action is attained by a series of chemical reactions such as DNA base substitution. During chlorine dioxide preoxidation, less halogentated compounds (only one species) are formed due to different reaction mechanism that is oxidation-reduction reaction, not electrophilic substitution reaction. Hence, mutagenicity of ClO2-treated water was obviously lower than that of Cl2-treated water. In addition, the mutagenicity of ClO2-treated water occurs at the doses of 7 L equivalent/plate, which was different from some previous Ames test results (Fiessinger, 1991; Huang and Li, 1998), probably because of impurities produced by chlorine dioxide gas generator (Table 1). It can be concluded that chlorine accounts for 10.4% of the total chlorine dioxide content, resulting in the mutagenic activity analogous to the result of prechlorination. This can be improved by enhancing chlorine dioxide gas purity by using advanced generator.
CONCLUSION
Among five oxidants used in the bench scale test, chlorine dioxide had the most inactivation effect on Mesocyclops leukarti and the efficiency of potassium permanganate was the lowest under the same conditions. One-hundred percent of Mesocyclops leukarti could be inactivated by 1.5 mg/L of chlorine dioxide contact for 30 min. Chlorine dioxide preoxidation has very good inactivation effect on Mesocyclops leukarti.
In full-scale study, 100% of removal effect in treated water from sand filter can be achieved by using 1.0 mg/L of ClO2. Mesocyclops leukarti can be effectively removed from water by chlorine dioxide preoxidation combined with conventional physical processes such as filtration or sedimentation. The existing process of prechlorination cannot achieve the same removal effect as chlorine dioxide.
Chlorite, the by-product of ClO2, is constant at 0.45 mg/L after filtration, which is lower than the critical value of USEPA.
GC-MS examination and Ames test further showed that the sort and amount of organic substances in treated water after chlorine dioxide preoxidation were evidently less than those of prechlorination, and that the mutagenicity of drinking water was greatly reduced. The advantage of chlorine dioxide disinfection may be further improved by enhancing chlorine dioxide gas purity (>95%).
Footnotes
Project (No. 2003AA601120) supported by the Hi-Tech Research and Development Program (863) of China
References
- 1.Benjamin W, Lukins JR, Mark HG. Using chlorine dioxide for trihalomethane control. Journal of the American Water Works Association. 1986;78(6):88–93. [Google Scholar]
- 2.Bernhardt H, Lusse B. Elimination of zooplankton by flocculation and filtration. Journal of Water Supply: Research & Technology AQUA. 1989;38(1):23–31. [Google Scholar]
- 3.Chinese EPA. Water and Wastewater Monitoring Methods. 3rd Ed. Beijing: Chinese Environmental Science Publishing House; 1997. pp. 50–150. (in Chinese) [Google Scholar]
- 4.Cui FY, Lin T, Ma F. The excess propagation and research on ecological control of the water flea of zooplankton in raw water. Journal of Harbin Institute of Technology. 2002;34(3):399–403. (in Chinese) [Google Scholar]
- 5.Fiessinger F. Advantages and disadvantages of chemical oxidation and disinfection of ozone and chlorine dioxide. Science of the Total Environment. 1991;18:245–246. doi: 10.1016/s0048-9697(81)80062-9. [DOI] [PubMed] [Google Scholar]
- 6.Huang JL, Li BX. Comparison of the mutagenicity of drinking water with chlorine dioxide and chlorine disinfections. Environmental Chemistry. 1998;17(4):34–38. (in Chinese) [Google Scholar]
- 7.Huang JL, Wang L, Ren NQ, Liu XL, Sun RF, Yang GL. Disinfection effect of chlorine dioxide on viruses, algae and animal plankton in water. Water Research. 1997;31(3):455–460. doi: 10.1016/S0043-1354(96)00275-8. [DOI] [Google Scholar]
- 8.Kouame Y, Haas CN. Inactivation of E. coli by combined action of free chlorine and monochlorine. Water Research. 1991;25(9):1027–1032. doi: 10.1016/0043-1354(91)90195-V. [DOI] [Google Scholar]
- 9.Långvik V, Holmbom B. Formation of mutagentic organic by-products and aox by chlorination of fractions of humic water. Water Research. 1994;28(3):553–557. doi: 10.1016/0043-1354(94)90006-X. [DOI] [Google Scholar]
- 10.Laplanche A, Orta de Velasquez M, Boisdon V, Martin N, Martin G. Modelisation of micropollutant removal in drinking water treatment by ozonation or advanced oxidation processes. Ozone Science Engineering. 1995;17:97–117. [Google Scholar]
- 11.Li JW, Yu Z, Cai X, Gao M, Chao F. Trihalomethanes formation in water treated with chlorine dioxide. Water Research. 1996;30(10):2371–2376. doi: 10.1016/0043-1354(96)00146-7. [DOI] [PubMed] [Google Scholar]
- 12.Liyanage LRJ, Gyurek LL, Finch GR, et al. Modeling Cryptosporidium parvum Inactivation by Chlorine Dioxide. Proceedings of the Forth Environmental Engineering Speciality Conference; Edmonton, the Netherlands. 1996. pp. 17–25. [Google Scholar]
- 13.Lupi E, Ricci V, Burrini D. Occurrence of nematodes in surface water used in a drinking water plant. Journal of Water Supply Research Technology. 1994;43(3):107–112. [Google Scholar]
- 14.Ma J, Liu W. Effectiveness and mechanism of potassium ferrate (VI) preoxidation for algae removal removal by coagulation. Water Research. 2002;36(4):871–878. doi: 10.1016/S0043-1354(01)00282-2. [DOI] [PubMed] [Google Scholar]
- 15.Mitcham RP, Shelley MW, Wheadon CM. Free chlorine versus ammonia-chlorine: disinfection, trihalomethane formation, and zooplankton removal. Journal of the American Water Works Association. 1983;75(4):196–198. [Google Scholar]
- 16.Olajide I, Sridhar MK. Guineaworm control in an endemic area in western nigeria. Journal of Water Supply: Research & Technology AQUA. 1987;6:333–339. [Google Scholar]
- 17.Park HS, Hwang TM, Kang JW, Choi H, Oh HJ. Characterization of raw water for the ozone application measuring ozone consumption rate. Water Research. 2001;35(11):2607–2614. doi: 10.1016/S0043-1354(00)00564-9. [DOI] [PubMed] [Google Scholar]
- 18.Reckhow DA, Singer PC, Malcolm RL. Chlorination of humic materials: byproduct formation and chemical interpretations. Environmental Science & Technology. 1990;24(11):1655–1664. doi: 10.1021/es00081a005. [DOI] [Google Scholar]
- 19.Sung W. Corrosion Control and Chloramination, Discolored Water and Nitrification. Proceedings–Water Quality Technology Conference; MWRA, Southborough, MA, USA. 2002. pp. 1683–1686. [Google Scholar]
- 20.USEPA. National Primary Drinking Water Regulations, Disinfectants and Disinfection Byproducts: Final Rule. 1998. pp. 69390–69476. 40 CFR Parts 9, 141 & 142, FED Reg. 63 No. 241. [Google Scholar]
- 21.Xing H. The removal of Cyclops in water treatment process. China Water and Wastewater. 2004;20(7):88. (in Chinese) [Google Scholar]