Abstract
The mutagenic effects of microwave and chromium trioxide (CrO3) on Vicia faba root tip were studied. Micronucleus assay and chromosomal aberration assay were used to determine the mitotic index, the micronucleus frequency and chromosomal aberration frequency of Vicia faba root tip cells induced by microwave and CrO3. The results showed that the micronucleus frequency decreased, and that the mitotic index and chromosomal aberration frequency showed linear dose responses to CrO3, in treatment of microwave for 5 s. In microwave of 25 s, the mitotic index decreased, the micronucleus frequency and chromosomal aberration frequency increased with increase of CrO3 concentration. We concluded that microwave and CrO3 had antagonistic effect on the mitotic index of Vicia faba root tip cells, but had synergetic effect on micronucleus frequency and chromosomal aberration frequency of Vicia faba root tip cells.
Keywords: Microwave, Chromium trioxide (CrO3), Antagonistic effect, Synergetic effect
INTRODUCTION
Electromagnetic radiation and microwave are human survival environmental factors. Their harmful effects on biology had been reported (Lacy-Hulbert et al., 1998; Sher, 2000; Forgacs et al., 2005; Sripakdee et al., 2005). Along with the popularization of network communication, broad-cast television, electric appliance, people accept more and more electromagnetic radiation, which has already become an environmental pollution problem, affecting humanity’s living conditions directly. Harm created by chromium alone has already been reported (Qian, 2004a; Kim et al., 2004; Garcia-Rodriguez et al., 2001). But the joint effects of two factors have not been reported yet. This work studied the effects of microwave in different treatment time and chromium trioxide in different concentration on the mitotic index, the micronucleus frequency, and chromosomal aberration frequency of Vicia fabe root tip cells.
MATERIALS AND METHODS
Materials
Vicia faba L. (collected from Hesheng Town, Yongjia of Zhejiang Province), CrO3 (produced by Xilong Chemical Plant, Guangdong, at 12.5 mg/L and 50.0 mg/L in distilled water, Batch number 20010216). Microwave oven used was an NN-S547WFS model, made in Shanghai Panasonic Microwave L. CN. in 1997.
Methods
Full size Vicia faba seeds were chosen. Table 1 presents the experimental grouping and treatment. The excised root tips were fixed in Carnoy’s fluid (anhydrous alcohol:glacial acetic acid=3:1, V/V) for 24 h, and kept in 70% alcohol in refrigerator at 4 °C. These samples were sectioned routinely and stained with modified carbol fuchsin. The cell mitotic index, micronucleus permillage and chromosomal aberration percentage were examined and counted microscopically on squashes, and the aberrant cells were microphotographed (Qian, 2004b). Each treatment as described above was repeated at least thrice; analysis of variance used SPSS software (SPSS, 10.0 version).
Table 1.
Grouping and treatment of the Vicia faba root tips
| Group | Number of root tip (n) | Time in oven (s)+concentration of CrO3 (mg/L) |
| A (control) | 10 | 0+0 |
| B | 10 | 5+0 |
| C | 10 | 25+0 |
| D | 10 | 0+12.5 |
| E | 10 | 0+50.0 |
| F | 10 | 5+12.5 |
| G | 10 | 5+50.0 |
| H | 10 | 25+12.5 |
| I | 10 | 25+50.0 |
RESULTS
Mitotic indexes of Vicia faba root tip cells as induced by microwave and CrO3
Table 2 shows obvious antagonistic effects. The mitotic indexes of Groups B and C were less than that of control (Group A) (P<0.05, P<0.001). The mitotic index decreased significantly prolongation of microwave treatment time increased from 5 s to 25 s. Compared with Group A, the mitotic indexes of Groups D and E increased remarkably (P<0.001) when CrO3 concentration increased. The mitotic indexes of Groups F and G were not remarkably different (P>0.05), showing that the mitotic index did not significantly change with increase of CrO3 concentration for 5 s of microwave treatment. In Groups H and I the mitotic index decreased significantly with increasing CrO3 concentration for 25 s of microwave treatment (P<0.001).
Table 2.
The mitotic indexes of Vicia faba root tip cells as induced by microwave and CrO3
| Group | Number of root tip (n) | Mitotic index for each root tip (x) (%) | Mitotic index (xݱs)(%) | |||||||||
| A (control) | 10 | 3.9 | 4.0 | 3.2 | 3.9 | 3.8 | 3.0 | 4.1 | 4.8 | 4.4 | 3.7 | 3.88±0.52 |
| B | 10 | 3.7 | 3.6 | 3.4 | 3.4 | 3.8 | 3.3 | 3.5 | 3.1 | 3.2 | 3.7 | 3.47±0.23* |
| C | 10 | 3.3 | 3.0 | 3.4 | 2.9 | 3.6 | 3.4 | 3.1 | 2.8 | 2.7 | 2.9 | 3.11±0.30*** |
| D | 10 | 5.4 | 5.5 | 5.1 | 5.6 | 5.1 | 5.2 | 4.5 | 4.5 | 4.8 | 4.9 | 5.06±0.39*** |
| E | 10 | 5.6 | 5.3 | 5.4 | 5.7 | 5.3 | 5.2 | 5.1 | 5.0 | 5.1 | 5.5 | 5.32±0.23*** |
| F | 10 | 3.7 | 4.1 | 3.7 | 3.1 | 3.7 | 3.7 | 3.2 | 4.4 | 3.3 | 3.8 | 3.67±0.40 |
| G | 10 | 4.2 | 4.0 | 4.1 | 4.2 | 3.9 | 4.5 | 4.0 | 4.3 | 3.9 | 3.9 | 4.10±0.20 |
| H | 10 | 2.8 | 3.2 | 2.9 | 2.9 | 3.2 | 3.4 | 2.8 | 3.7 | 3.5 | 3.5 | 3.19±0.33*** |
| I | 10 | 2.2 | 2.8 | 3.0 | 2.4 | 3.6 | 3.4 | 2.7 | 3.4 | 3.6 | 2.6 | 2.97±0.51*** |
The other groups compared with Group A.
P<0.05
P<0.001
Micronucleus frequency of Vicia faba root tip cells as induced by microwave and CrO3
Table 3 shows the micronucleus frequencies of Groups B, C, D, and E were all higher than that of Group A (P<0.001). The micronucleus frequencies increased significantly with prolongation of microwave treatment time. The result revealed that treatment time determines the value of the micronucleus frequency (P<0.001). The micronucleus frequency of Group F was remarkably high compared to Groups B and D (P<0.001), and that of Group G was remarkably high compared to Groups B and E (P<0.001). These show combinations of 5 s microwave and 12.5 mg/L CrO3 and 5 s and 50.0 mg/L had synergetic effects. The micronucleus frequency of Group H was lower than that of Group C, but higher than that of Group D (P<0.001). The micronucleus frequency of Group I was remarkably higher that that of Groups C and E (P<0.001). Synergetic effects of 25 s microwave and 50.0 mg/L CrO3 on micronucleus frequency could be observed.
Table 3.
The micronucleus frequencies of Vicia faba root tip cells as induced by microwave and CrO3
| Group | Number of root tip (n) | Number of interphase cell observed | Number of cell with micronucleus per root tip | Micronucleus frequency (xݱs)(‰) | ||||
| A (control) | 10 | 1000 | 2 | 3 | 1 | 2 | 2 | 2.20±0.79 |
| 3 | 3 | 1 | 2 | 3 | ||||
| B | 10 | 1000 | 11 | 11 | 10 | 12 | 10 | 10.60±0.97*** |
| 10 | 11 | 12 | 10 | 9 | ||||
| C | 10 | 1000 | 14 | 15 | 16 | 17 | 16 | 15.40±0.97*** |
| 15 | 16 | 15 | 16 | 14 | ||||
| D | 10 | 1000 | 9 | 11 | 12 | 13 | 10 | 10.40±1.35*** |
| 9 | 9 | 10 | 11 | 10 | ||||
| E | 10 | 1000 | 5 | 6 | 6 | 7 | 5 | 6.60±1.17*** |
| 8 | 6 | 7 | 8 | 8 | ||||
| F | 10 | 1000 | 25 | 26 | 28 | 27 | 26 | 25.10±1.79*** |
| 22 | 25 | 23 | 24 | 25 | ||||
| G | 10 | 1000 | 17 | 16 | 14 | 15 | 16 | 14.80±1.23*** |
| 14 | 14 | 13 | 15 | 14 | ||||
| H | 10 | 1000 | 14 | 13 | 15 | 13 | 12 | 12.50±1.58*** |
| 14 | 11 | 12 | 11 | 10 | ||||
| I | 10 | 1000 | 20 | 20 | 18 | 20 | 17 | 19.20±1.23*** |
| 21 | 20 | 19 | 18 | 19 | ||||
The other groups compared with Group A,
P<0.001
Chromosomal aberration of Vicia faba root tip cells as induced by microwave and CrO3
Table 4 shows that the chromosomal aberration frequencies of the 8 test groups were remarkably higher than that of the control group (P<0.001). The chromosomal aberration frequency increased gradually with microwave treatment time and CrO3 concentration (P<0.001). The chromosomal aberration frequency of Group F was remarkably higher than that of Groups B and D (P<0.001), and that of Group G was remarkably higher than that of Groups B and E (P<0.001, P<0.01). This shows that two factors of 12.5 mg/L and 50.0 mg/L, 5 s had synergetic effect. The chromosomal aberration frequency of Group H was slightly higher than that of Group C (P>0.05), but apparently higher than that of Group D (P<0.001). These show that the two factors of 25 s and 12.5 mg/L had synergetic effect. The chromosome aberration frequency of Group I was slightly higher than that of Groups C (P<0.01) and E (P>0.05).
Table 4.
The chromosomal aberration of Vicia faba root tip cells as induced by microwave and CrO3
| Group | Number of root tips (n) | Number of cells with chromosomal aberration | Chromosomal aberration frequency (xݱs)(%) | |||||||||
| A (control) | 10 | 2 | 2 | 1 | 1 | 3 | 1 | 1 | 2 | 3 | 2 | 1.80±0.79 |
| B | 10 | 20 | 21 | 18 | 17 | 17 | 20 | 17 | 19 | 19 | 20 | 18.80±1.48*** |
| C | 10 | 26 | 27 | 27 | 28 | 30 | 28 | 28 | 29 | 30 | 29 | 28.20±1.32*** |
| D | 10 | 20 | 21 | 18 | 17 | 19 | 17 | 17 | 19 | 20 | 19 | 18.70±1.42*** |
| E | 10 | 31 | 30 | 32 | 28 | 29 | 33 | 31 | 32 | 29 | 28 | 30.30±1.77*** |
| F | 10 | 27 | 29 | 28 | 28 | 31 | 28 | 27 | 28 | 30 | 27 | 28.30±1.34*** |
| G | 10 | 35 | 31 | 32 | 36 | 31 | 30 | 33 | 32 | 34 | 31 | 32.50±1.96*** |
| H | 10 | 31 | 29 | 28 | 27 | 30 | 31 | 30 | 28 | 29 | 32 | 29.50±1.58*** |
| I | 10 | 32 | 30 | 29 | 34 | 28 | 27 | 30 | 31 | 30 | 33 | 30.40±2.17*** |
The other groups compared with Group A,
P<0.001
Mitoses of Vicia faba affected by microwave and CrO3
Microscope examination found abnormal cell mitosis phenomenon in prophase, metaphase, anaphase, telophase of Vicia faba root tip meristem cells in the presence of microwave and CrO3.
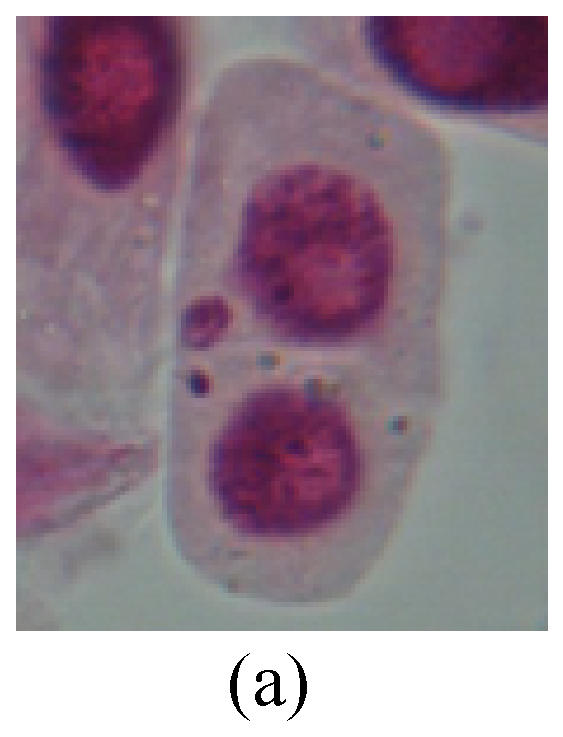
1. Prophase: The main feature is the occurrence of single or double micronuclei (Figs.1a, 1b, 2c, 3b and 4b). In the last mitosis the chromosome fragment is ejected from the cell nucleus, and then uncoils in the cytoplasm and becomes the micronucleus, formed in the cytoplasm.
Fig. 1.
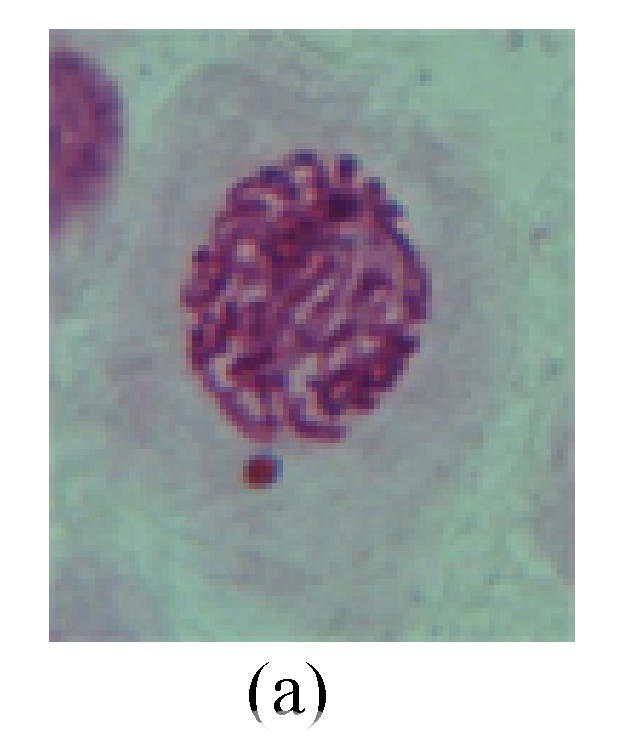
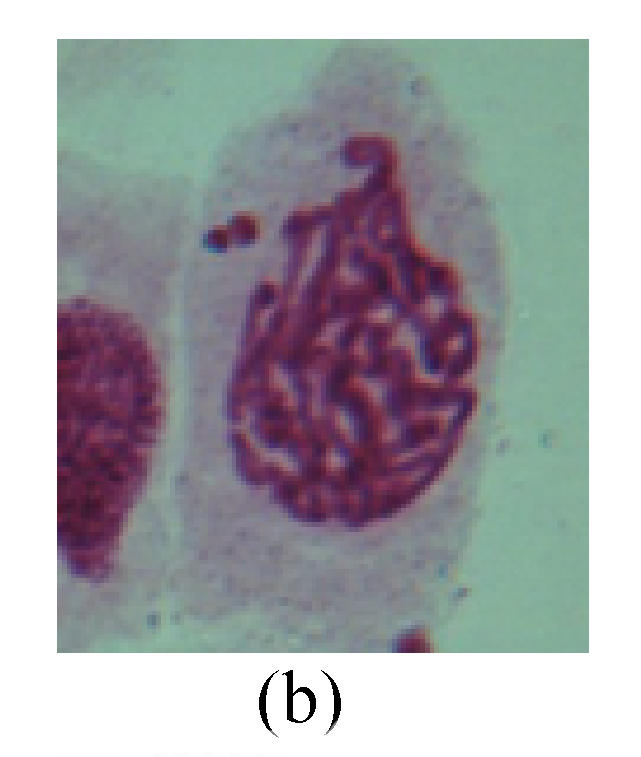
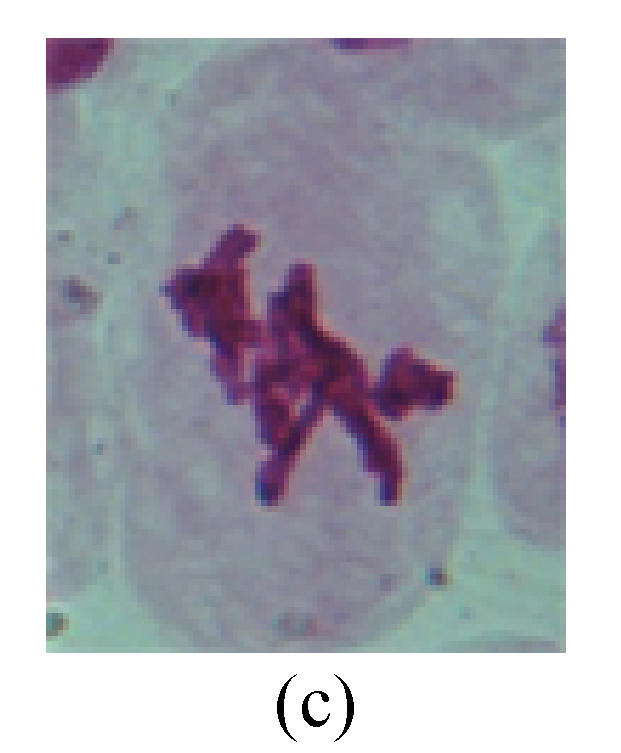
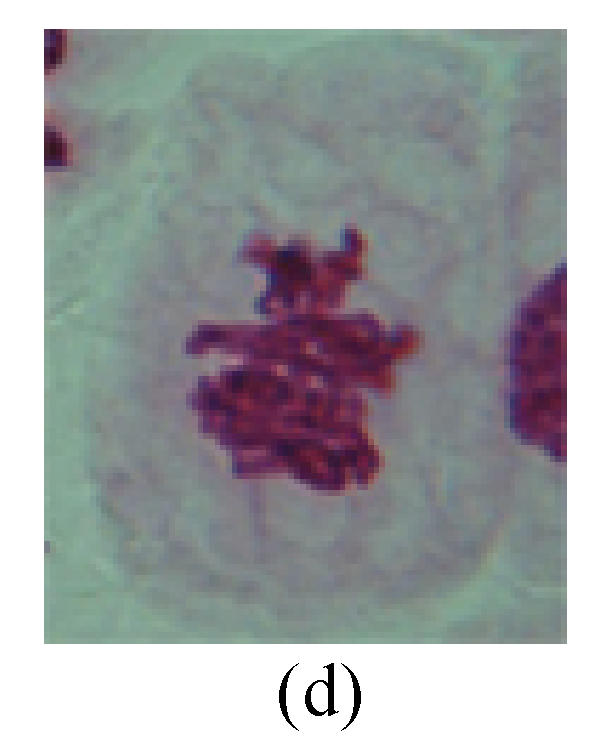

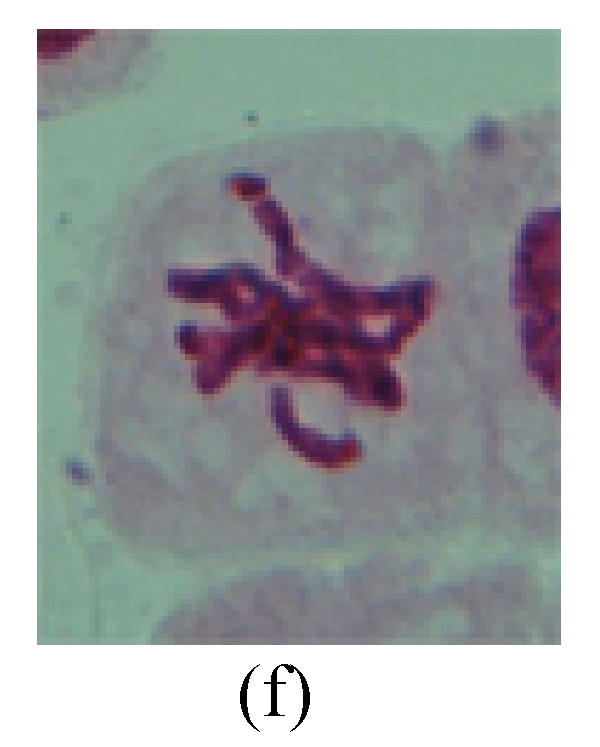
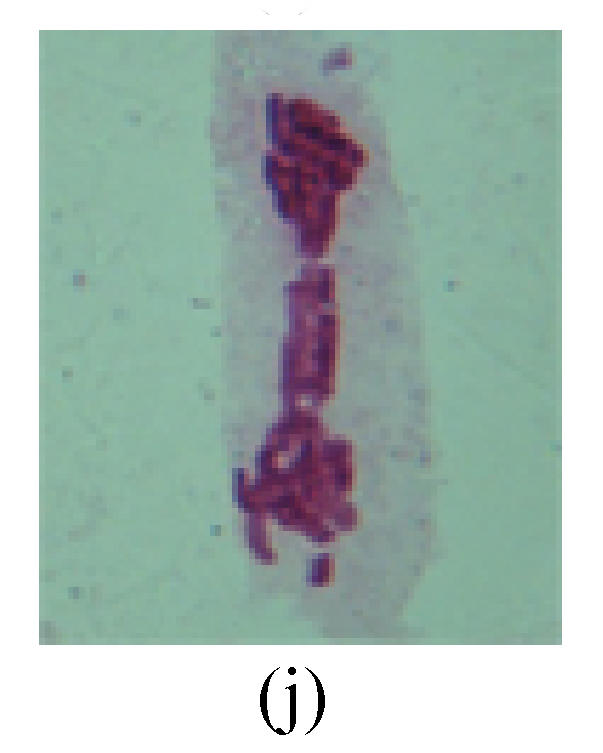
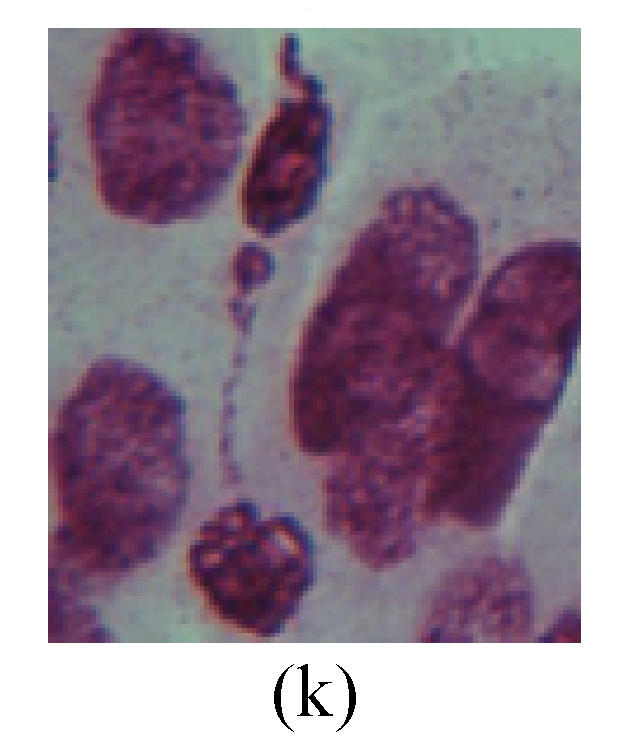
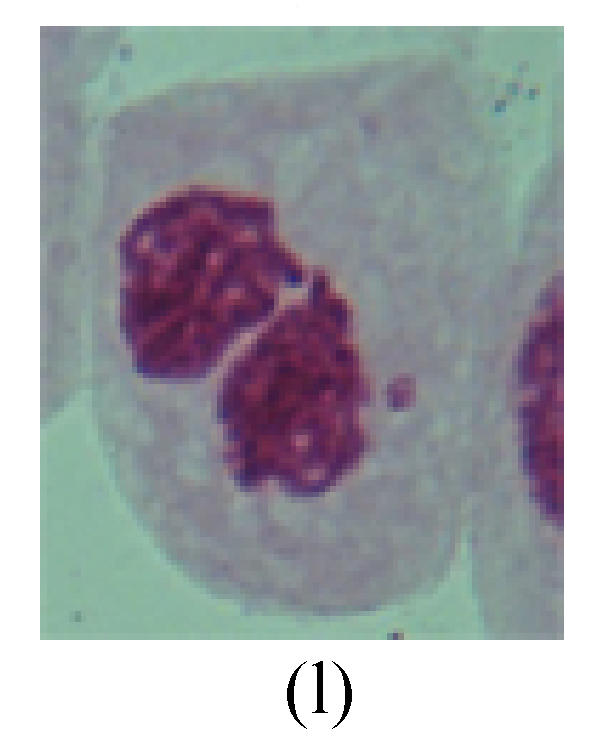
Chromosome aberration of Group F (a) Single micronuclei in prophase; (b) Double micronuclei in prophase; (c) Chromosome blending in metaphase; (d) Chromosome blending in metaphase; (e) Chromosome was divided into two parts on equatorial plane in metaphase; (f) Lagging chromosome in metaphase; (g) Micronucleus in metaphase; (h) Lagging chromosome in anaphase; (i) Chromosome uncoiling abnormally in telophase; (j) Two chromosome bridges and fragment in telophase; (k) One chromosome bridge and uncoiling abnormally in telophase; (l) Syncytium was formed in telophase
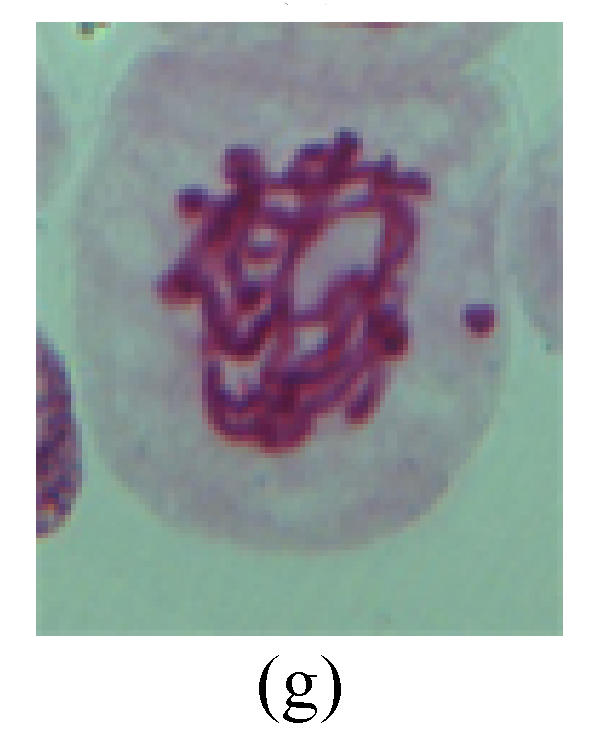
Fig. 2.
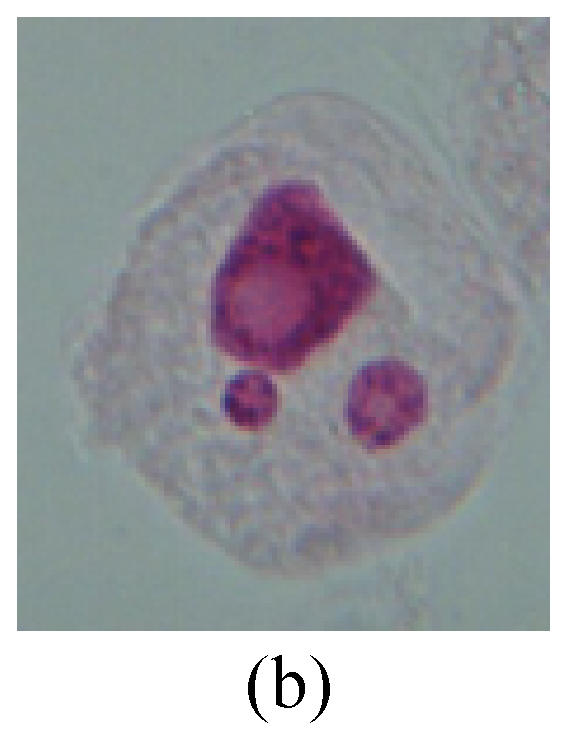
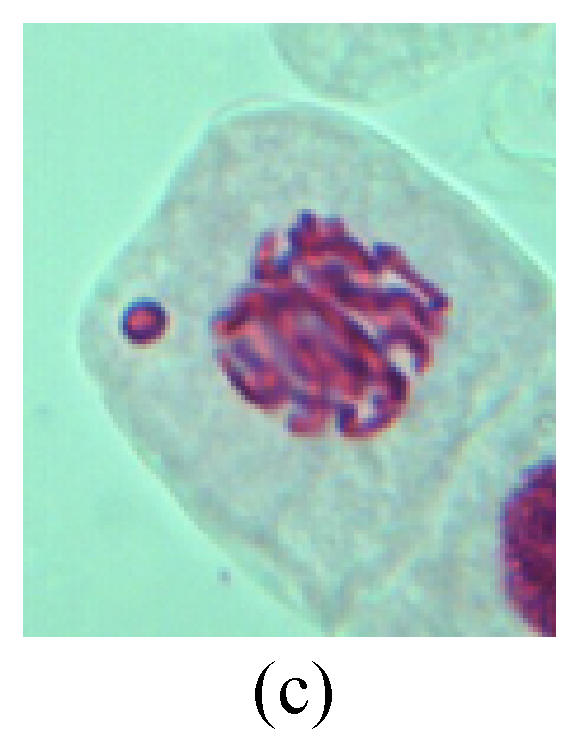
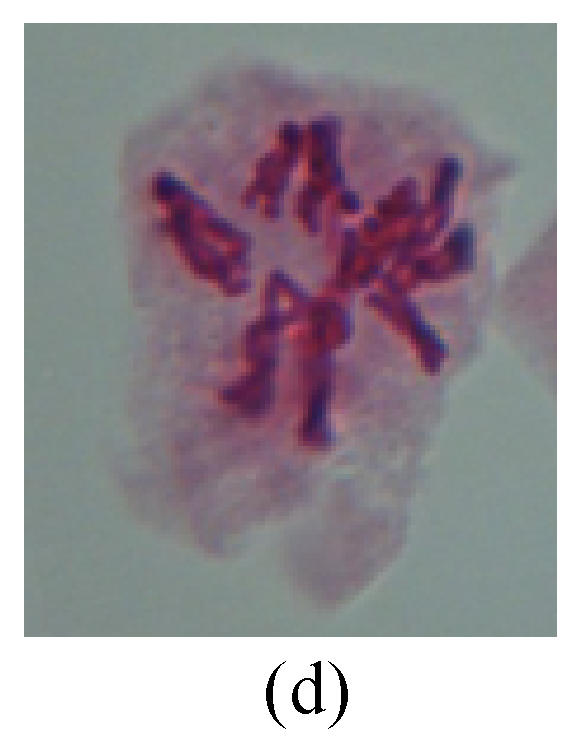
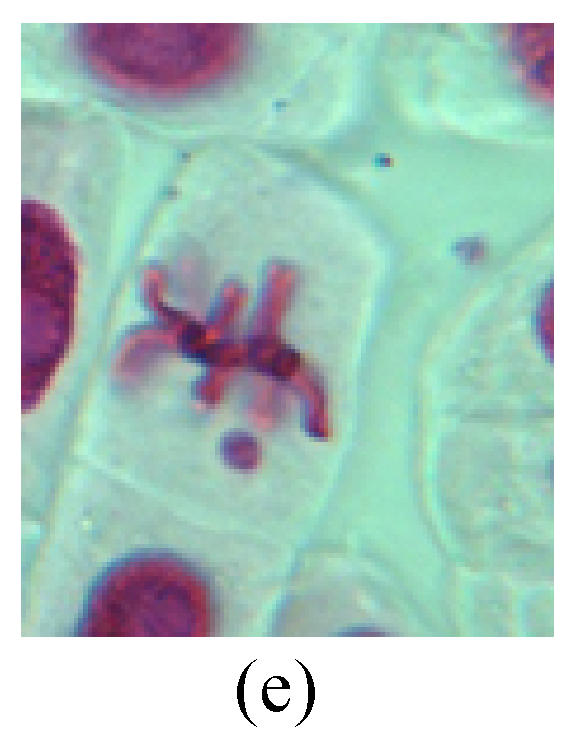
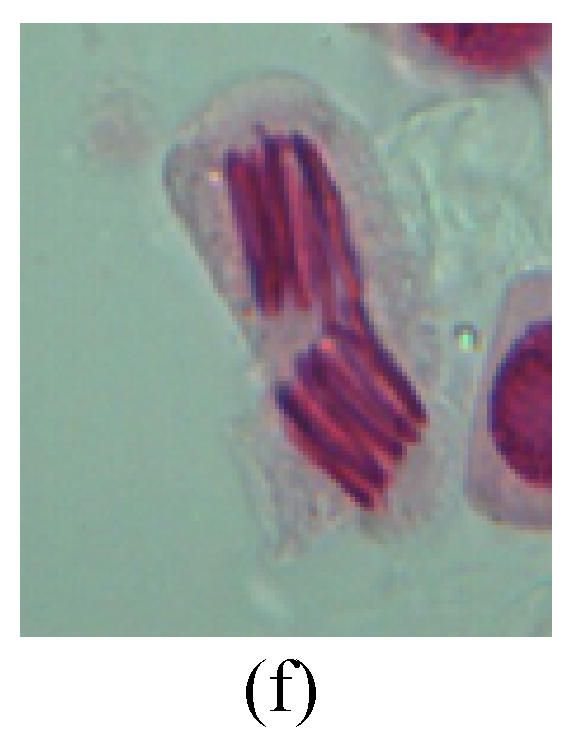
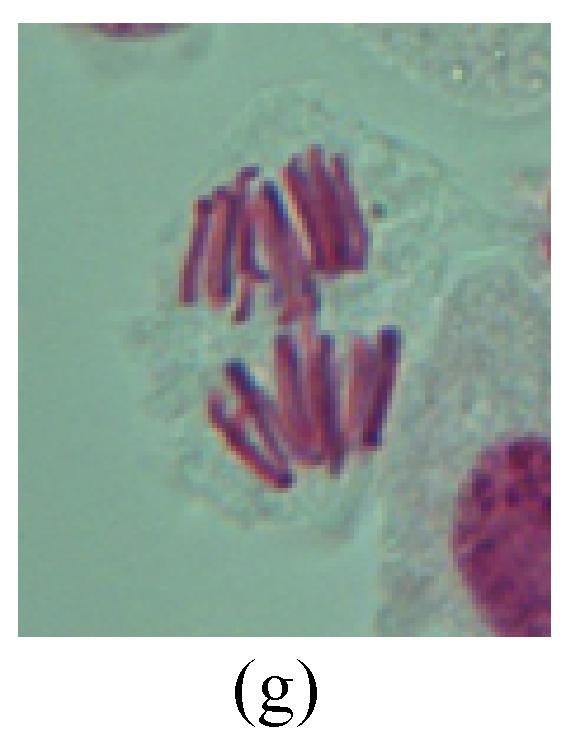
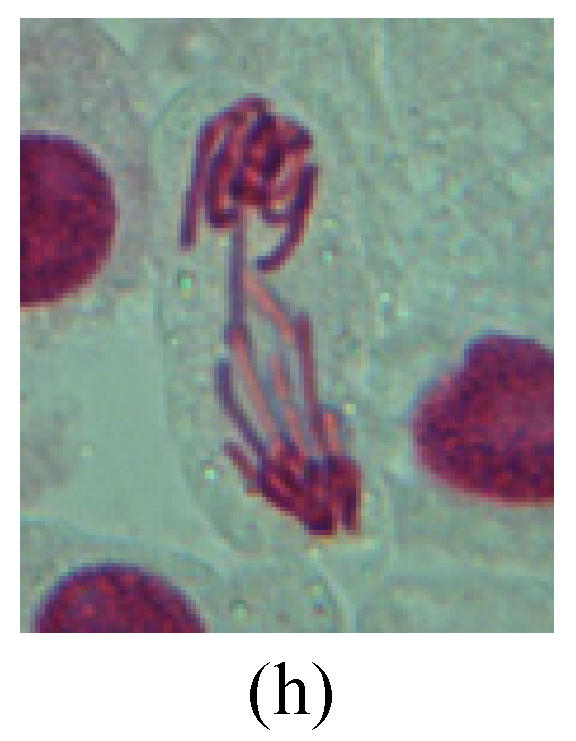
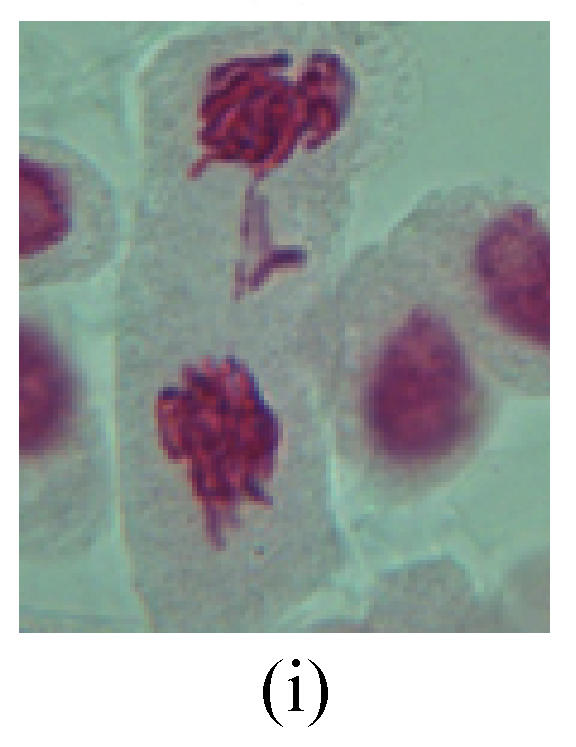
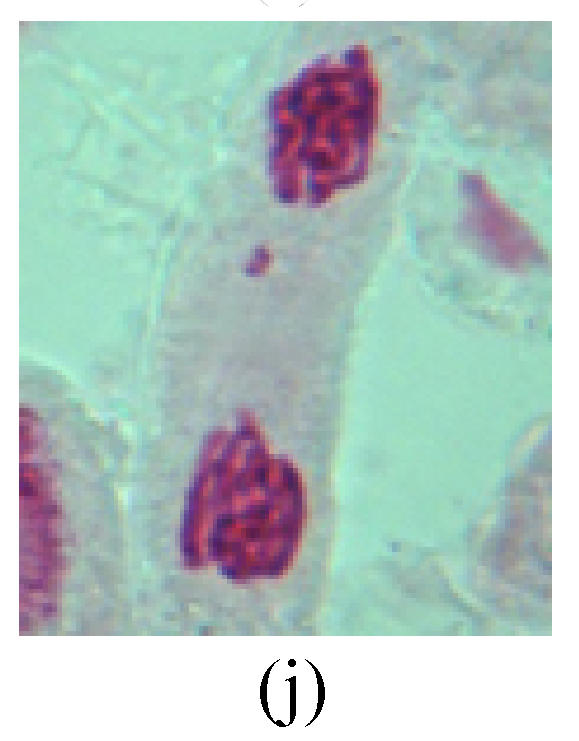
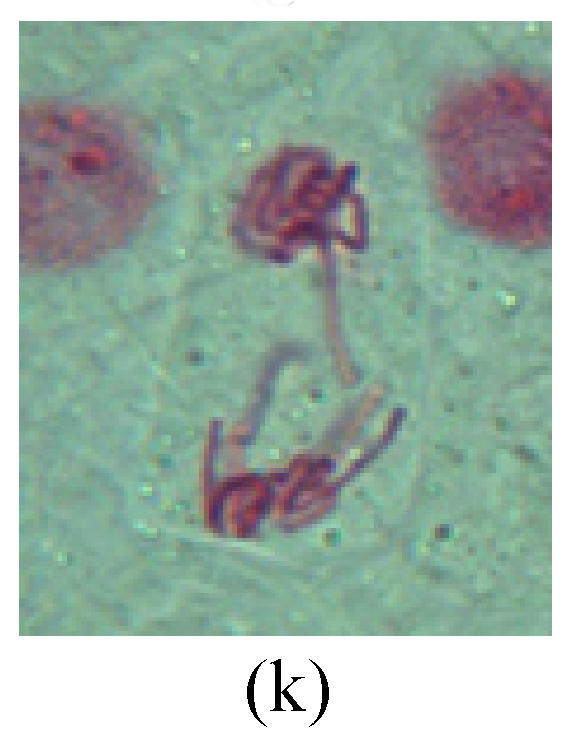
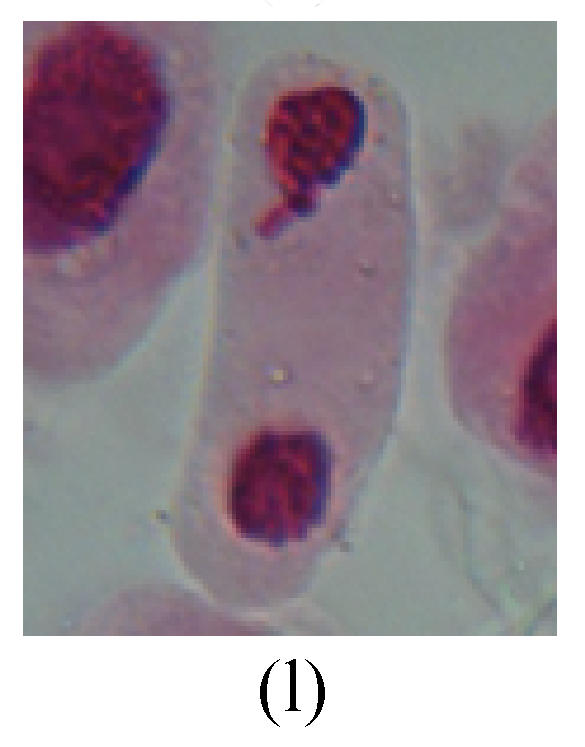
Chromosome aberration of Group G (a) Single micronuclei in interphase; (b) Syncytium; (c) Single micronuclei in prophase; (d) Chromosome divergence in metaphase; (e) Micronucleus in metaphase; (f) Chromosome bridge in anaphase; (g) Chromosome bridge and lagging in anaphase; (h) Chromosome bridge and fragment in anaphase; (i) Chromosome bridges and fragment in telophase; (j) Chromosome fragment in telophase; (k) Uncoiling abnormally in telophase; (l) Uncoiling abnormally in telophase
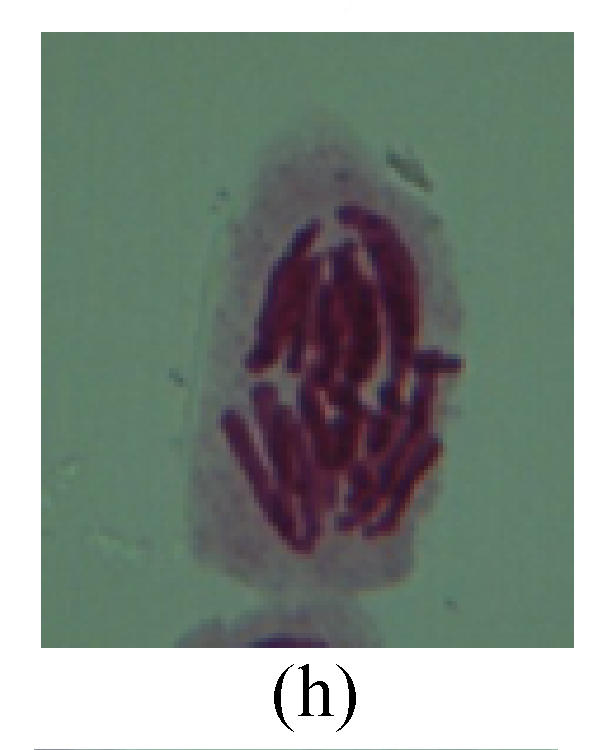
Fig. 3.
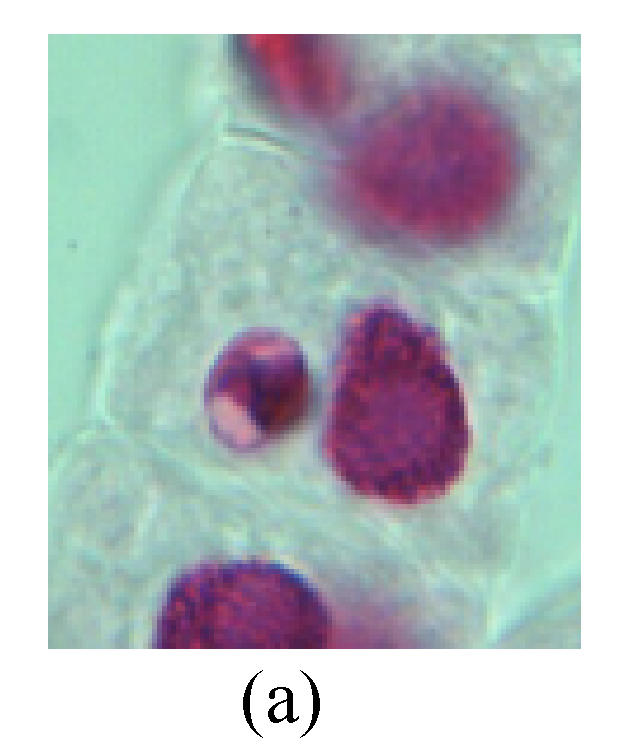
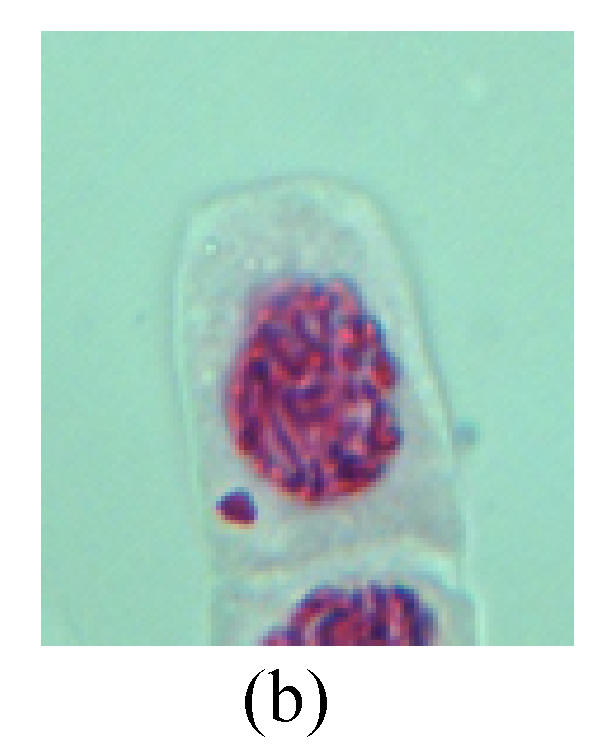
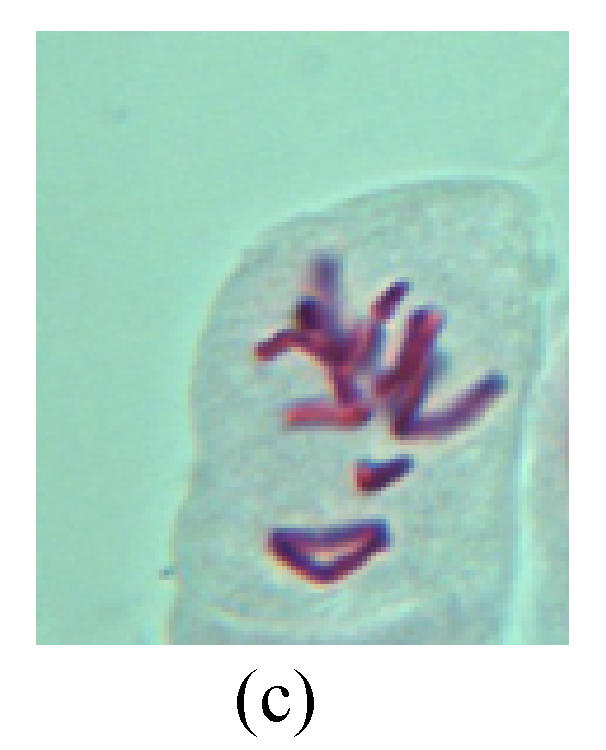
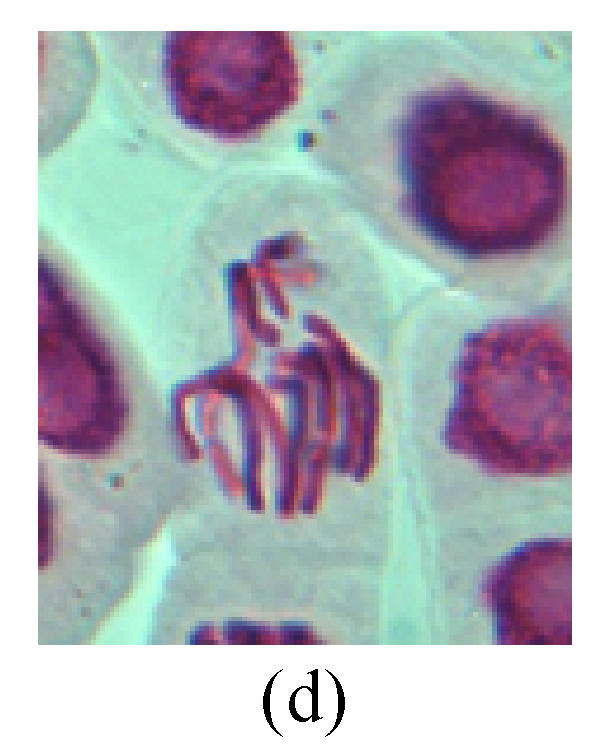
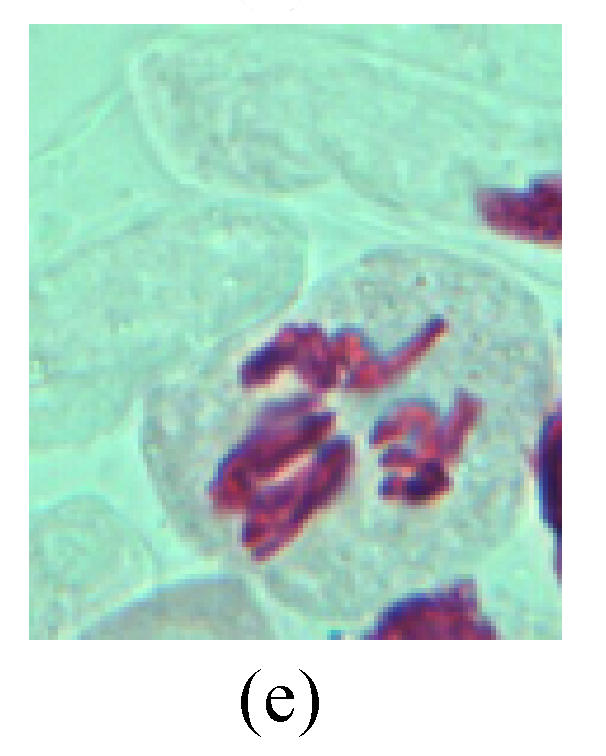
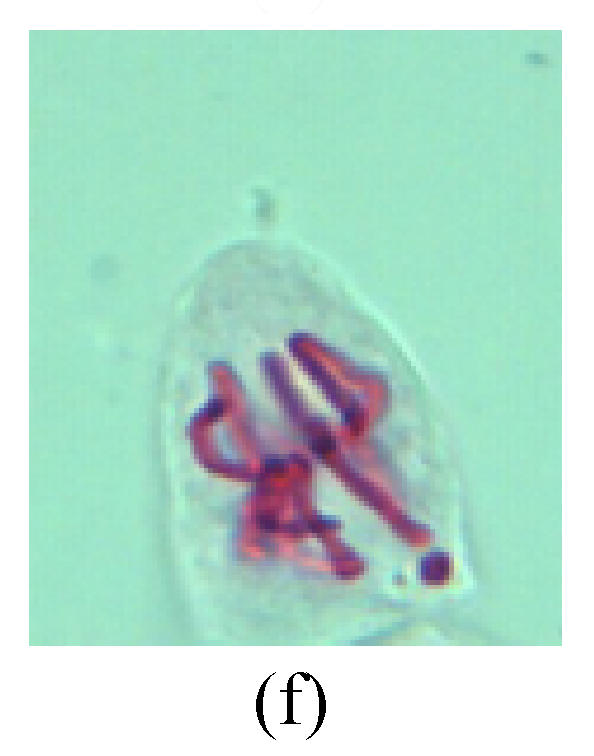
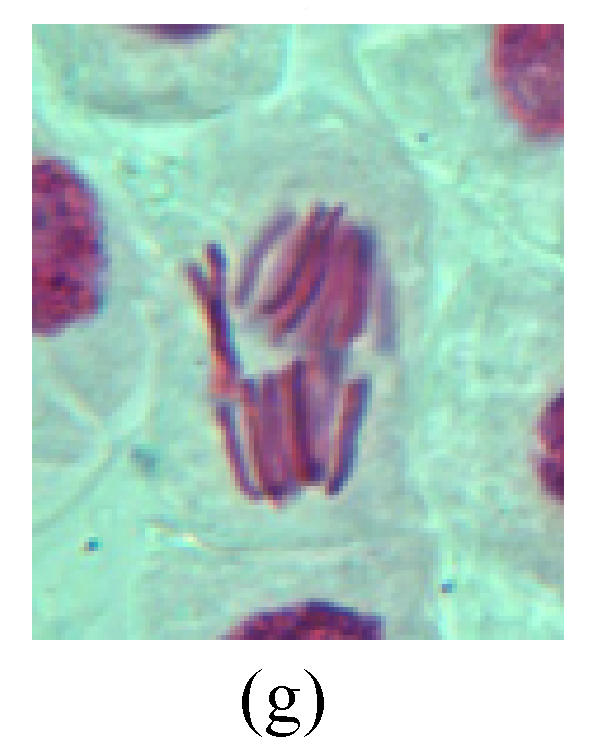
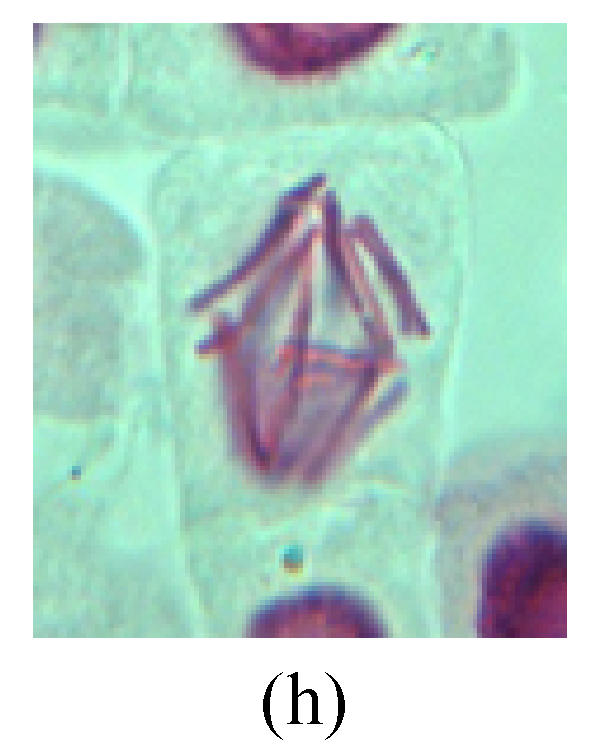
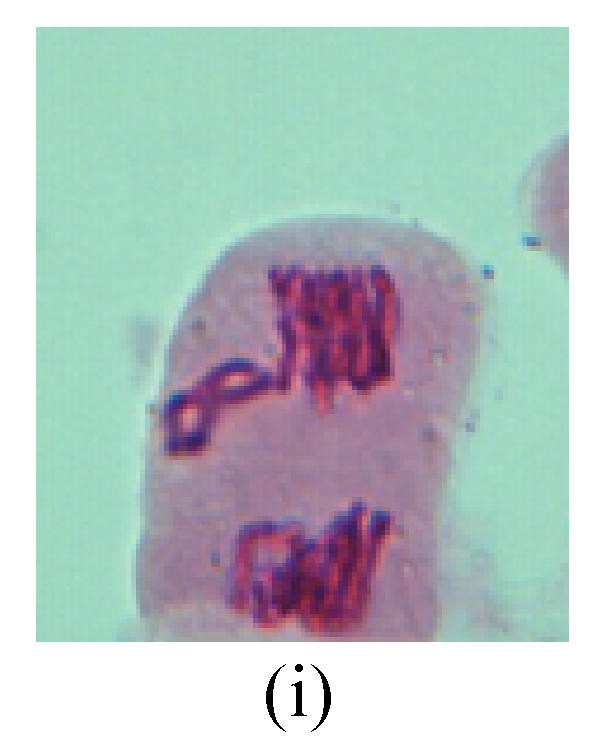
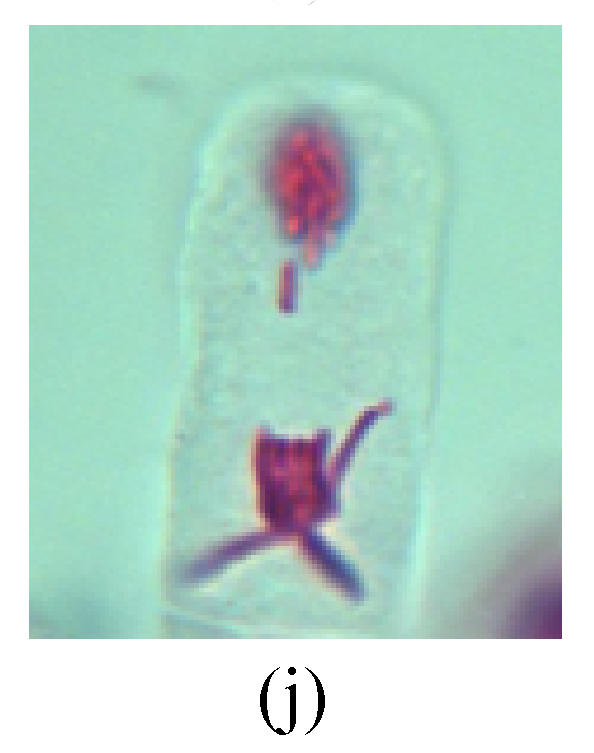
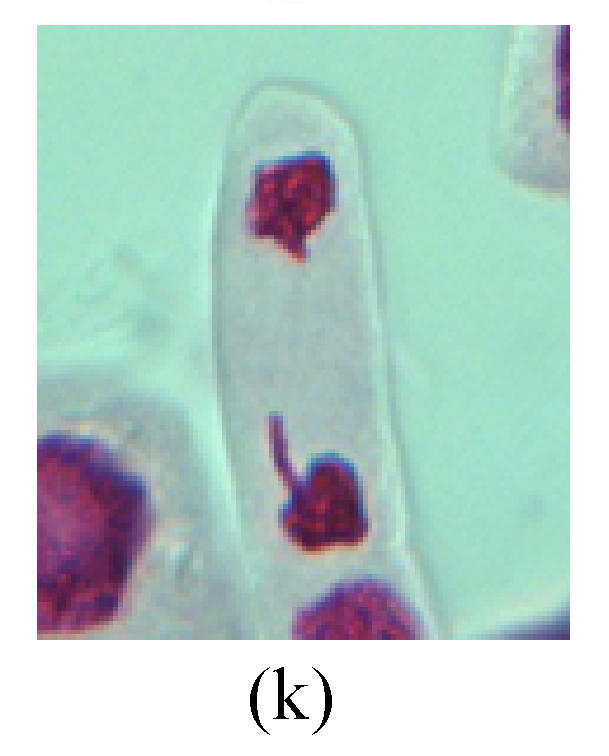
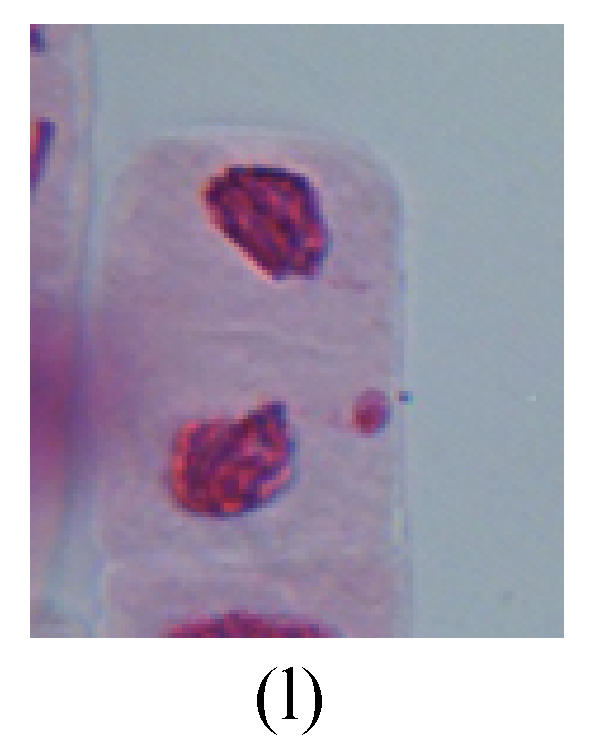
Chromosome aberration of Group H (a) Syncytium; (b) Micronuclei in prophase; (c) Resident chromosome in metaphase; (d) Resident chromosome in metaphase; (e) Chromosome was divided into three parts on equatorial plane in metaphase; (f) Micronucleus in metaphase; (g) Resident chromosome in anaphase; (h) Resident chromosome in anaphase; (i) Resident chromosome in telophase; (j) Uncoiling abnormally in telophase; (k) Uncoiling abnormally in telophase; (l) Micronucleus in telophase
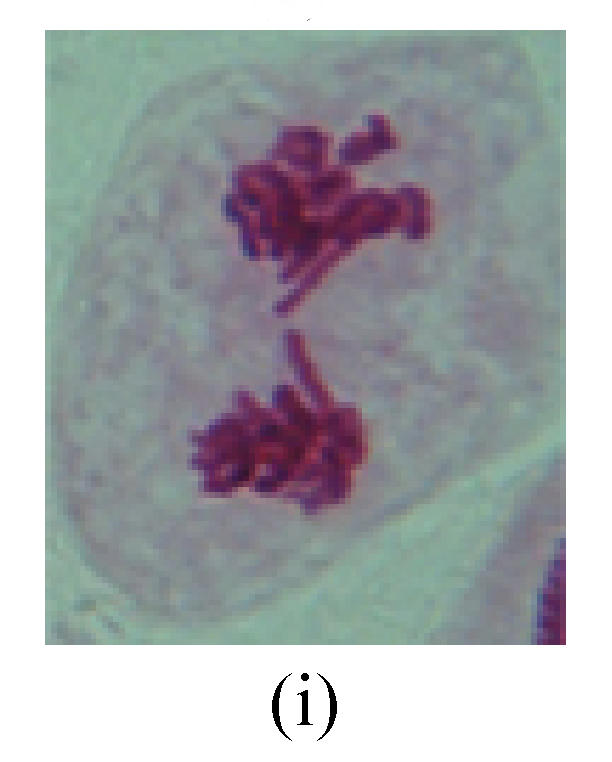
Fig. 4.
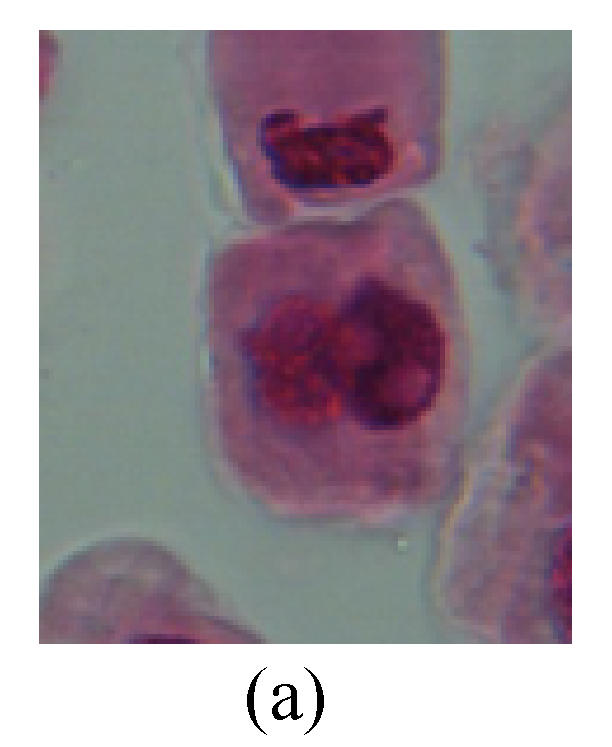
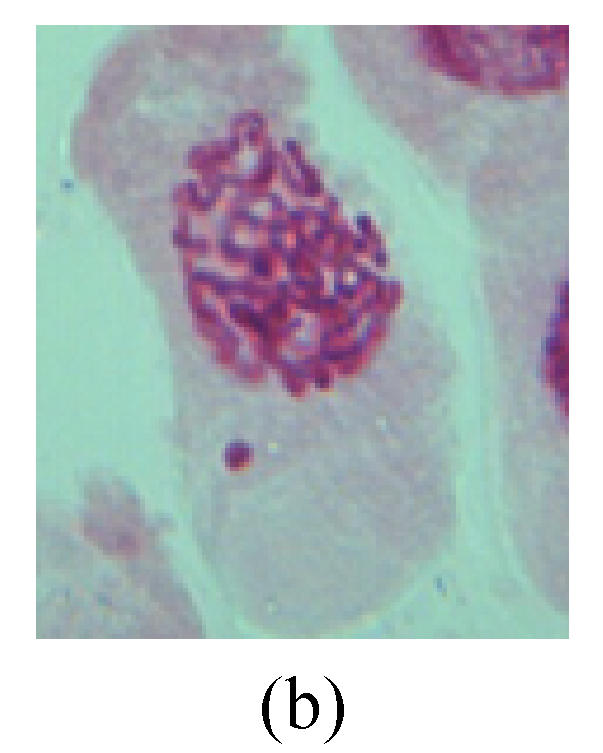
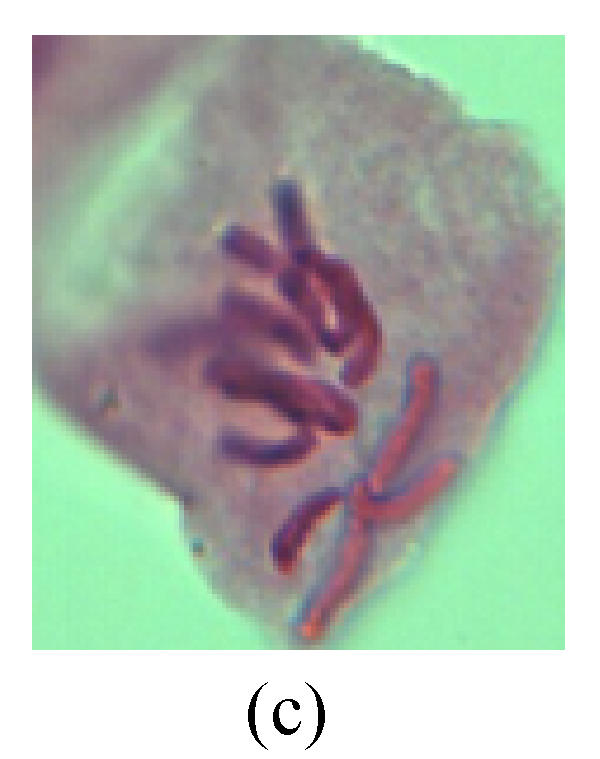
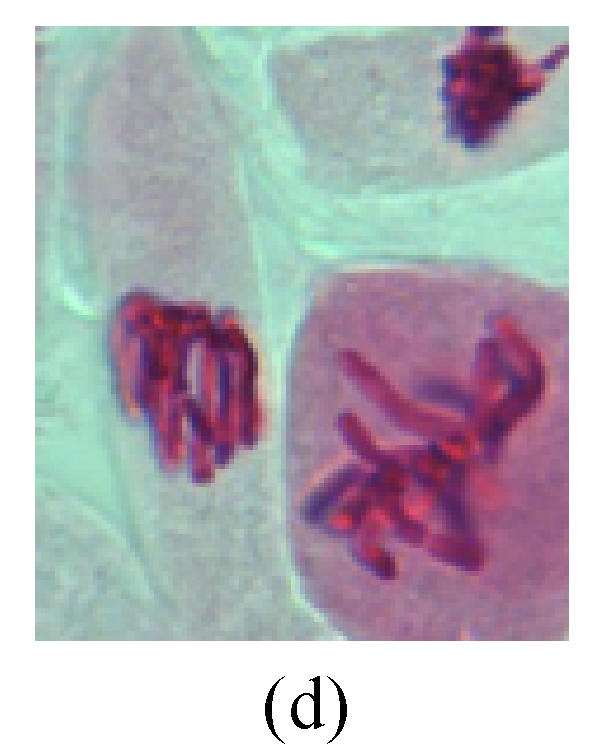
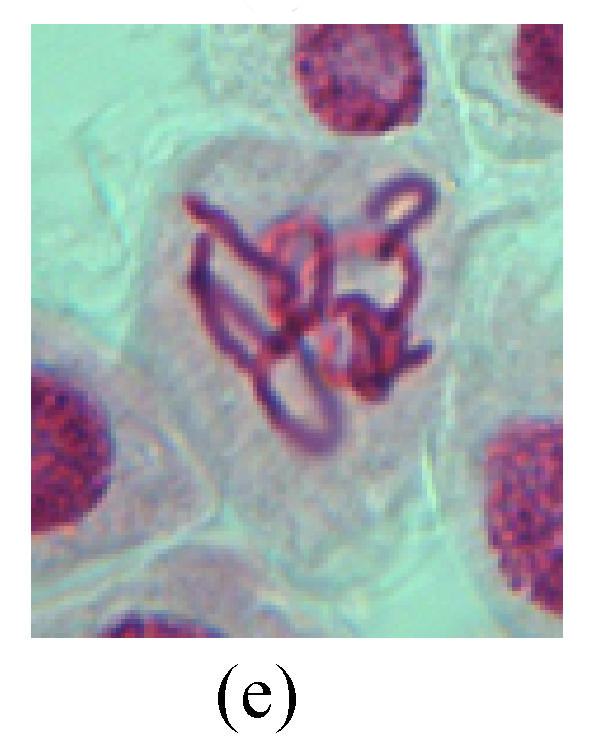
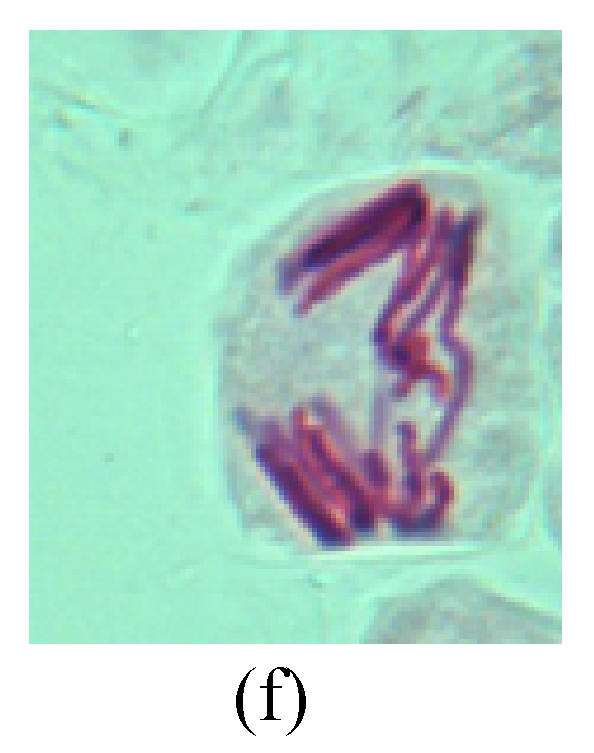
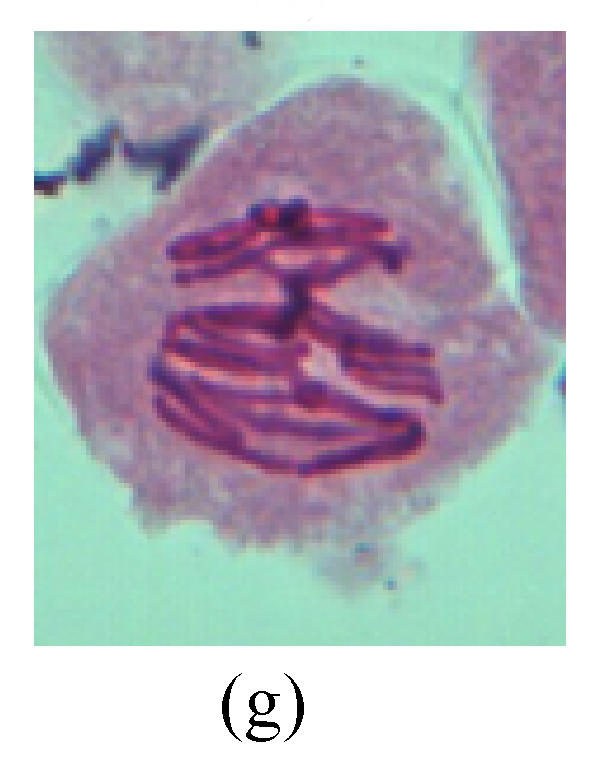
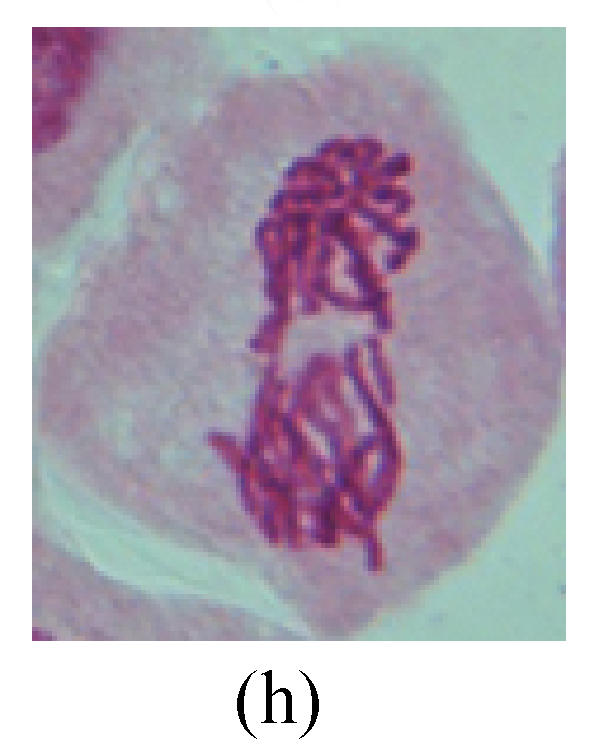
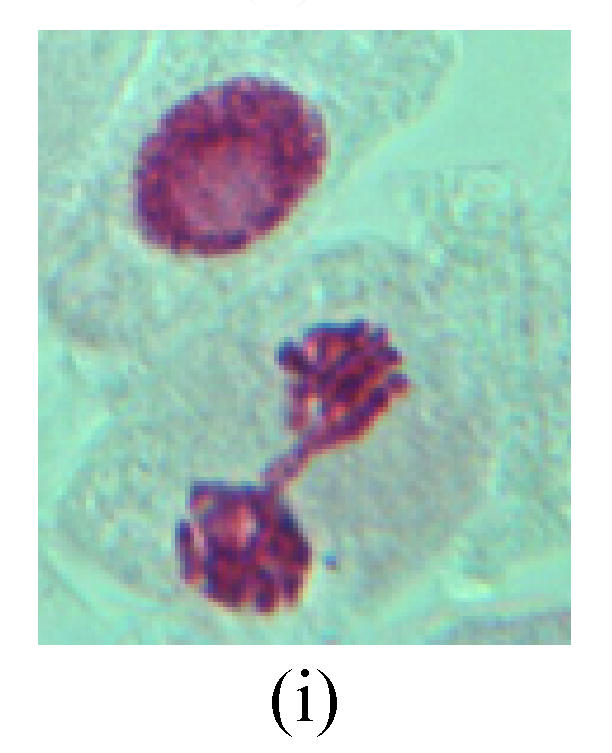
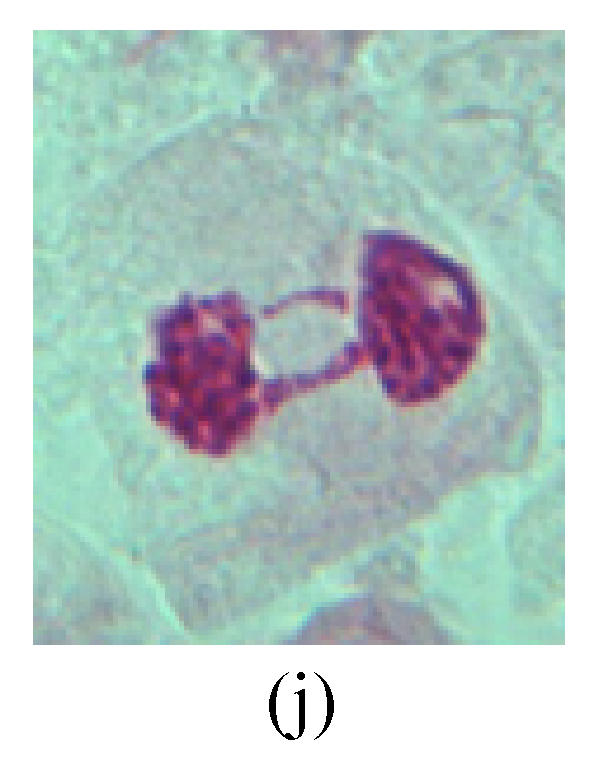
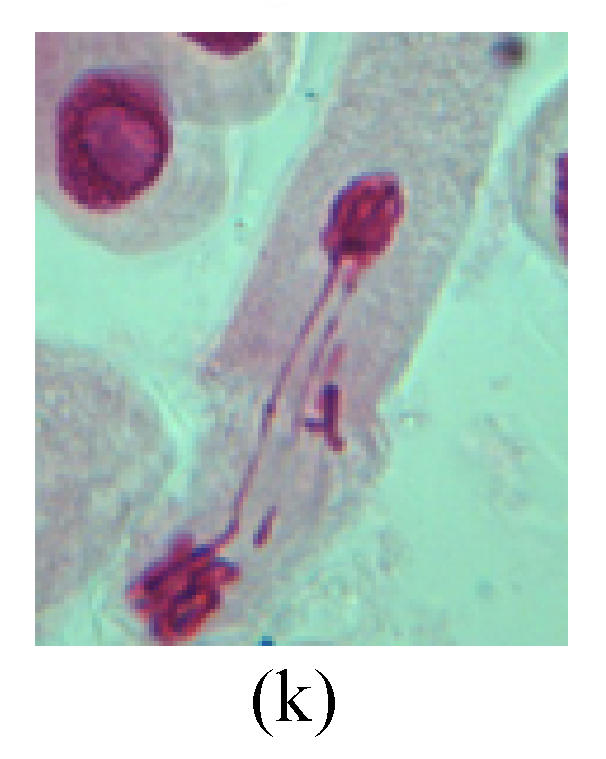
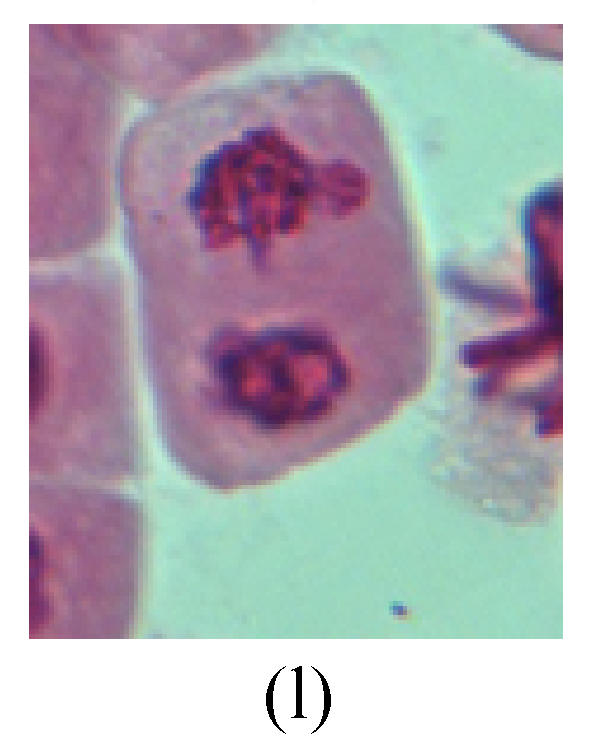
Chromosome aberration of Group I (a) Syncytium; (b) Micronuclei in prophase; (c) Resident chromosome in metaphase; (d) Chromosome blending in metaphase; (e) Chromosome blending in metaphase; (f) Chromosome bridge and lagging in anaphase; (g) Chromosome multi-polar and sticking in anaphase; (h) Polyploid; (i) Chromosome single bridge in telophase; (j) Chromosome double bridges in telophase; (k) Chromosome double bridges and fragment in telophase; (l) Micronucleus in telophase
2. Metaphase: Aside from the micronucleus (Figs.1g, 2e and 3f), there is a chromosome that remained and diverged and was formed by spindle fiber broken in the mitosis process (Figs.1f, 3c, 3d, 4c and 2d). The chromosomes in groups on the equatorial planes are resulted from the abnormal activities of chromosomes (Figs.1e and 3e) and multi-polar phenomena in these kinds of cells will appear in anaphase, when there is also chromosome blending (Figs.1c, 1d, 4d and 4e).
3. Anaphase: The abnormality has mainly four features. The first is the occurrence of chromosomal bridge (Figs.2f, 2g, 2h and 4f), one of the main features of abnormal cell division and chromosomal aberration, results from the formation of dicentric chromosome and acentric fragment. The formation of chromosomal bridges is accompanied by the occurrence of the chromosomal fragment (Fig.2h). Secondly, chromosomal lagging appears (Figs.1h, 3g and 3h). Most chromosomes move to both poles normally, only a few chromosomes or chromosomal fragments remained between the two poles. This reflects that the moving speed of the chromosomes differs from the rest. Thirdly, multi-polar phenomena occurs. In the anaphase of a normal mitosis, the chromosomes move equally to the opposite poles with the traction of the spindle fibers. However abnormal multi-poles unequal distribution was observed in the Vicia faba root tip cells which had been treated with microwave and CrO3 (Fig.4g). Fourthly, chromosomes stick to each other (Fig.4g).
4. Telophase: The abnormality mainly includes the micronucleus (Figs.3l and 4l), the chromosomal fragment (Figs.1j, 2i, 2j and 4k), the chromosomal single bridge (Figs.1k, 2i and 4i), the chromosomal double bridges (Figs.1j, 4j and 4k), the chromosome resident (Fig.3i), and abnormal uncoiling (Figs.1i, 1k, 2k, 2l, 3j and 3k). The telophase micronucleus is possibly formed in the last mitosis or in the present mitosis (Figs.3l and 4l). Some chromosomes uncoil unsynchronously. Most chromosomes have already uncoiled spirally and became the chromatin, chromosomes which have not uncoiled spirally still exist as chromosome.
5. Polyploid: The spindle fiber is broken in the mitosis process, so that daughter chromosomes cannot reach the two poles. The mitosis process is prevented, as a result, daughter cell does not form and becomes polyploid (Fig.4h).
6. Syncytium: Abnormal multi-poles unequal distribution and damage or abnormality of individual chromosome lead to syncytium in anaphase (Figs.1l, 2b, 3a and 4a).
DISCUSSION
CrO3 alone has a turnover dose-effect on the micronucleus frequency, possibly because the highly concentrated solution causes the cell mitosis to pause so it fails to enter into the next cell cycle, and consequently has lower micronucleus frequency. This accords with the results of previous work (Qian, 2004a).
Hexavalent chromium causes DNA damage in animals and men (Knasmuller et al., 1998) and also shows higher toxicity than its trivalent compounds since chromium ions in the +6 oxidation state easily cross biological membranes (Szelag et al., 2003). The results obtained in the present study showed that antagonistic effect appeared between specific concentrations of CrO3 and microwave exposure time in the mitotic index, micronucleus frequency, and chromosomal aberration frequency. Implying that microwave weakened the effect of chromium on cell cycle by prolonging the mitosis cycle. Moreover, microwave may be the cause of antagonistic effect by reducing chromium ions from the +6 to the +3 oxidation state, which is of potential value in combating chromium poisoning.
Repetitive high power microwave can exert inhibitory influence on the process of DNA and RNA syntheses in tumor cells of P-815 mastocytoma (Litviakov et al., 2005). It was reported that microwaves can preserve not only cell and nuclear shapes but also immunohistochemical antigenicity. However, their enzyme function was lost, which indicated cell dysfunction and death (Ozaki et al., 2003). So microwave may have effect on some enzymes and influence CrO3 action.
Fig.1 through Fig.4 depict varieties of chromosomal aberration. Microwave and CrO3 may play a role in destruction of microtubules and/or some enzymes in cell (Pavicic, 2004).
This experimental result shows that coexistence of microwave and chromium trioxide has obvious mutagenic effects, but display antagonistic effect on some indices. Its mechanism needs further studies.
Footnotes
Project supported by Wenzhou Technology Bureau (No. S2002A015) and Wenzhou Normal College (No. 2003Z20), China
References
- 1.Forgacs Z, Kubinyi G, Sinay G. Effects of 1800 MHz GSM-like exposure on the gonadal function and hematological parameters of male mice. Magy Onkol. 2005;49(2):149–151. [PubMed] [Google Scholar]
- 2.Garcia-Rodriguez MC, Lopez-Santiago V, Altamirano-Lozano M. Effect of chlorophyllin on chromium trioxide-induced micronuclei in polychromatic erythrocytes in mouse peripheral blood. Mutat Res. 2001;496(1-2):145–151. doi: 10.1016/s1383-5718(01)00225-x. [DOI] [PubMed] [Google Scholar]
- 3.Kim HY, Lee SB, Jang BC. Subchronic inhalation toxicity of soluble hexavalent chromium trioxide in rats. Arch Toxicol. 2004;78(7):363–368. doi: 10.1007/s00204-004-0553-4. [DOI] [PubMed] [Google Scholar]
- 4.Knasmuller S, Gottmann E, Steinkellner H, Fomin A, Pcickl C, Paschke A, God R, Kundi M. Detection of genotoxic effects of heavy metal contaminated soils with plant bioassays. Mutat Res. 1998;420(1-3):37–48. doi: 10.1016/s1383-5718(98)00145-4. [DOI] [PubMed] [Google Scholar]
- 5.Lacy-Hulbert A, Metcalfe JC, Hesketh R. Biological responses to electromagnetic field. Faseb J. 1998;12(6):395–420. doi: 10.1096/fasebj.12.6.395. [DOI] [PubMed] [Google Scholar]
- 6.Litviakov NV, Buldakov MA, Cherdyntseva NV, Rostov VV, Klimov AI, Bol′shakov MA. Effect of impulse-intermittent ultrahigh frequency irradiation on synthesis of nucleic acids in tumor cells. Radiats Biol Radioecol. 2005;45(4):460–463. [PubMed] [Google Scholar]
- 7.Ozaki T, Tabuse K, Tsuji T, Nakamura Y, Kakudo K, Mori I. Microwave cell death: enzyme histo-chemical evaluation for metastatic carcinoma of the liver. Pathol Int. 2003;53(12):837–845. doi: 10.1046/j.1440-1827.2003.01571.x. [DOI] [PubMed] [Google Scholar]
- 8.Pavicic I. Impact of radiofrequency/microwave radiation on cell and cytoskeleton structure. Arh Hig Rada Toksikol. 2004;55(4):321–328. [PubMed] [Google Scholar]
- 9.Qian XW. Mutagenic effects of chromium trioxide on root tip cells of Vicia faba . Journal of Zhejiang University (SCIENCE) 2004;5(12):1570–1576. doi: 10.1631/jzus.2004.1570. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 10.Qian XW. Study on teratogenic effect of potassium dichromate on Vicia faba root tip cells. Hereditas. 2004;26(3):337–342. (in Chinese) [PubMed] [Google Scholar]
- 11.Sher L. The effects of natural and man-made electromagnetic fields on mood and behavior: the role of sleep disturbances. Med Hypotheses. 2000;54(4):630–633. doi: 10.1054/mehy.1999.0912. [DOI] [PubMed] [Google Scholar]
- 12.Sripakdee D, Sukontason KL, Piangjai S, Ngern-klun R, Sukontason K. Effect of microwave irradiation on the blow fly Chrysomya megacephala (F.) (Diptera: Calliphoridae) Southeast Asian J Trop Med Public Heslth. 2005;36(4):893–895. [PubMed] [Google Scholar]
- 13.Szelag A, Magdalan J, Kopacz M. Assessment of efficacy of quercetin-5'-sulfonic acid sodium salt in the treatment of acute chromium poisoning: experimental studies. Pol J Pharmacol. 2003;55(6):1097–1103. [PubMed] [Google Scholar]


