Abstract
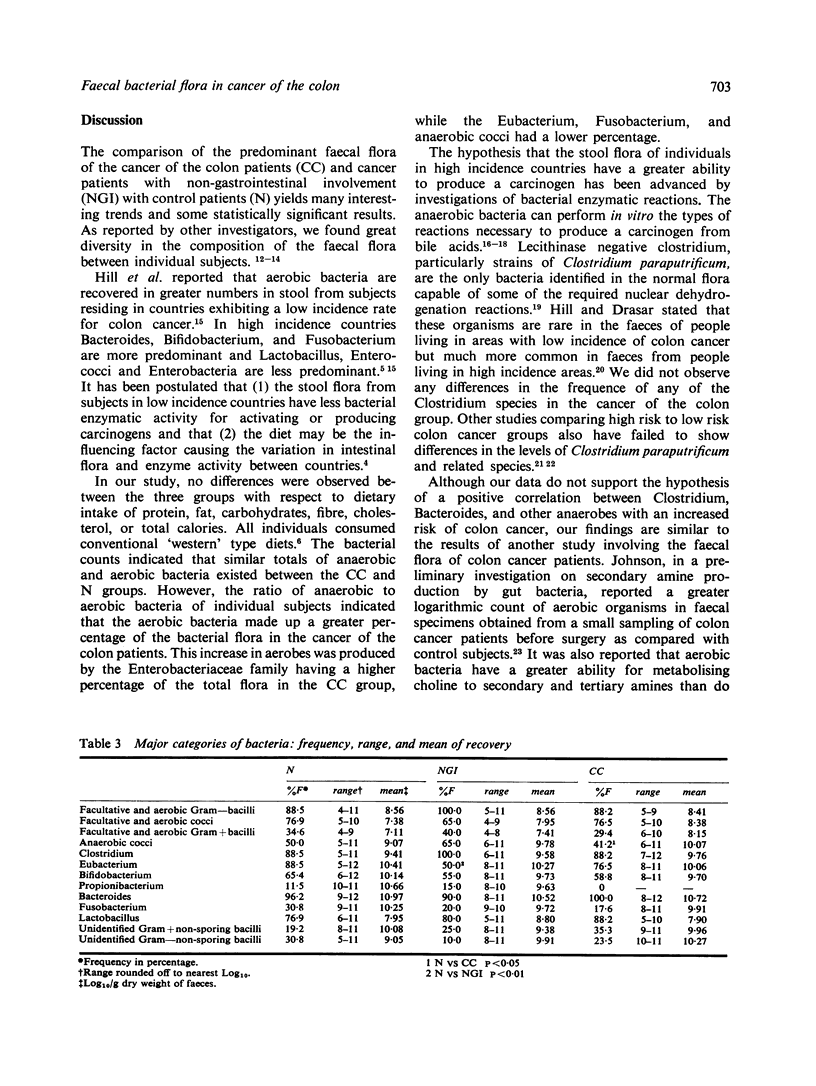
Selective aerobic and anaerobic plate media were employed to isolate the predominant faecal flora of patients with cancer of the colon (CC), cancer with non-gastrointestinal involvement (NGI), and with non-malignant diseases (N). The CC and N groups did not differ significantly in either total aerobic or anaerobic counts. The CC group did have a significantly lower anaerobic/aerobic ratio compared with the N group (2.42 vs. 2.96, P less than 0.05). This was the result of a greater predominance of aerobic bacteria and a decrease in anaerobic cocci, Eubacterium and Fusobacterium in the CC group. Previous studies report that aerobic organisms have a greater ability to produce amines than non-spore forming anaerobes. If the intestinal flora can produce carcinogenic nitrosamines in vivo from amines and nitrites, the aerobic bacterium in the faeces may be of importance in supplying the amine substrate for nitrosation. The comparison of the NGI group with the N group showed a significant variation in the total anaerobic count (11.02 vs. 11.41, P less than 0.05) and in the composition of the faecal flora. This indicates that discretion must be used in analysing the data obtained from cancer patients, as the presence of a carcinoma may be responsible for changes in bacterial flora.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aries V., Crowther J. S., Drasar B. S., Hill M. J., Williams R. E. Bacteria and the aetiology of cancer of the large bowel. Gut. 1969 May;10(5):334–335. doi: 10.1136/gut.10.5.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best W. R. On the logarithmid transformation of intestinal bacterial counts. Am J Clin Nutr. 1970 Dec;23(12):1608–1609. doi: 10.1093/ajcn/23.12.1608. [DOI] [PubMed] [Google Scholar]
- Burkitt D. P. Epidemiology of cancer of the colon and rectum. Cancer. 1971 Jul;28(1):3–13. doi: 10.1002/1097-0142(197107)28:1<3::aid-cncr2820280104>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Crowther J. S., Drasar B. S., Hill M. J., Maclennan R., Magnin D., Peach S., Teoh-chan C. H. Faecal steroids and bacteria and large bowel cancer in Hong Kong by socio-economic groups. Br J Cancer. 1976 Aug;34(2):191–198. doi: 10.1038/bjc.1976.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasar B. S., Hill M. J. Intestinal bacteria and cancer. Am J Clin Nutr. 1972 Dec;25(12):1399–1404. doi: 10.1093/ajcn/25.12.1399. [DOI] [PubMed] [Google Scholar]
- Finegold S. M., Flora D. J., Attebery H. R., Sutter V. L. Fecal bacteriology of colonic polyp patients and control patients. Cancer Res. 1975 Nov;35(11 Pt 2):3407–3417. [PubMed] [Google Scholar]
- Finegold S. M., Sutter V. L., Sugihara P. T., Elder H. A., Lehmann S. M., Phillips R. L. Fecal microbial flora in Seventh Day Adventist populations and control subjects. Am J Clin Nutr. 1977 Nov;30(11):1781–1792. doi: 10.1093/ajcn/30.11.1781. [DOI] [PubMed] [Google Scholar]
- Floch M. H., Gershengoren W., Freedman L. R. Methods for the quantitative study of the aerobic and anaerobic intestinal bacterial flora of man. Yale J Biol Med. 1968 Aug;41(1):50–61. [PMC free article] [PubMed] [Google Scholar]
- Fuchs H-M, Dorfman S., Floch M. H. The effect of dietary fiber supplementation in man. II. Alteration in fecal physiology and bacterial flora. Am J Clin Nutr. 1976 Dec;29(12):1443–1447. doi: 10.1093/ajcn/29.12.1443. [DOI] [PubMed] [Google Scholar]
- Goddard P., Fernandez F., West B., Hill M. J., Barnes P. The nuclear dehydrogenation of steroids by intestinal bacteria. J Med Microbiol. 1975 Aug;8(3):429–435. doi: 10.1099/00222615-8-3-429. [DOI] [PubMed] [Google Scholar]
- Goddard P., Hill M. J. Degradation of steroids by intestinal bacteria. IV. The aromatisation of ring A. Biochim Biophys Acta. 1972 Oct 5;280(2):336–342. doi: 10.1016/0005-2760(72)90101-4. [DOI] [PubMed] [Google Scholar]
- Goldin B., Gorbach S. L. Alterations in fecal microflora enzymes related to diet, age, lactobacillus supplements, and dimethylhydrazine. Cancer. 1977 Nov;40(5 Suppl):2421–2426. doi: 10.1002/1097-0142(197711)40:5+<2421::aid-cncr2820400905>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hill M. J., Drasar B. S., Hawksworth G., Aries V., Crowther J. S., Williams R. E. Bacteria and aetiology of cancer of large bowel. Lancet. 1971 Jan 16;1(7690):95–100. doi: 10.1016/s0140-6736(71)90837-3. [DOI] [PubMed] [Google Scholar]
- Hill M. J., Drasar B. S. The normal colonic bacterial flora. Gut. 1975 Apr;16(4):318–323. doi: 10.1136/gut.16.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. J., Drasar B. S., Williams R. E., Meade T. W., Cox A. G., Simpson J. E., Morson B. C. Faecal bile-acids and clostridia in patients with cancer of the large bowel. Lancet. 1975 Mar 8;1(7906):535–539. doi: 10.1016/s0140-6736(75)91556-1. [DOI] [PubMed] [Google Scholar]
- Hill M. J. Steroid nuclear dehydrogenation and colon cancer. Am J Clin Nutr. 1974 Dec;27(12):1475–1480. doi: 10.1093/ajcn/27.12.1475. [DOI] [PubMed] [Google Scholar]
- Holdeman L. V., Good I. J., Moore W. E. Human fecal flora: variation in bacterial composition within individuals and a possible effect of emotional stress. Appl Environ Microbiol. 1976 Mar;31(3):359–375. doi: 10.1128/aem.31.3.359-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. A. The production of secondary amines by the human gut bacteria and its possible relevance to carcinogenesis. Med Lab Sci. 1977 Apr;34(2):131–143. [PubMed] [Google Scholar]
- Kahaner N., Fuchs H-M, Floch M. H. The effect of dietary fiber supplementation in man. I. Modification of eating habits. Am J Clin Nutr. 1976 Dec;29(12):1437–1442. doi: 10.1093/ajcn/29.12.1437. [DOI] [PubMed] [Google Scholar]
- Mastromarino A., Reddy B. S., Wynder E. L. Metabolic epidemiology of colon cancer: enzymic activity of fecal flora. Am J Clin Nutr. 1976 Dec;29(12):1455–1460. doi: 10.1093/ajcn/29.12.1455. [DOI] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz M., White C., Barnett R. N., Stevens S., Russell E., Vargo D., Floch M. H. Diet, fecal bile acids, and neutral sterols in carcinoma of the colon. Dig Dis Sci. 1979 Oct;24(10):746–751. doi: 10.1007/BF01317206. [DOI] [PubMed] [Google Scholar]
- Reddy B. S., Weisburger J. H., Wynder E. L. Fecal bacterial beta-glucuronidase: control by diet. Science. 1974 Feb 1;183(4123):416–417. doi: 10.1126/science.183.4123.416. [DOI] [PubMed] [Google Scholar]
- Walker A. R. Colon cancer and diet, with special reference to intakes of fat and fiber. Am J Clin Nutr. 1976 Dec;29(12):1417–1426. doi: 10.1093/ajcn/29.12.1417. [DOI] [PubMed] [Google Scholar]
- Weisburger J. H. Colon carcinogens: their metabolism and mode of action. Cancer. 1971 Jul;28(1):60–70. doi: 10.1002/1097-0142(197107)28:1<60::aid-cncr2820280113>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Wynder E. L., Reddy B. Studies of large-bowel cancer: human leads to experimental application. J Natl Cancer Inst. 1973 May;50(5):1099–1106. doi: 10.1093/jnci/50.5.1099. [DOI] [PubMed] [Google Scholar]
- Wynder E. L., Shigematsu T. Environmental factors of cancer of the colon and rectum. Cancer. 1967 Sep;20(9):1520–1561. doi: 10.1002/1097-0142(196709)20:9<1520::aid-cncr2820200920>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]


