Abstract
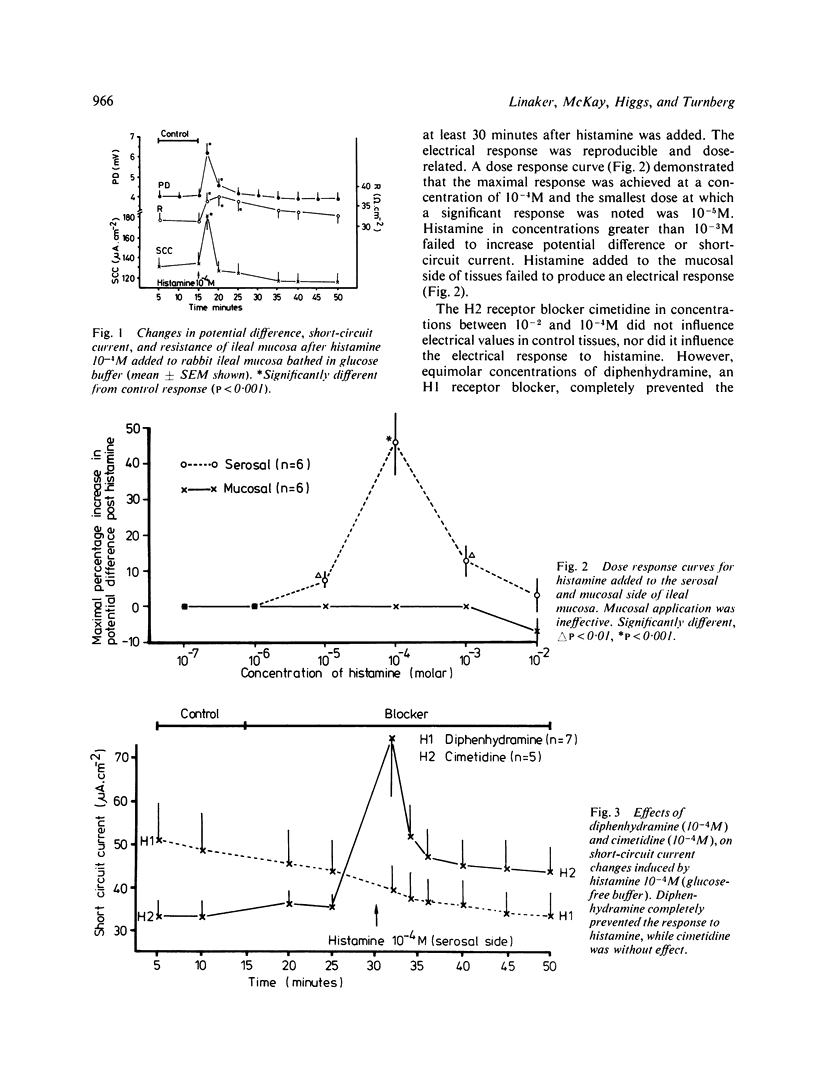
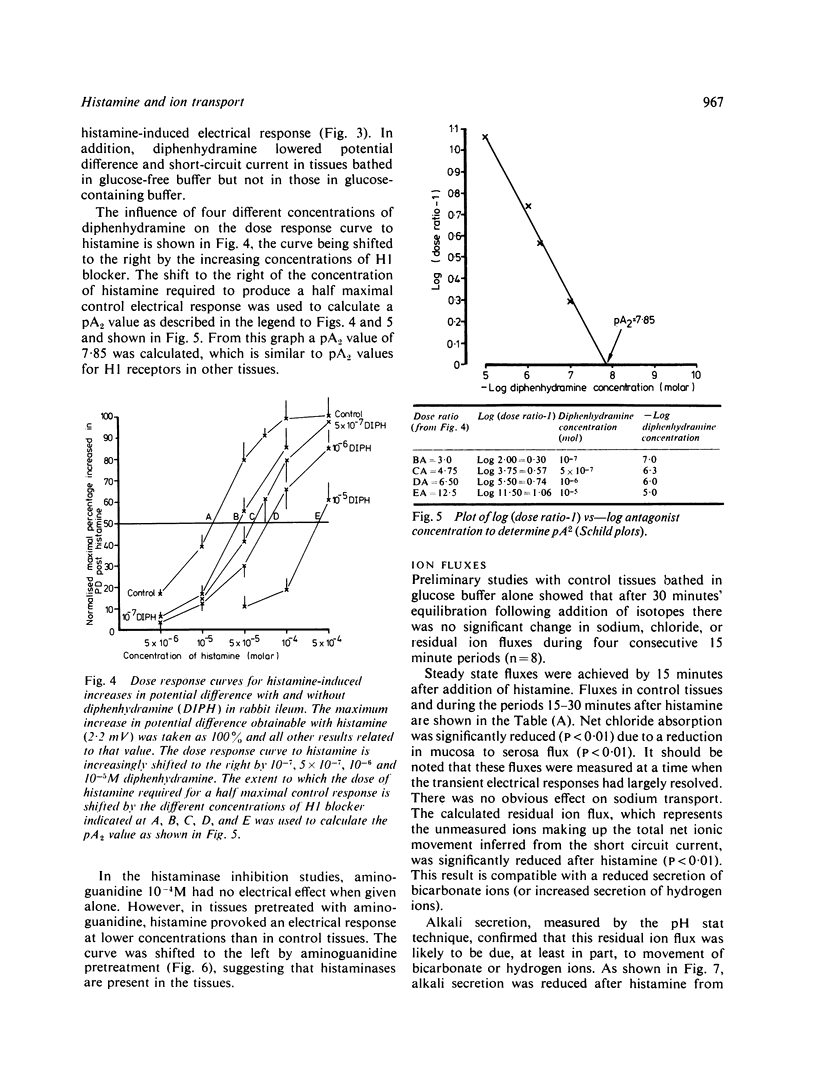
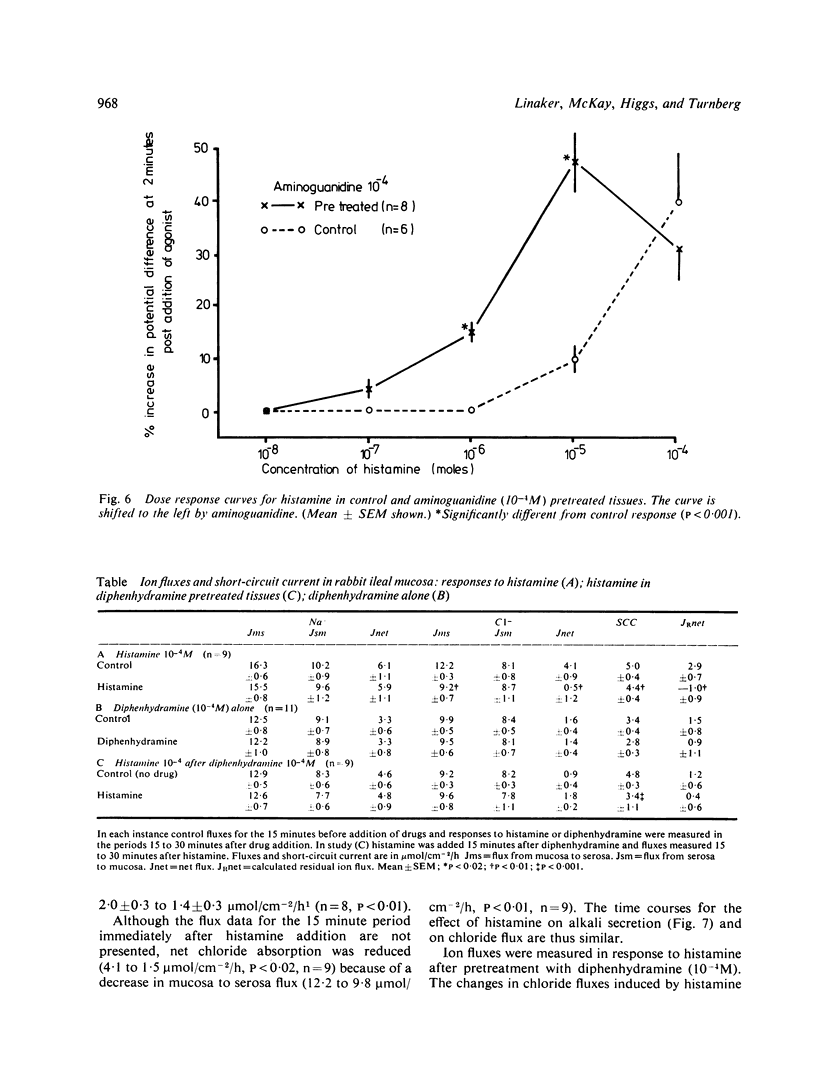
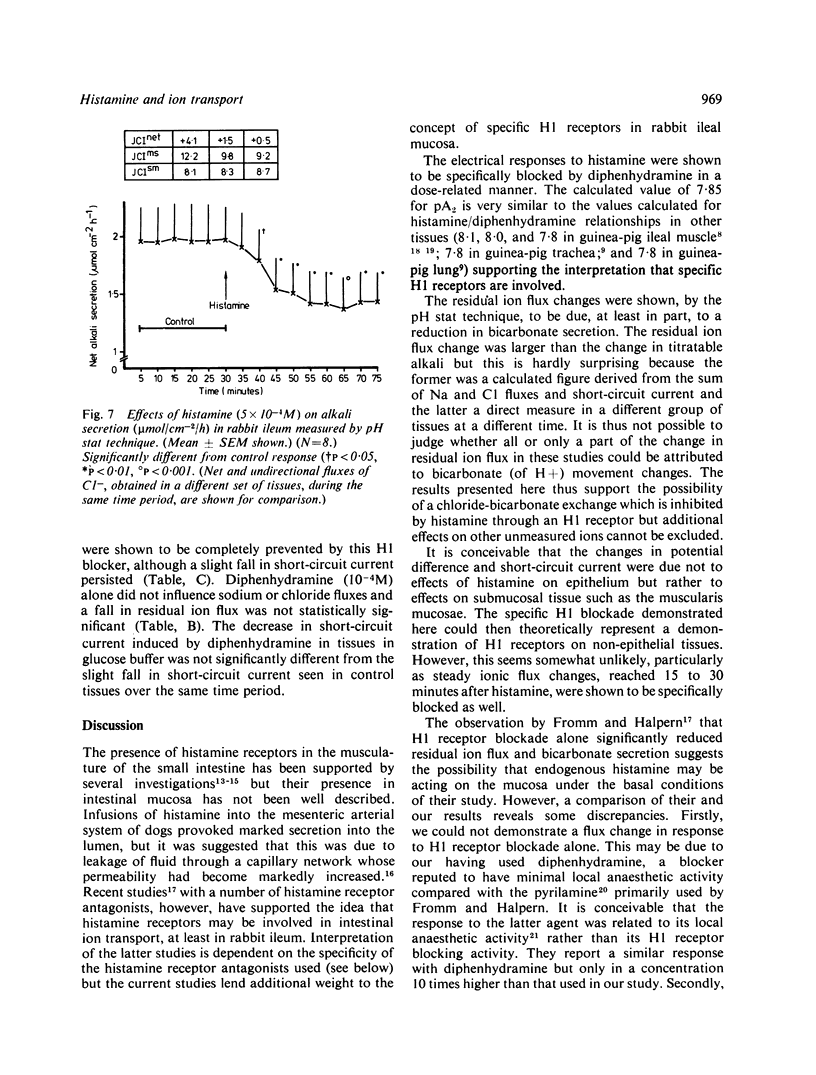
Histamine is present in high concentrations in the intestine and we investigated the possibility that it might have a role here in intestinal transport. When added to the basal side of rabbit ileal mucosa in vitro histamine (10(-4)M) induced a short-lived increase in electrical potential difference and short circuit current. It inhibited net chloride absorption but did not influence sodium transport. Alkali secretion, measured by a pH stat technique, was inhibited, suggesting that bicarbonate secretion was reduced. Both the electrical and ion flux responses to histamine were blocked by the H1 receptor blocker diphenhydramine, but not by the H2 receptor blocker cimetidine. The presence of specific H1 histamine receptors was further supported by shifts in the dose-response curve to histamine by four different concentrations of diphenhydramine. Calculation of a pA2 value from these "Schild' plots provided a figure of 7.85, which is similar to that for H1 receptors in other tissues. Aminoguanidine, a histaminase blocker, had no electrical effects alone but shifted the histamine dose response curve to the left. These studies indicate that histamine inhibits chloride absorption and alkali secretion, possibly by influencing a chloride/bicarbonate exchange process, through specific mucosal H1 receptors. Enhancement of histamine effects by a histaminase inhibitor suggests that histaminases are present in the intestinal mucosa and supports the possibility of a role for endogenous histamine in influencing ion transport. The observations indicate a mechanism by which absorption might be impaired in diseases in which histamine is liberated locally in the intestine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash A. S., Schild H. O. Receptors mediating some actions of histamine. Br J Pharmacol Chemother. 1966 Aug;27(2):427–439. doi: 10.1111/j.1476-5381.1966.tb01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareicha I., Silva M. R. Occurrence of H2-receptors for histamine in the guinea-pig intestine. Biochem Pharmacol. 1975 Jun 15;24(11-12):1215–1219. doi: 10.1016/0006-2952(75)90065-9. [DOI] [PubMed] [Google Scholar]
- Black J. W., Duncan W. A., Durant C. J., Ganellin C. R., Parsons E. M. Definition and antagonism of histamine H 2 -receptors. Nature. 1972 Apr 21;236(5347):385–390. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- Corbett C. L., Isaacs P. E., Riley A. K., Turnberg L. A. Human intestinal ion transport in vitro. Gut. 1977 Feb;18(2):136–140. doi: 10.1136/gut.18.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., FELDBERG W., PATON W. D. M., SCHACHTER M. Distribution of histamine and substance P in the wall of the dog's digestive tract. J Physiol. 1951 Oct 29;115(2):163–176. doi: 10.1113/jphysiol.1951.sp004661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURBIN R. P., HEINZ E. Electromotive chloride transport and gastric acid secretion in the frog. J Gen Physiol. 1958 May 20;41(5):1035–1047. doi: 10.1085/jgp.41.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Fromm D., McColl I. Ion transport in rabbit ileal mucosa. I. Na and Cl fluxes and short-circuit current. Am J Physiol. 1971 May;220(5):1388–1396. doi: 10.1152/ajplegacy.1971.220.5.1388. [DOI] [PubMed] [Google Scholar]
- Fromm D., Halpern N. Effects of histamine receptor antagonists on ion transport by isolated ileum of the rabbit. Gastroenterology. 1979 Nov;77(5):1034–1038. [PubMed] [Google Scholar]
- Hill S. J., Young J. M., Marrian D. H. Specific binding of 3H-mepyramine to histamine H1 receptors in intestinal smooth muscle. Nature. 1977 Nov 24;270(5635):361–363. doi: 10.1038/270361a0. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Silverberg J. W. Effect of histamine on intestinal fluid secretion in the dog. Am J Physiol. 1976 Sep;231(3):793–798. doi: 10.1152/ajplegacy.1976.231.3.793. [DOI] [PubMed] [Google Scholar]
- Lorenz W., Matejka E., Schmal A., Seidel W., Reimann H. J., Uhlig R., Mann G. A phylogenetic study on the occurrence and distribution of histamine in the gastro-intestinal tract and other tissues of man and various animals. Comp Gen Pharmacol. 1973 Sep;4(15):229–250. doi: 10.1016/0010-4035(73)90004-9. [DOI] [PubMed] [Google Scholar]
- MARSHALL P. B. Some chemical and physical properties associated with histamine antagonism. Br J Pharmacol Chemother. 1955 Sep;10(3):270–278. doi: 10.1111/j.1476-5381.1955.tb00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakir K. M., Margolis S., Baylin S. B. Localization of histamine (diamine oxidase) in rat small intestinal mucosa: site of release by heparin. Biochem Pharmacol. 1977 Dec 15;26(24):2343–2347. doi: 10.1016/0006-2952(77)90438-5. [DOI] [PubMed] [Google Scholar]
- WATON N. G. Studies on mammalian histidine decarboxylase. Br J Pharmacol Chemother. 1956 Jun;11(2):119–127. doi: 10.1111/j.1476-5381.1956.tb01039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILBRANDT W. Zur Frage des Wirkungsmechanismus der Antihistaminsubstan zen. Helv Physiol Pharmacol Acta. 1950;8(4):399–408. [PubMed] [Google Scholar]


