Abstract
Intraepithelial lymphocytes (IEL) of the normal human stomach, small intestine, and large intestine have been characterised in tissue sections by a double marker immunofluorescent technique. A panel of reagents was used in combination, including antisera to T lymphocyte antigen (HuTLA), Ia-like (p28, 33) antigens and immunoglobulin subclasses, as well as a mouse monoclonal antibody to a human leucocyte antigen (HLe-1). In stomach and proximal small intestine over 95% of IEL were T lymphocytes (HLe-1+, HuTLA+). The proportion was slightly lower in the colon and rectum (85--95%). IEL rarely expressed Ia-like antigens. B lymphocytes were not seen within the epithelium of any of the tissues examined. The functions of IEL must be assessed in the light of the finding that they are predominantly T cells.
Full text
PDF


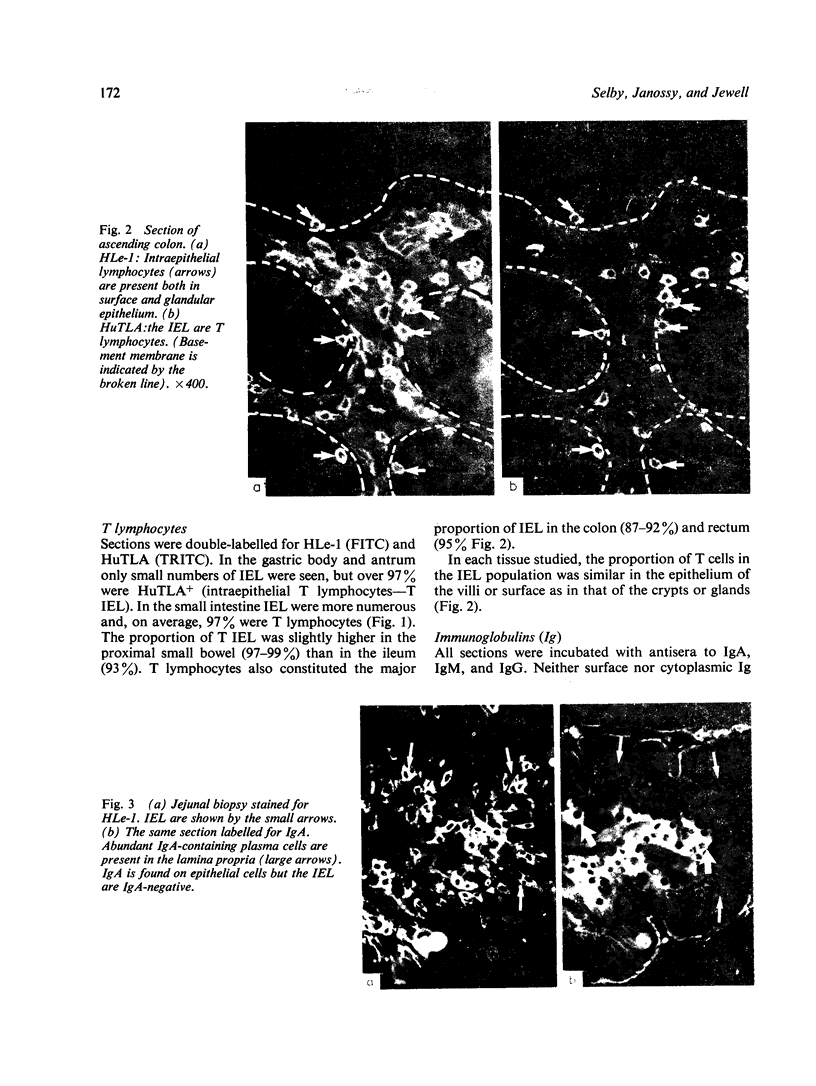




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaud-Battandier F., Bundy B. M., O'Neill M., Bienenstock J., Nelson D. L. Cytotoxic activities of gut mucosal lymphoid cells in guinea pigs. J Immunol. 1978 Sep;121(3):1059–1065. [PubMed] [Google Scholar]
- Bartnik W., ReMine S. G., Chiba M., Thayer W. R., Shorter R. G. Isolation and characterization of colonic intraepithelial and lamina proprial lymphocytes. Gastroenterology. 1980 May;78(5 Pt 1):976–985. [PubMed] [Google Scholar]
- Evans R. L., Faldetta T. J., Humphreys R. E., Pratt D. M., Yunis E. J., Schlossman S. F. Peripheral human T cells sensitized in mixed leukocyte culture synthesize and express Ia-like antigens. J Exp Med. 1978 Nov 1;148(5):1440–1445. doi: 10.1084/jem.148.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A. Intraepithelial lymphocytes of the small intestine. Gut. 1977 Nov;18(11):921–937. doi: 10.1136/gut.18.11.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A., Parrott D. M. The effect of antigen deprivation on thymus-dependent and thymus-independent lymphocytes in the small intestine of the mouse. Clin Exp Immunol. 1972 Dec;12(4):477–488. [PMC free article] [PubMed] [Google Scholar]
- Fichtelius K. E., Yunis E. J., Good R. A. Occurrence of lymphocytes within the gut epithelium of normal and neonatally thymectomized mice. Proc Soc Exp Biol Med. 1968 May;128(1):185–188. doi: 10.3181/00379727-128-32974. [DOI] [PubMed] [Google Scholar]
- Fu S. M., Chiorazzi N., Wang C. Y., Montrazeri G., Kunkel H. G., Ko H. S., Gottlieb A. B. Ia-bearing T lymphocytes in man. Their identification and role in the generation of allogeneic helper activity. J Exp Med. 1978 Nov 1;148(5):1423–1428. doi: 10.1084/jem.148.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The gut-associated lymphoid system: nature and properties of the large dividing cells. Eur J Immunol. 1974 Jun;4(6):435–443. doi: 10.1002/eji.1830040610. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The mouse gut T lymphocyte, a novel type of T cell. Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J Exp Med. 1978 Dec 1;148(6):1661–1677. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegert D. G., Coombs R. R. Do human B and null lymphocytes form a single immunoglobulin-bearing population? Lancet. 1979 Nov 17;2(8151):1051–1053. doi: 10.1016/s0140-6736(79)92446-2. [DOI] [PubMed] [Google Scholar]
- Janossy G., Bollum F. J., Bradstock K. F., McMichael A., Rapson N., Greaves M. F. Terminal transferase-positive human bone marrow cells exhibit the antigenic phenotype of common acute lymphoblastic leukemia. J Immunol. 1979 Oct;123(4):1525–1529. [PubMed] [Google Scholar]
- Mayrhofer G. Thymus-dependent and thymus-independent subpopulations of intestinal intraepithelial lymphocytes: a granular subpopulation of probable bone marrow origin and relationship to mucosal mast cells. Blood. 1980 Mar;55(3):532–535. [PubMed] [Google Scholar]
- McMichael A. J., Pilch J. R., Galfré G., Mason D. Y., Fabre J. W., Milstein C. A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. Eur J Immunol. 1979 Mar;9(3):205–210. doi: 10.1002/eji.1830090307. [DOI] [PubMed] [Google Scholar]
- Meader R. D., Landers D. F. Electron and light microscopic observations on relationships between lymphocytes and intestinal epithelium. Am J Anat. 1967 Nov;121(3):763–773. doi: 10.1002/aja.1001210318. [DOI] [PubMed] [Google Scholar]
- Meuwissen S. G., Feltkamp-Vroom T. M., De La Rivière A. B., Von Dem Borne A. E., Tytgat G. N. Analysis of the lympho-plasmacytic infiltrate in Crohn's disease with special reference to identification of lymphocyte-subpopulations. Gut. 1976 Oct;17(10):770–780. doi: 10.1136/gut.17.10.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaudet P., Greaves M., Horwitz D. Phenotypes of 'null' lymphoid cells in human blood. Scand J Immunol. 1979;9(4):387–393. doi: 10.1111/j.1365-3083.1979.tb03178.x. [DOI] [PubMed] [Google Scholar]
- Pfeiffer C. J., Weibel J. The antral clear cell--a new cell type discovered in the pyloric-antral mucosa of the ferret. J Ultrastruct Res. 1969 Dec;29(5):550–562. doi: 10.1016/s0022-5320(69)90073-2. [DOI] [PubMed] [Google Scholar]
- Pizzolo G., Sloane J., Beverley P., Thomas J. A., Bradstock K. F., Mattingly S., Janossy G. Differential diagnosis of malignant lymphoma and nonlymphoid tumors using monoclonal anti-leucocyte antibody. Cancer. 1980 Dec 15;46(12):2640–2647. doi: 10.1002/1097-0142(19801215)46:12<2640::aid-cncr2820461218>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Rudzik O., Bienenstock J. Isolation and characteristics of gut mucosal lymphocytes. Lab Invest. 1974 Mar;30(3):260–266. [PubMed] [Google Scholar]
- Röpke C., Everett N. B. Kinetics of intraepithelial lymphocytes in the small intestine of thymus-deprived mice and antigen-deprived mice. Anat Rec. 1976 May;185(1):101–108. doi: 10.1002/ar.1091850110. [DOI] [PubMed] [Google Scholar]
- Strickland R. G., Husby G., Black W. C., Williams R. C., Jr Peripheral blood and intestinal lymphocyte sub-populations in Crohn's disease. Gut. 1975 Nov;16(11):847–853. doi: 10.1136/gut.16.11.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarbrick E. T., Stokes C. R., Soothill J. F. Absorption of antigens after oral immunisation and the simultaneous induction of specific systemic tolerance. Gut. 1979 Feb;20(2):121–125. doi: 10.1136/gut.20.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner P. G., Ferguson A. Intraepithelial cells in the human intestinal mucosa. J Ultrastruct Res. 1971 Feb;34(3):329–344. doi: 10.1016/s0022-5320(71)80076-x. [DOI] [PubMed] [Google Scholar]
- Yu D. T., Winchester R. J., Fu S. M., Gibofsky A., Ko H. S., Kunkel H. G. Peripheral blood Ia-positive T cells. Increases in certain diseases and after immunization. J Exp Med. 1980 Jan 1;151(1):91–100. doi: 10.1084/jem.151.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]