Abstract
Portions of jejunal biopsies from control subjects and from patients with coeliac disease were cultured for 24 hours using an in vitro organ culture technique. Alkaline phosphatase activity was measured in the tissue and medium before and after culture; enzyme activities were expressed per microgram tissue DNA. The increase in enzyme activity during the culture period was taken to represent net enzyme synthesis. Alkaline phosphatase synthesis by mucosa from patients with untreated gluten-sensitive coeliac disease and by mucosa from patients with non-responsive coeliac disease was significantly less than that by normal mucosa. Alkaline phosphatase synthesis by mucosa from patients with treated gluten-sensitive coeliac disease was greater than that by untreated coeliac mucosa but was less than normal mucosa. Sequential studies of alkaline phosphatase synthesis by jejunal mucosa from seven patients with coeliac disease, before and after successful treatment by gluten withdrawal, showed an increase in synthesis in all patients. Study, by analytical subcellular fractionation with sucrose density gradient centrifugation, of the properties of the organelles of cultured control tissue showed good preservation of their integrity. A striking finding, however, was the decrease in malate dehydrogenase with a corresponding marked increase in lactate dehydrogenase. This would be expected to be followed by a shift from aerobic to anaerobic metabolism. Analytical subcellular fractionation of cultured mucosa from patients with coeliac disease gave similar conclusions. There was, however, a marked improvement of the brush border abnormalities, characteristic of coeliac disease, during culture with increased enzyme activities and membrane equilibrium density in the sucrose gradients.
Full text
PDF
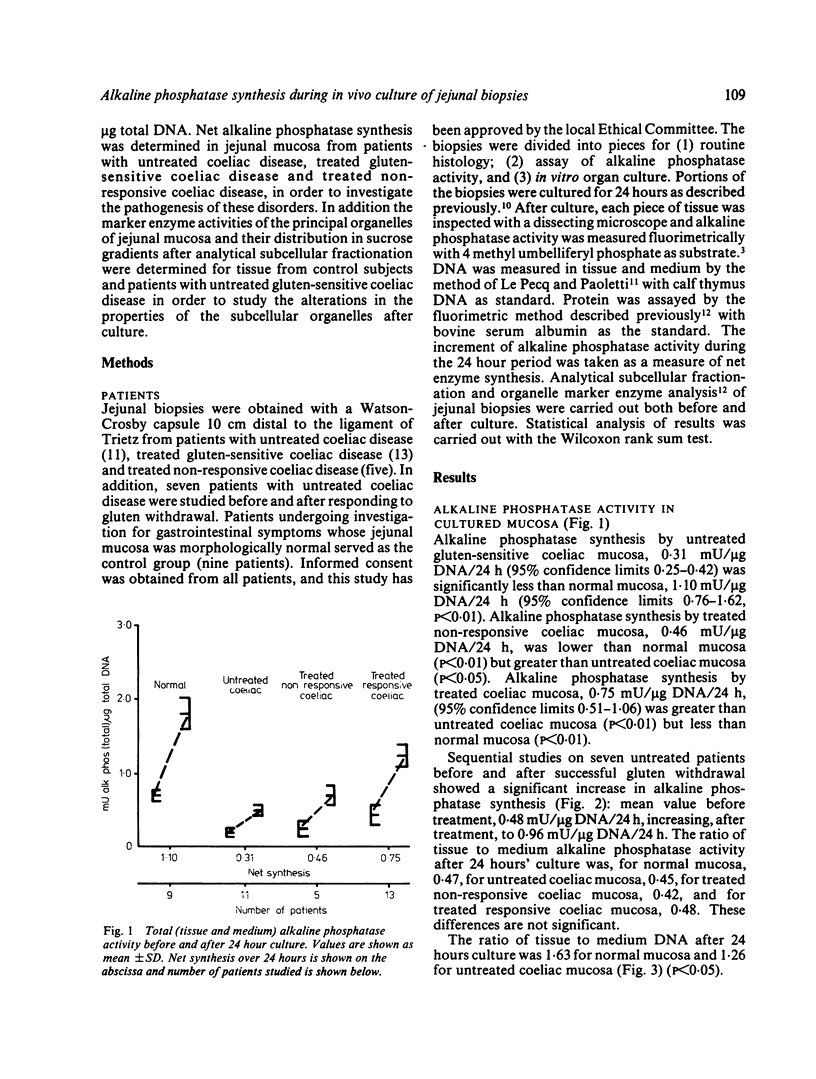
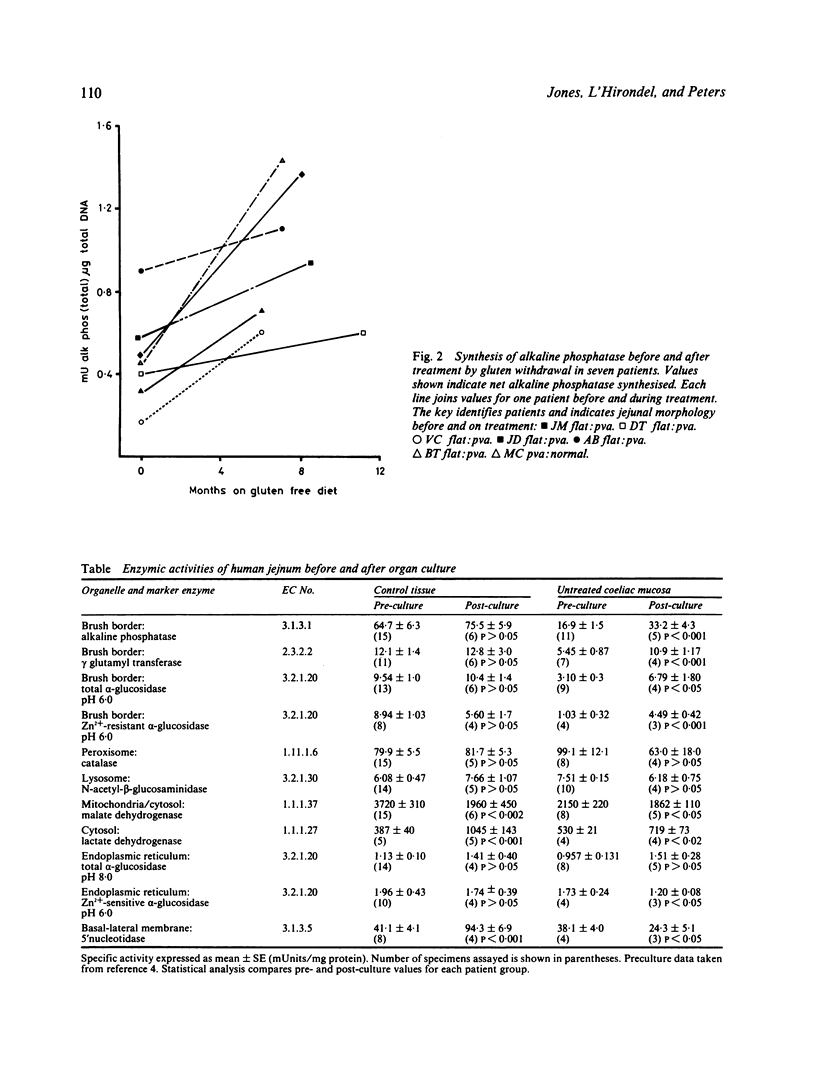




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell C. B., Cowen A. E., McGeary H. M., Gaffney T. J. Mucosal enzyme activity as a quantitative index of early functional improvement in the management of coeliac disease and other small intestinal diseases. Aust N Z J Med. 1972 Aug;2(3):220–227. doi: 10.1111/j.1445-5994.1972.tb03066.x. [DOI] [PubMed] [Google Scholar]
- Falchuk Z. M., Gebhard R. L., Sessoms C., Strober W. An in vitro model of gluten-sensitive enteropathy. Effect of gliadin on intestinal epithelial cells of patients with gluten-sensitive enteropathy in organ culture. J Clin Invest. 1974 Feb;53(2):487–500. doi: 10.1172/JCI107582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A., Watson W. C., Maxwell J. D., Fell G. S. Alkaline phosphatase levels in normal and diseased small bowel. Gut. 1968 Feb;9(1):96–98. doi: 10.1136/gut.9.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Kedinger M., Haffen K., Gaze H., Hadorn B., Hekkens W. Re-evaluation of the techique of organ culture for studying gluten toxicity in coeliac disease. Gut. 1978 Dec;19(12):1090–1098. doi: 10.1136/gut.19.12.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. E., Peters T. J. DNA synthesis by jejunal mucosa in responsive and non-responsive coeliac disease. Br Med J. 1977 Apr 30;1(6069):1130–1131. doi: 10.1136/bmj.1.6069.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jos J., Lenoir G., Ritis G D., Rey J. In vitro pathogenetic studies of coeliac disease. Effects of protein digests on coeliac intestinal biopsy specimens maintained in culture for 48 hours. Scand J Gastroenterol. 1975;10(2):121–128. [PubMed] [Google Scholar]
- L'Hirondel C., Doe W. F., Peters T. J. Biochemical and morphological studies on human jejunal mucosa maintained in culture. Clin Sci Mol Med. 1976 May;50(5):425–429. doi: 10.1042/cs0500425. [DOI] [PubMed] [Google Scholar]
- Le Pecq J. B., Paoletti C. A new fluorometric method for RNA and DNA determination. Anal Biochem. 1966 Oct;17(1):100–107. doi: 10.1016/0003-2697(66)90012-1. [DOI] [PubMed] [Google Scholar]
- Mitchell J. D., Mitchell J., Peters T. J. Enzyme changes in human small bowel mucosa during culture in vitro. Gut. 1974 Oct;15(10):805–811. doi: 10.1136/gut.15.10.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T. J. Analytical subcellular fractionation of jejunal biopsy specimens: methodology and characterization of the organelles in normal tissue. Clin Sci Mol Med. 1976 Dec;51(6):557–574. doi: 10.1042/cs0510557. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Heath J. R., Wansbrough-Jones M. H., Foe W. F. Enzyme activities and properties of lysosomes and brush borders in jejunal biopsies from control subjects and patients with coeliac disease. Clin Sci Mol Med. 1975 Apr;48(4):259–267. doi: 10.1042/cs0480259. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Jones P. E., Wells G. Analytical subcellular fractionation of jejunal biopsy specimens: enzyme activities, organelle pathology and response to gluten withdrawal in patients with coeliac disease. Clin Sci Mol Med. 1978 Sep;55(3):285–292. doi: 10.1042/cs0550285. [DOI] [PubMed] [Google Scholar]
- Stevens F. M., Keane R., Fottrell P. F., McNicholl B., McCarthy C. F. Organ culture of coeliac and non-coeliac small intestinal mucosa. Ir J Med Sci. 1978 Jan;147(1):11–14. doi: 10.1007/BF02939361. [DOI] [PubMed] [Google Scholar]
- Trier J. S., Browning T. H. Epithelial-cell renewal in cultured duodenal biopsies in celiac sprue. N Engl J Med. 1970 Dec 3;283(23):1245–1250. doi: 10.1056/NEJM197012032832302. [DOI] [PubMed] [Google Scholar]



