Abstract
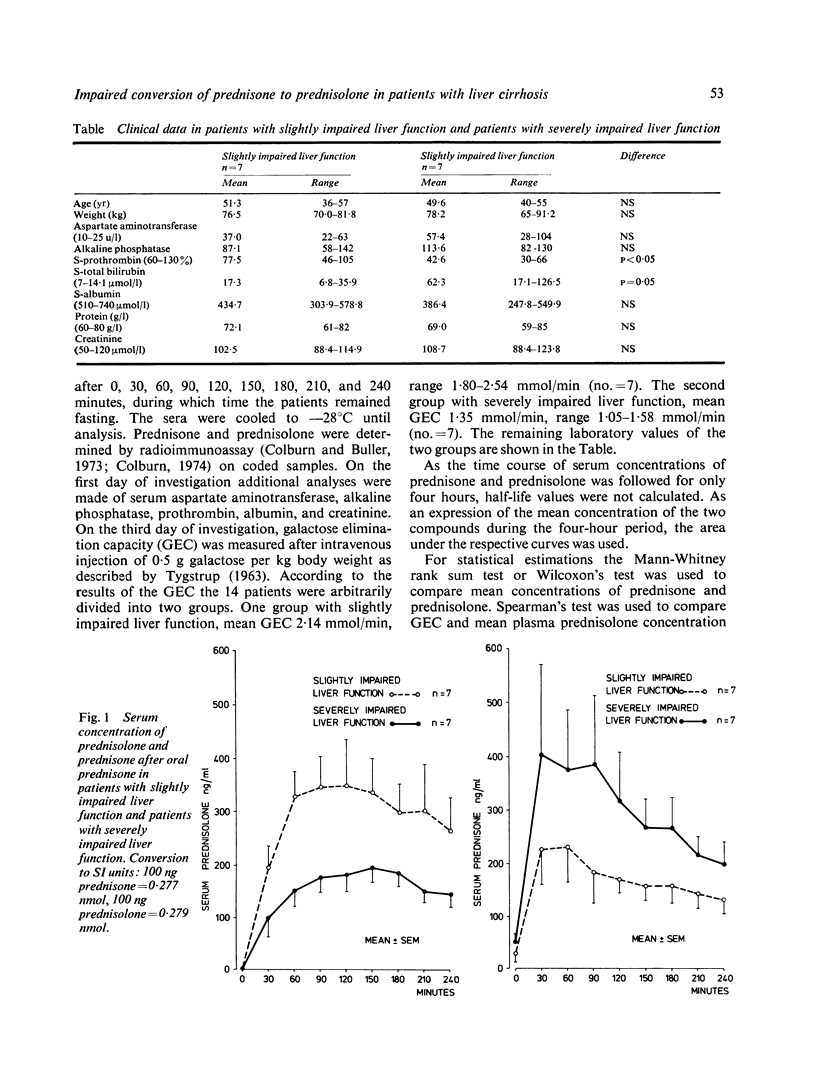
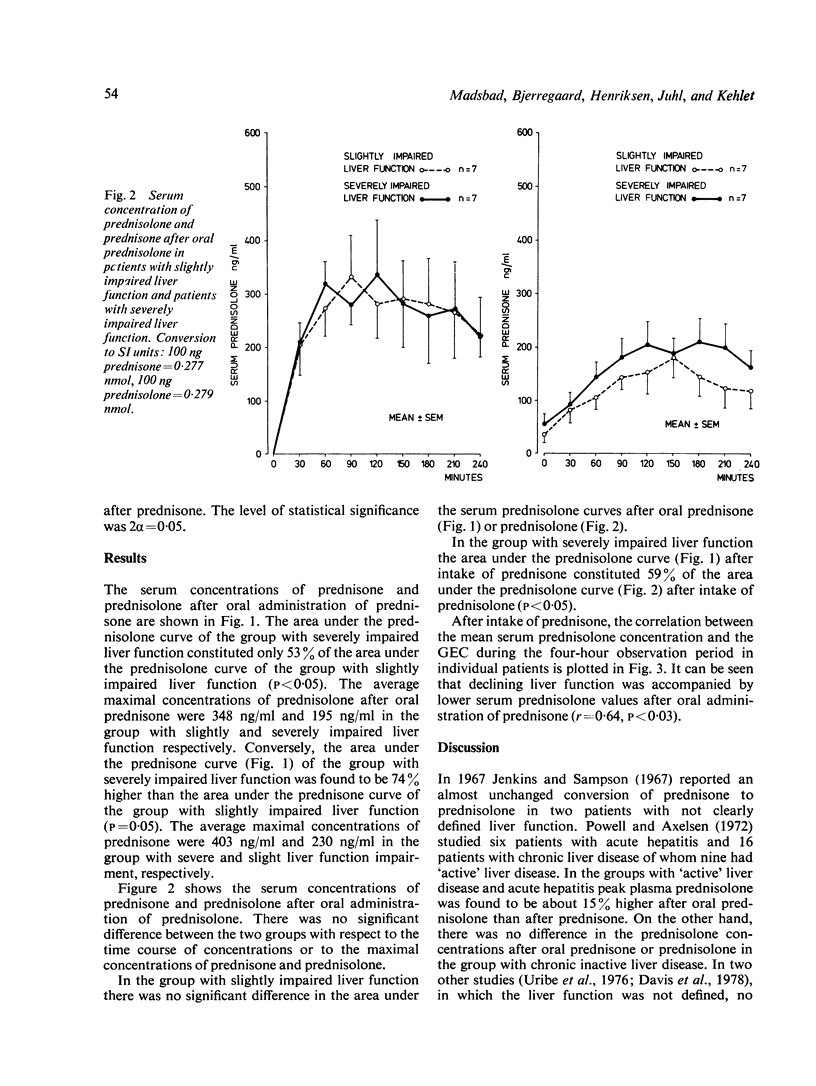
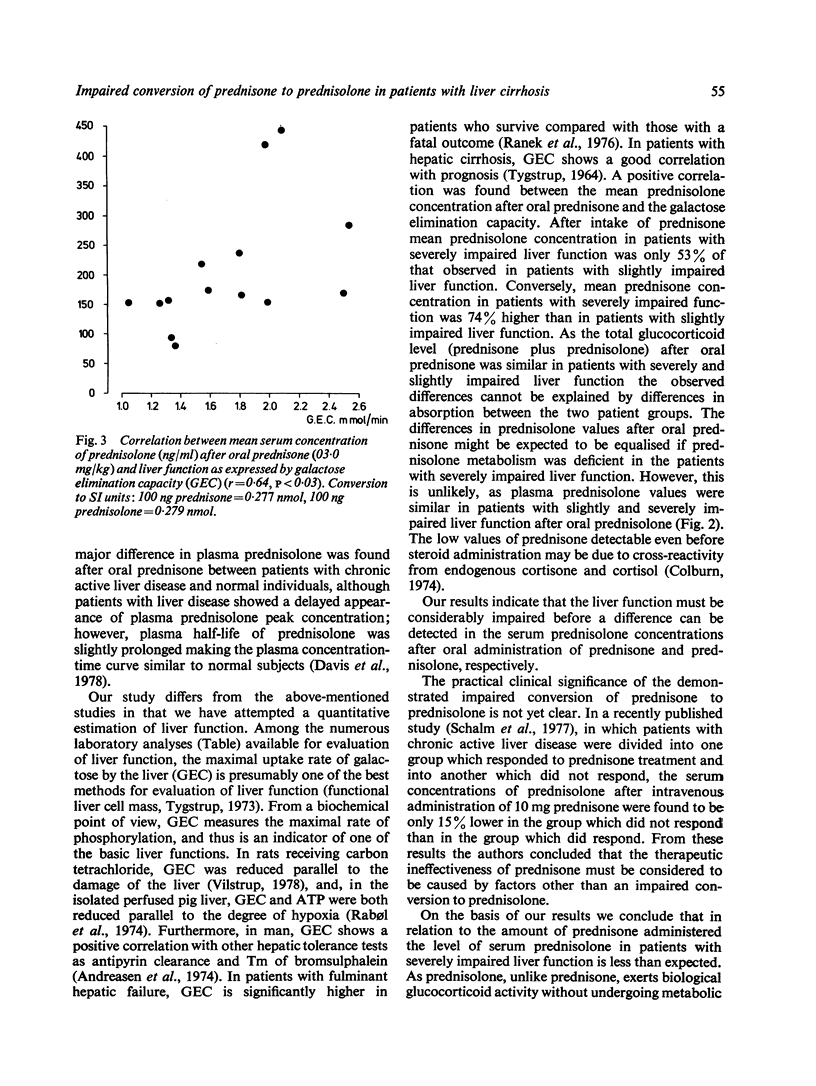
Fourteen patients with liver cirrhosis received oral prednisone or prednisolone (0.3 mg per kg) randomised on two consecutive days. Serum prednisone and prednisolone were measured over the following four hours. Mean serum prednisolone concentration after oral prednisone decreased with impaired liver function estimated by galactose elimination capacity (r = 0.64, P less than 0.03). Mean serum prednisolone concentration after oral prednisone in the seven patients with severely impaired liver function was only 53% (P less than 0.05) of that observed in the seven patients with slightly impaired liver function. Conversely, mean serum prednisone concentration after oral prednisone in the patients with severely impaired liver function was 74% higher (P = 0.05) than in patients with slightly impaired liver function. Mean serum prednisolone after oral prednisolone was independent of liver function. As only prednisolone exerts glucocorticoid activity, our results indicate that prednisolone should be preferred to prednisone in the treatment of patients with impaired liver function.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen P. B., Ranek L., Statland B. E., Tygstrup N. Clearance of antipyrine-dependence of quantitative liver function. Eur J Clin Invest. 1974 Apr;4(2):129–134. doi: 10.1111/j.1365-2362.1974.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Colburn W. A., Buller R. H. Radioimmunoassay for prednisolone. Steroids. 1973 Jun;21(6):833–846. doi: 10.1016/0039-128x(73)90124-4. [DOI] [PubMed] [Google Scholar]
- Colburn W. A. Radioimmunoassay for prednisone. Steroids. 1974 Jul;24(1):95–106. doi: 10.1016/0039-128x(74)90048-8. [DOI] [PubMed] [Google Scholar]
- Davis M., Williams R., Chakraborty J., English J., Marks V., Ideo G., Tempini S. Prednisone or prednisolone for the treatment of chronic active hepatitis? A comparison of plasma availability. Br J Clin Pharmacol. 1978 Jun;5(6):501–505. doi: 10.1111/j.1365-2125.1978.tb01664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins J. S., Sampson P. A. Conversion of cortisone to cortisol and prednisone to prednisolone. Br Med J. 1967 Apr 22;2(5546):205–207. doi: 10.1136/bmj.2.5546.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray-Lyon I. M., Stern R. B., Williams R. Controlled trial of prednisone and azathioprine in active chronic hepatitis. Lancet. 1973 Apr 7;1(7806):735–737. doi: 10.1016/s0140-6736(73)92125-9. [DOI] [PubMed] [Google Scholar]
- Powell L. W., Axelsen E. Corticosteroids in liver disease: studies on the biological conversion of prednisone to prednisolone and plasma protein binding. Gut. 1972 Sep;13(9):690–696. doi: 10.1136/gut.13.9.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranek L., Andreasen P. B., Tygstrup N. Galactose elimination capacity as a prognostic index in patients with fulminant liver failure. Gut. 1976 Dec;17(12):959–964. doi: 10.1136/gut.17.12.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARETT L. H., PATCHETT A. A., STEELMAN S. L. THE EFFECTS OF STRUCTURAL ALTERATION ON THE ANTI-INFLAMMATORY PROPERTIES OF HYDROCORTISONE. Fortschr Arzneimittelforsch. 1963;5:11–153. doi: 10.1007/978-3-0348-7047-4_1. [DOI] [PubMed] [Google Scholar]
- Schalm S. W., Summerskill W. H., Go V. L. Prednisone for chronic active liver disease: pharmacokinetics, including conversion to prednisolone. Gastroenterology. 1977 May;72(5 Pt 1):910–913. [PubMed] [Google Scholar]
- Summerskill W. H., Korman M. G., Ammon H. V., Baggenstoss A. H. Prednisone for chronic active liver disease: dose titration, standard dose, and combination with azathioprine compared. Gut. 1975 Nov;16(11):876–883. doi: 10.1136/gut.16.11.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYGSTRUP N. Determination of the hepatic galactose elimination capacity after a single intravenous injection in man: the reproducibility and the influence of uneven distribution. Acta Physiol Scand. 1963 Jun-Jul;58:162–172. doi: 10.1111/j.1748-1716.1963.tb02638.x. [DOI] [PubMed] [Google Scholar]
- TYGSTRUP N. THE GALACTOSE ELIMINATION CAPACITY IN RELATION TO CLINICAL AND LABORATORY FINDINGS IN PATIENTS WITH CIRRHOSIS. Acta Med Scand. 1964 Mar;175:291–300. doi: 10.1111/j.0954-6820.1964.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Vilstrup H. The galactose elimination capacity as a quantitative measure of liver function in acute carbon tetrachloride intoxication of rats. Eur J Clin Invest. 1978 Oct;8(5):317–319. doi: 10.1111/j.1365-2362.1978.tb00848.x. [DOI] [PubMed] [Google Scholar]


