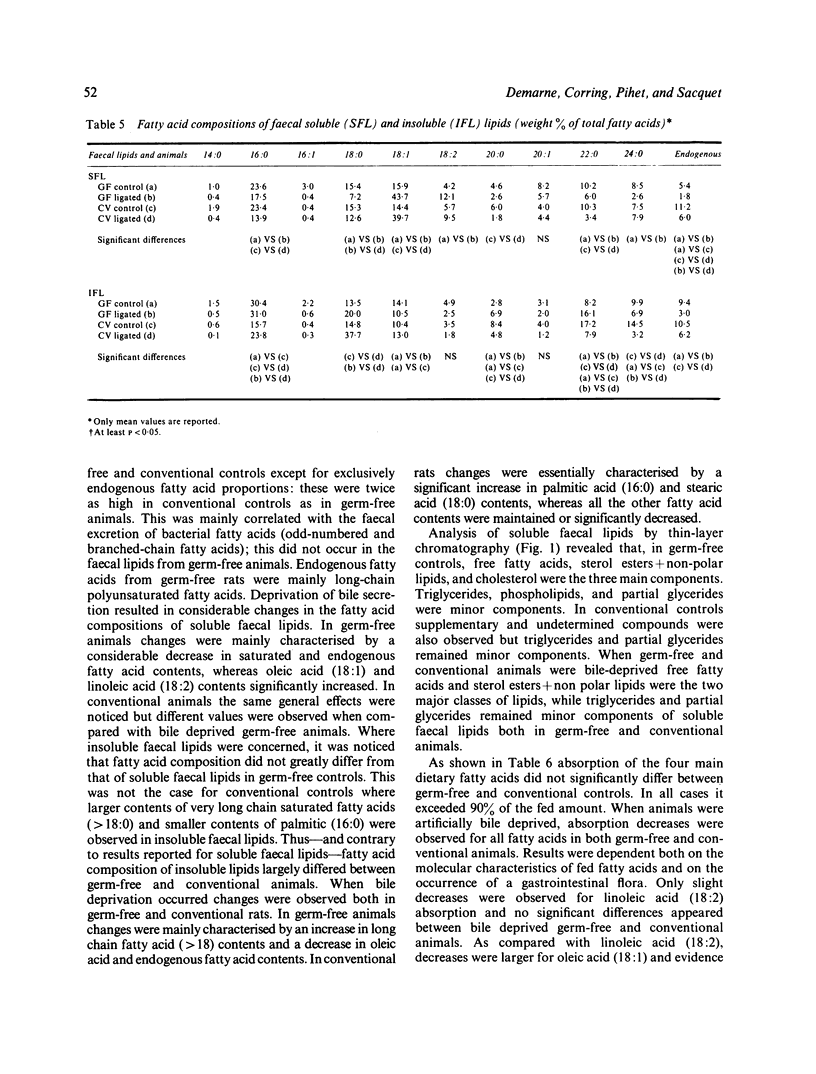
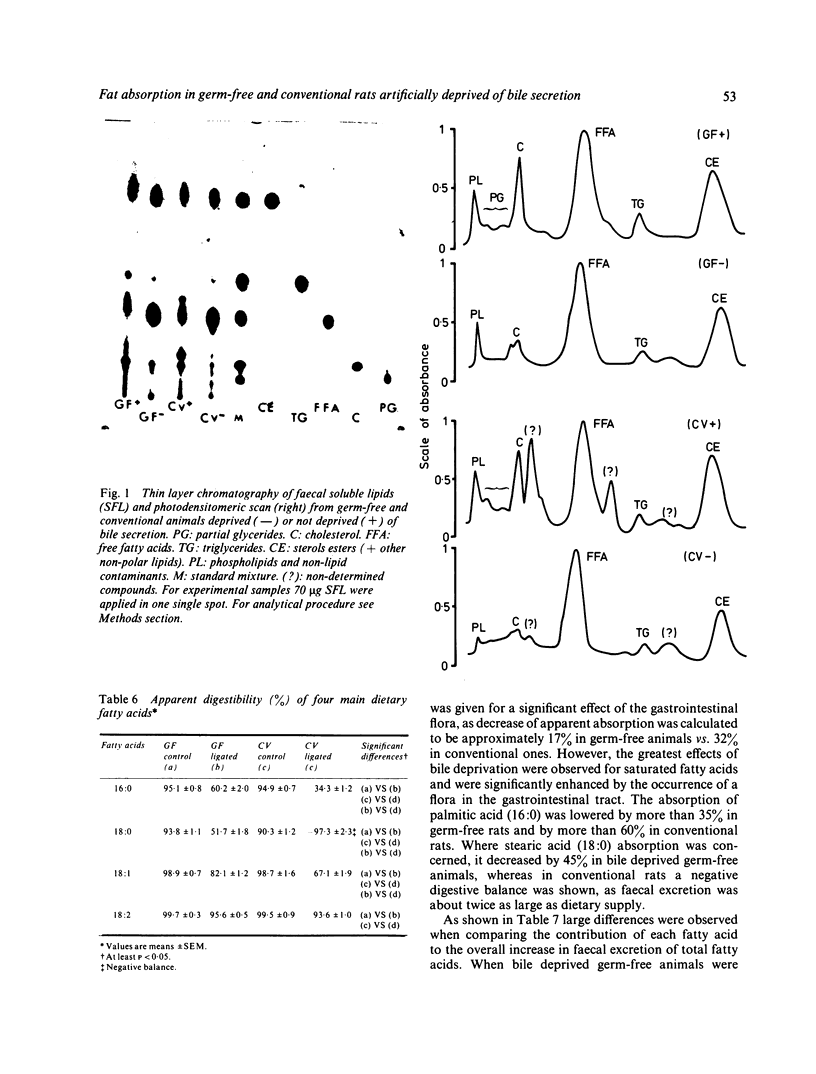
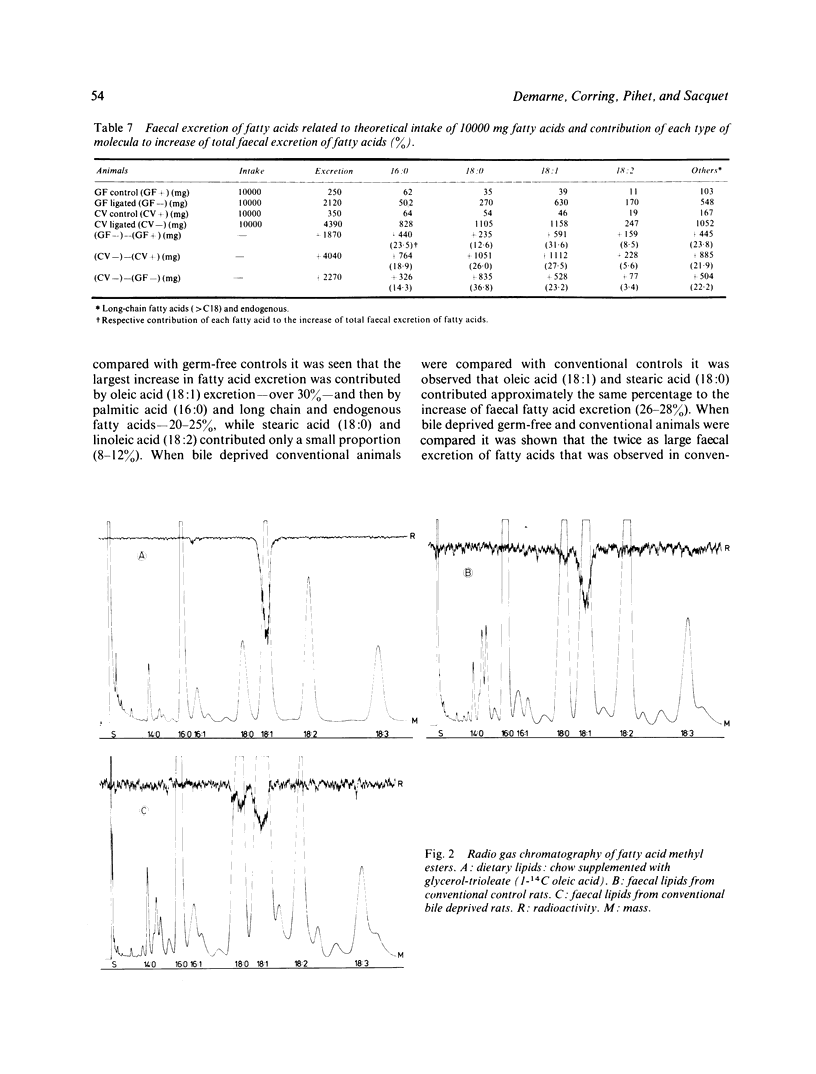
Abstract
Bile duct ligation was performed in germ-free and conventional rats in order to study the effects of bile deprivation on the absorption of dietary lipids and the excretion of faecal lipids in the presence or the absence of gastrointestinal flora. The main consequence of bile duct ligation in conventional rats was decrease of about 50% in the apparent absorption of dietary lipids (peanut oil). In germ-free rats, absorption decreased by only about 25%.In conventional as well as in germ-free controls, faecal lipids were mainly excreted as compounds directly soluble in organic solvents that is, free fatty acids, triglycerides, partial glycerides, cholesterol, cholesterol esters. Deprivation of bile secretion significantly increased the faecal excretion of 'insoluble' compounds-that is, calcium soaps-both in germ-free and conventional rats. Free fatty acids and sterol esters were the two main class of soluble faecal lipids both in germ-free and conventional rats deprived of bile secretion. Faecal excretion of triglycerides remained low in germ-free as well as in conventional animals. No significant difference of fatty acid absorption was observed between germ-free and conventional controls. Deprivation of bile secretion resulted in a significant decrease in the absorption of all fatty acids in germ-free as well as in conventional animals. However, the decrease was larger for saturated fatty acids-that is, 16:0 or 18:0- than for unsaturated fatty acids-that is, 18:1 or 18:2. The absorption of all fatty acids, except linoleic acid (18:2), was significantly lower in conventional rats artificially deprived of bile secretion than in their germ-free counterparts. Evidence was given for a negative digestive balance of stearic acid (18:0) in bile deprived conventional animals. This observation was correlated with a very efficient biohydrogenation of dietary unsaturated fatty acids as revealed by radio gas chromatography of faecal acids in bile deprived conventional rats fed a diet containing 1-14C oleic acid (18:1) as homogeneous triglycerides. Nevertheless, biohydrogenation of unsaturated dietary fatty acids by the gastrointestinal flora was not considered to be the only factor involved in the origin of the difference of fat absorption between bile deprived germ-free and conventional animals.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cesano L., Dawson A. M. Absorption of 14C triolein in the bile fistula rat. Proc Soc Exp Biol Med. 1966 May;122(1):96–99. doi: 10.3181/00379727-122-31061. [DOI] [PubMed] [Google Scholar]
- Combe E., Demarne Y., Gueguen L., Ivorec-Szylit O., Meslin J. C., Sacquet E. Some aspects of the relationships between gastrointestinal flora and host nutrition. World Rev Nutr Diet. 1976;24:1–57. doi: 10.1159/000399403. [DOI] [PubMed] [Google Scholar]
- Corring T., Moreau C., Ducluzeau R. Comparative apparent digestibility of casein in holoxenic, axenic, and Clostridium bifermentans monoassociated rats. Am J Clin Nutr. 1979 Jun;32(6):1231–1237. doi: 10.1093/ajcn/32.6.1231. [DOI] [PubMed] [Google Scholar]
- Demarne Y., Sacquet E., Lecourtier M. J., Flanzy J. Comparative study of endogenous fecal fatty acids in germ-free and conventional rats. Am J Clin Nutr. 1979 Oct;32(10):2027–2032. doi: 10.1093/ajcn/32.10.2027. [DOI] [PubMed] [Google Scholar]
- Demarne Y., Sacquet E., Van Heijenoort Y., Mathis C. Influence d'une supplémentation de la ration en sels biliaires conjugués sur l'équipement en acides biliaires de l'intestin grêle du rat. Modifications de l'absorption apparente des acides gras. Ann Biol Anim Biochim Biophys. 1974;14(2):239–250. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fakambi L., Flanzy J., François A. C. Compétition in vivo entre acides gras et phosphore pour la formation de composés insolubles de calcium. C R Acad Sci Hebd Seances Acad Sci D. 1969 Dec 3;269(22):2233–2235. [PubMed] [Google Scholar]
- Fewster M. E., Burns B. J., Mead J. F. Quantitative densitometric thin-layer chromatography of lipids using copper acetate reagent. J Chromatogr. 1969 Aug 5;43(1):120–126. doi: 10.1016/s0021-9673(00)99173-8. [DOI] [PubMed] [Google Scholar]
- Floch M. H., Gershengoren W., Elliott S., Spiro H. M. Bile acid inhibition of the intestinal microflora--a function for simple bile acids? Gastroenterology. 1971 Aug;61(2):228–233. [PubMed] [Google Scholar]
- Friedman H. I., Nylund B. Intestinal fat digestion, absorption, and transport. A review. Am J Clin Nutr. 1980 May;33(5):1108–1139. doi: 10.1093/ajcn/33.5.1108. [DOI] [PubMed] [Google Scholar]
- GRABER C. D., O'NEAL R. M., RABIN E. R. EFFECT OF HIGH FAT DIETS ON INTESTINAL MICROFLORA AND SERUM CHOLESTEROL IN RATS. J Bacteriol. 1965 Jan;89:47–51. doi: 10.1128/jb.89.1.47-51.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo-Torres H. E. Obligatory role of bile for the intestinal absorption of vitamin E. Lipids. 1970 Apr;5(4):379–384. doi: 10.1007/BF02532102. [DOI] [PubMed] [Google Scholar]
- Holt P. R. The roles of bile acids during the process of normal fat and cholesterol absorption. Arch Intern Med. 1972 Oct;130(4):574–583. [PubMed] [Google Scholar]
- KNOEBEL L. K., RYAN J. M. Digestion and mucosal absorption of fat in normal and bile-deficient dogs. Am J Physiol. 1963 Mar;204:509–514. doi: 10.1152/ajplegacy.1963.204.3.509. [DOI] [PubMed] [Google Scholar]
- Porter H. P., Saunders D. R., Tytgat G., Brunser O., Rubin C. E. Fat absorption in bile fistula man. A morphological and biochemical study. Gastroenterology. 1971 Jun;60(6):1008–1019. [PubMed] [Google Scholar]
- Sacquet E., Raibaud P., Garnier J. Etude comparée de la microflore de l'estomac, de l'intestin grêle et du caecum du rat "holoxénique" (conventionnel), et de ses modifications à la suite de diverses interventions chirurgicales: anse aveugle jéjunale, déviations biliaires. Ann Inst Pasteur (Paris) 1971 Apr;120(4):501–524. [PubMed] [Google Scholar]
- Thieulin C. Les divers facteurs influant sur l'utilisation digestive des matières grasses. Ann Nutr Aliment. 1968;22(6):245–258. [PubMed] [Google Scholar]
- VAHOUNY G. V., TREADWELL C. R. ABSOLUTE REQUIREMENT FOR FREE STEROL FOR ABSORPTION BY RAT INTESTINAL MUCOSA. Proc Soc Exp Biol Med. 1964 Jun;116:496–498. doi: 10.3181/00379727-116-29289. [DOI] [PubMed] [Google Scholar]
- van Heijenoort Y., Sacquet E., Raibaud P., Demarne Y., Mathis C. Action de la flore microbienne sur le contenu de l'intestin grêle du rat en acides biliaires. C R Acad Sci Hebd Seances Acad Sci D. 1972 Jul 10;275(2):271–274. [PubMed] [Google Scholar]


