Abstract
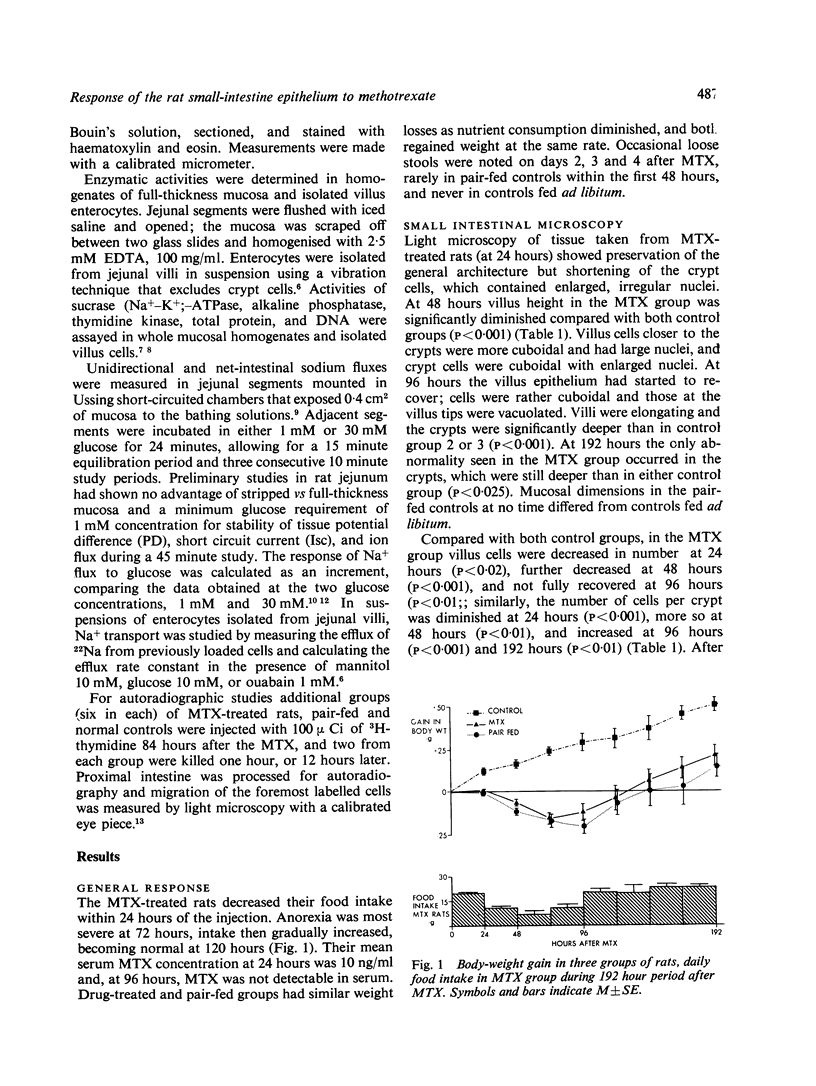
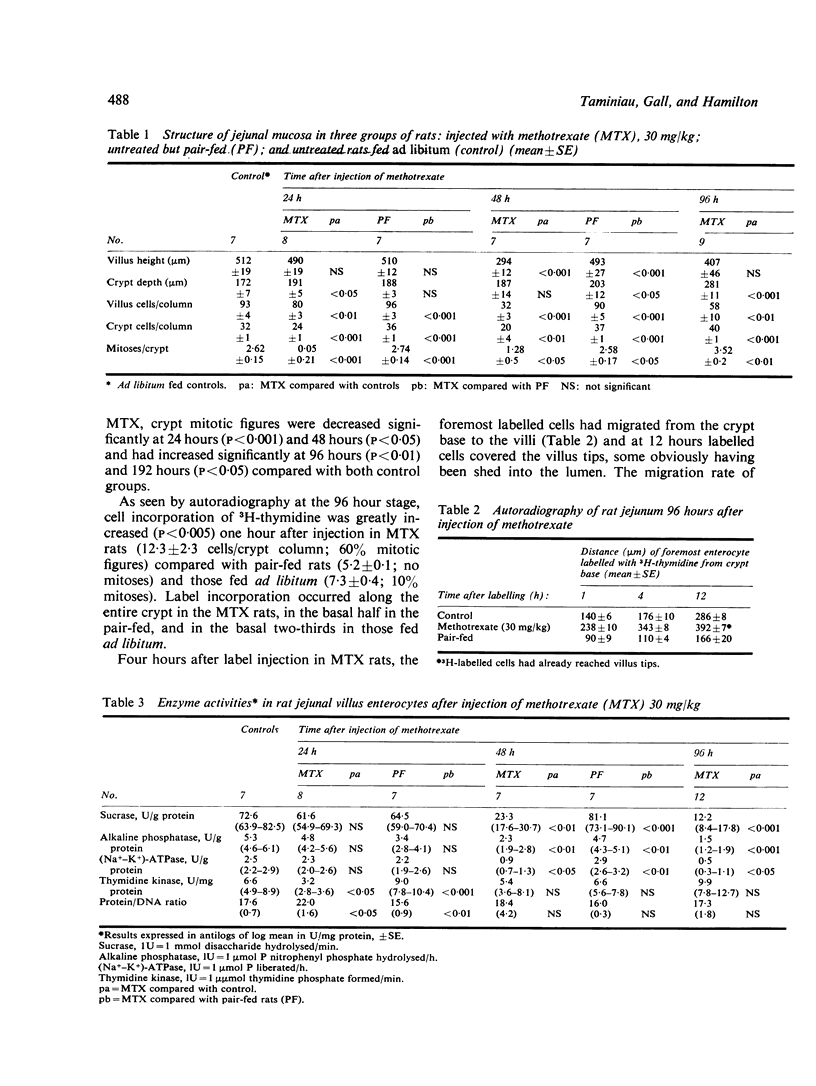
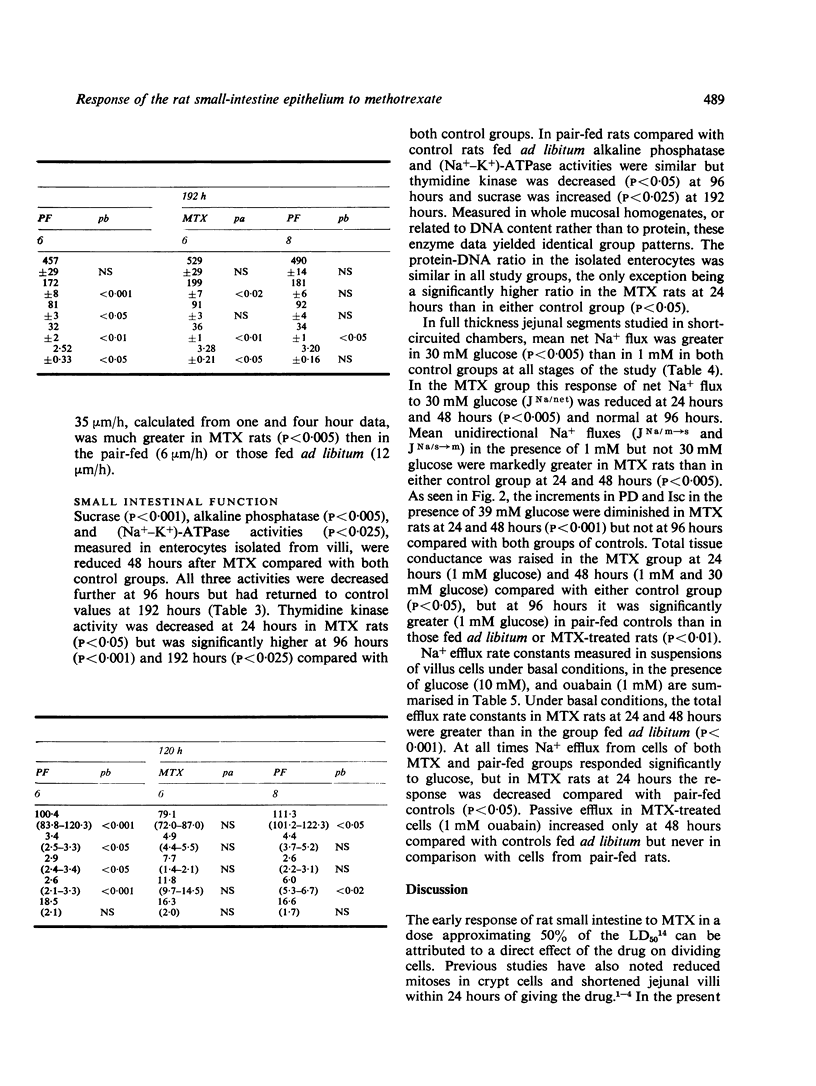
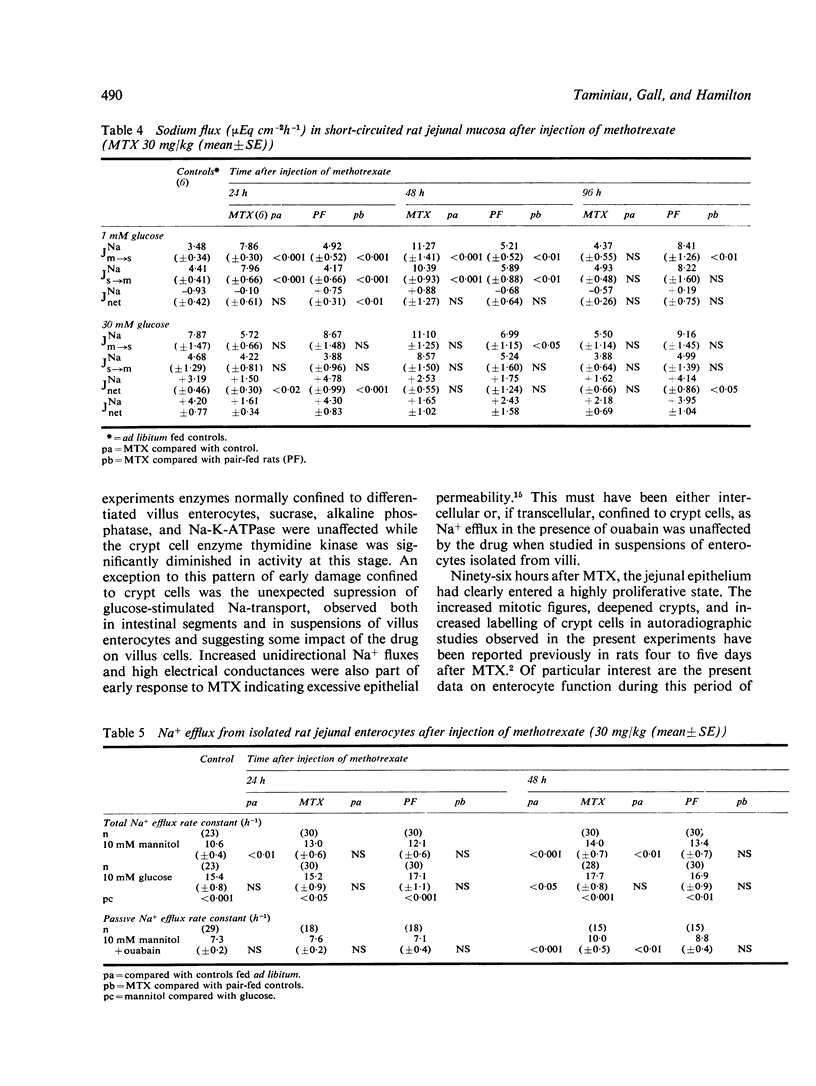
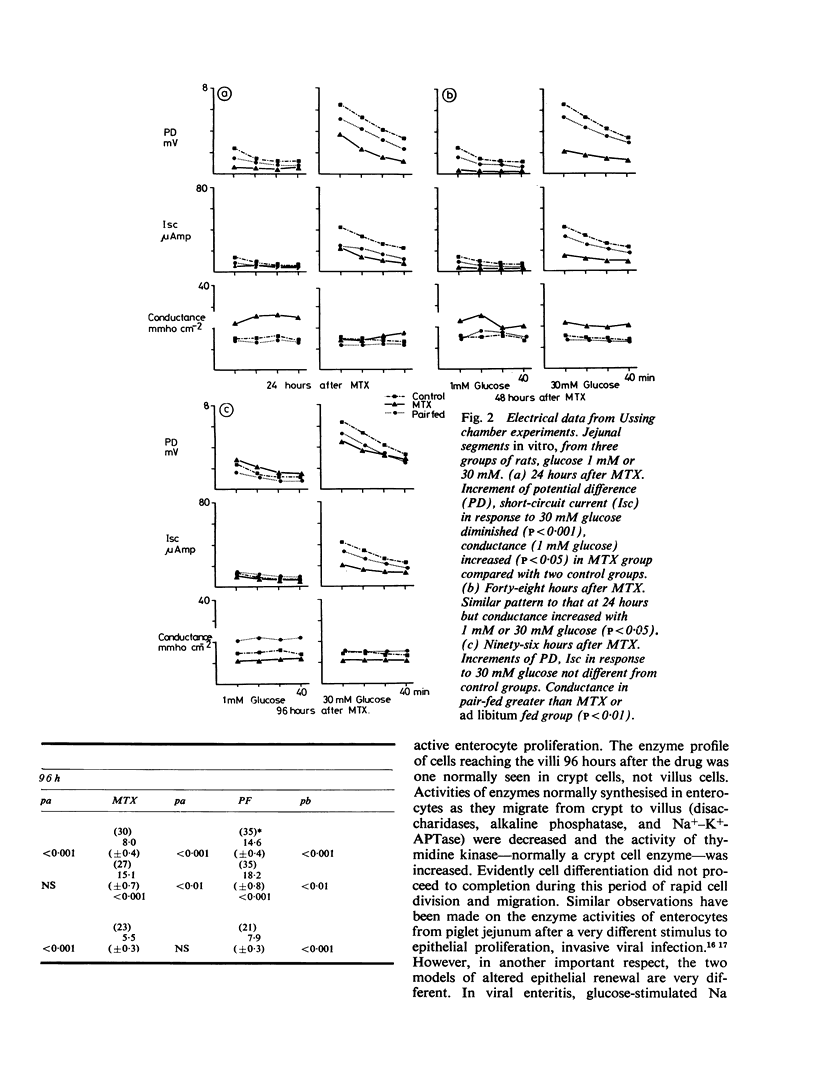
We studied jejunal epithelial structure and function in rats 24, 48, 96, and 192 hours after a single intravenous injection of methotrexate (MTX) 30 mg/kg. The acute effect of the drug on the gut at 24 and 48 hours was characterised, as expected, by reduced mitoses in crypts, shortened villi, and depressed activity of thymidine kinase (an enzyme normally confined to intestinal crypt cells). At 96 hours, when MTX was no longer detectable in serum, the intestine had entered a proliferative phase characterised by increased crypt mitoses, accelerated migration of enterocytes along villi, and the presence on villi of epithelial cells with the enzyme profile of crypt cells, decreased disaccharidase, alkaline phosphatase, and Na+-K+ATPase activities and increased thymidine kinase activity. Although the enzyme data suggested that enterocyte maturation was defective during this proliferative phase, glucose-stimulated Na+ transport, normally a function of fully differentiated villus cells, was normal at 96 hours. Measured both in Ussing chambers and in suspensions of enterocytes isolated from villi, Na+ transport responded normally to glucose at 96 hours, although the response had been significantly depressed at 24 hours. These findings cannot be attributed to MTX-induced malnutrition, as all comparisons included pair-fed controls. We conclude that, in the MTX-induced malnutrition, as all comparisons included pair-fed controls. We conclude that, in the small intestine under conditions of altered epithelial renewal, some components of enterocyte function may be affected more than others. Comparing the present experimental model with another intestinal disorder, acute viral enteritis, in which proliferative activity is excessive, it is clear that the nature of the original intestinal injury is a significant determinant of the pattern of enterocyte response.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann G. G. Changes in the mucosa of the small intestine following methotrexate administration or abdominal x-irradiation. Am J Anat. 1974 Jun;140(2):263–279. doi: 10.1002/aja.1001400210. [DOI] [PubMed] [Google Scholar]
- Chabner B. A., Young R. C. Threshold methotrexate concentration for in vivo inhibition of DNA synthesis in normal and tumorous target tissues. J Clin Invest. 1973 Aug;52(8):1804–1811. doi: 10.1172/JCI107362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson G. P., Gall D. G., Petric M., Butler D. G., Hamilton J. R. Human rotavirus enteritis induced in conventional piglets. Intestinal structure and transport. J Clin Invest. 1977 Dec;60(6):1402–1409. doi: 10.1172/JCI108901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Fromm D., McColl I. Ion transport in rabbit ileal mucosa. I. Na and Cl fluxes and short-circuit current. Am J Physiol. 1971 May;220(5):1388–1396. doi: 10.1152/ajplegacy.1971.220.5.1388. [DOI] [PubMed] [Google Scholar]
- Gall D. G., Butler D. G., Tepperman F., Hamilton J. Sodium ion transport in isolated intestinal epithelial cells. The effect of actively transported sugars on sodium ion efflux. Biochim Biophys Acta. 1974 Mar 29;339(3):291–302. doi: 10.1016/0005-2736(74)90156-4. [DOI] [PubMed] [Google Scholar]
- Gall D. G., Chapman D., Kelly M., Hamilton J. R. Na+ transport in jejunal crypt cells. Gastroenterology. 1977 Mar;72(3):452–456. [PubMed] [Google Scholar]
- Kerzner B., Kelly M. H., Gall D. G., Butler D. G., Hamilton J. R. Transmissible gastroenteritis: sodium transport and the intestinal epithelium during the course of viral enteritis. Gastroenterology. 1977 Mar;72(3):457–461. [PubMed] [Google Scholar]
- Loehry C. A., Creamer B. Three-demensional structure of the rat small intestinal mucosa related to mucosal dynamics. I. Mucosal structure and dynamics in the rat after the administration of methotrexate. Gut. 1969 Feb;10(2):112–116. doi: 10.1136/gut.10.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis S., Philips F. S., Sternberg S. S. The cytotoxicity of methotrexate in mouse small intestine in relation to inhibition of folic acid reductase and of DNA synthesis. Cancer Res. 1971 Dec;31(12):2037–2046. [PubMed] [Google Scholar]
- McClung H. J., Butler D. G., Kerzner B., Gall D. G., Hamilton J. R. Transmissible gastroenteritis. Mucosal ion transport in acute viral enteritis. Gastroenterology. 1976 Jun;70(6):1091–1095. doi: 10.1016/S0016-5085(76)80317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill L. K., Hamilton J. R. The effect of fasting on disaccharidase activity in the rat small intestine. Pediatrics. 1971 Jan;47(1):65–72. [PubMed] [Google Scholar]
- Munck B. G. Effects of sugar and amino acid transport on transepithelial fluxes of sodium and chloride of short circuited rat jejunum. J Physiol. 1972 Jun;223(3):699–717. doi: 10.1113/jphysiol.1972.sp009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck B. G., Schultz S. G. Properties of the passive conductance pathway across in vitro rat jejunum. J Membr Biol. 1974;16(2):163–174. doi: 10.1007/BF01872412. [DOI] [PubMed] [Google Scholar]
- Robinson J. W., Antonioli J. A., Vannotti A. The effect of oral methotrexate on the rat intestine. Biochem Pharmacol. 1966 Oct;15(10):1479–1489. doi: 10.1016/0006-2952(66)90193-6. [DOI] [PubMed] [Google Scholar]
- SCHMID P., SCHMID C., BRODIE D. C. The determination of the total deoxyribose of deoxyribonucleic acid. J Biol Chem. 1963 Mar;238:1068–1072. [PubMed] [Google Scholar]
- Schultz S. G. Sodium-coupled solute transport of small intestine: a status report. Am J Physiol. 1977 Oct;233(4):E249–E254. doi: 10.1152/ajpendo.1977.233.4.E249. [DOI] [PubMed] [Google Scholar]
- Sirotnak F. M., Donsbach R. C. Comparative studies on the transport of aminopterin, methotrexate, and methasquin by the L1210 leukemia cell. Cancer Res. 1972 Oct;32(10):2120–2126. [PubMed] [Google Scholar]
- TRIER J. S. Morphologic alterations induced by methotrexate in the mucosa of human proximal intestine. I. Serial observations by light microscopy. Gastroenterology. 1962 Mar;42:295–305. [PubMed] [Google Scholar]


