Abstract
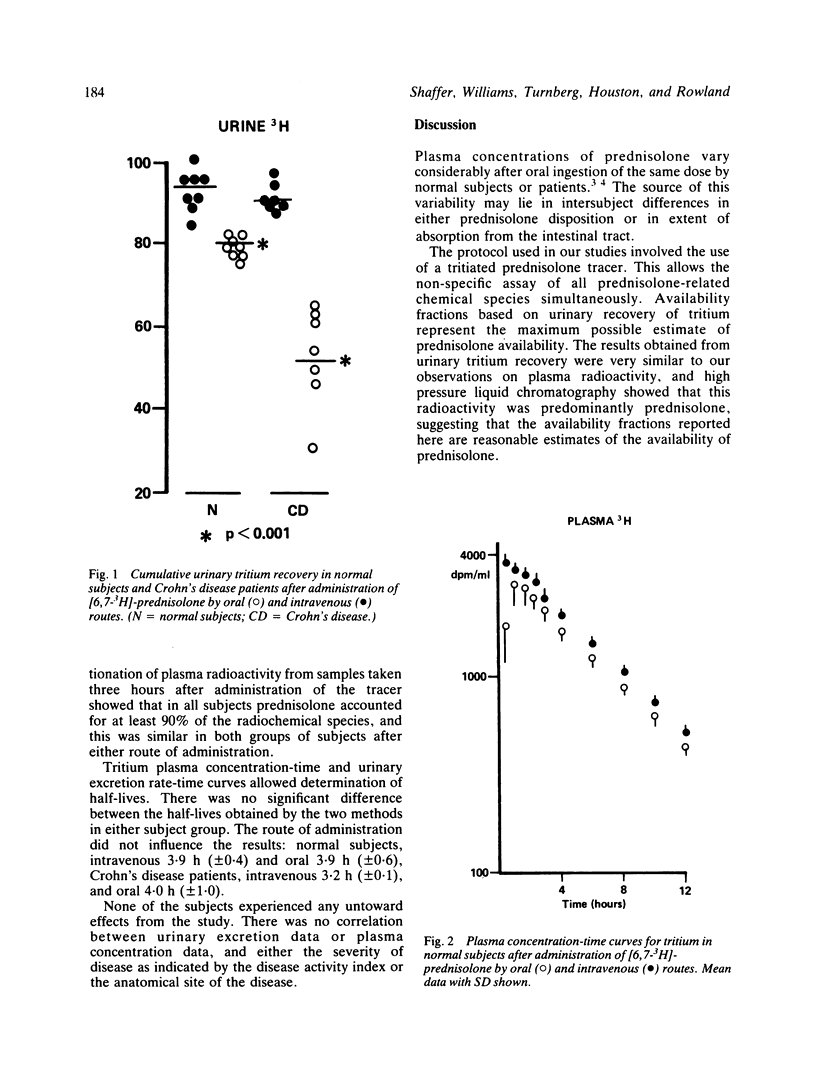
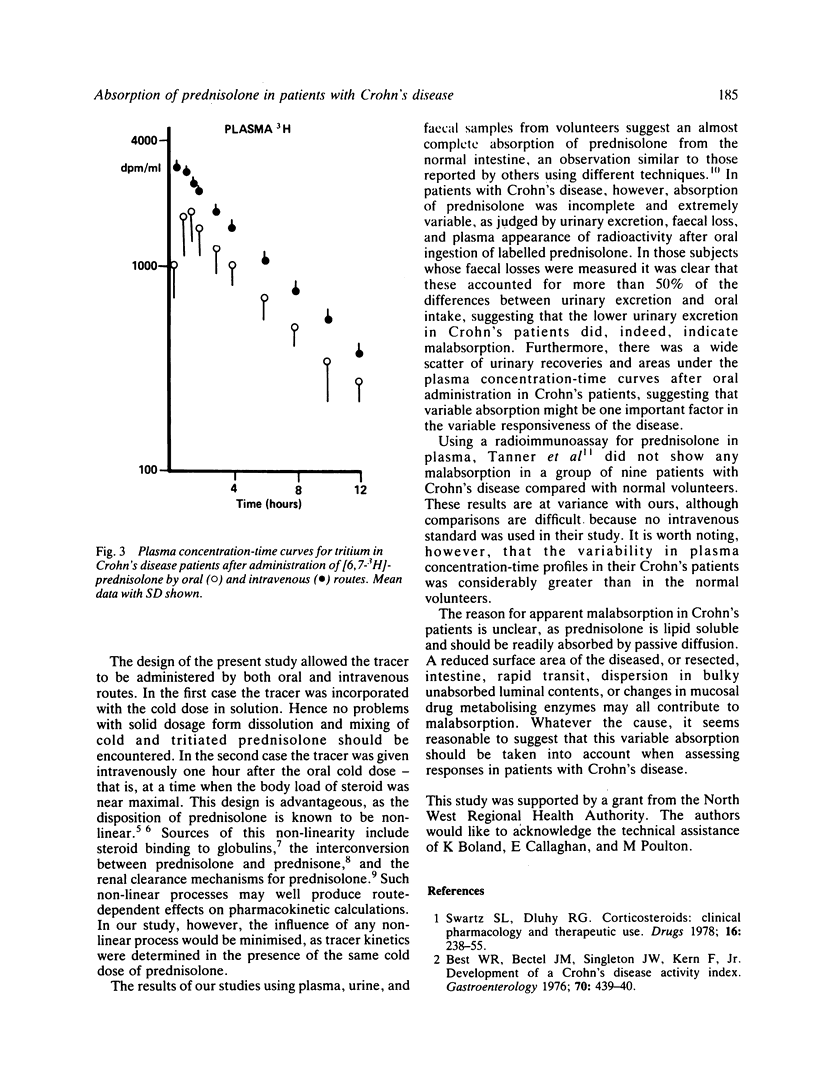
The absorption of prednisolone in patients with Crohn's disease was investigated. Seven patients with Crohn's disease and eight normal control subjects were given a tracer dose of tritiated prednisolone with 20 mg cold prednisolone by mouth. On a separate occasion they were given an intravenous injection of radiolabelled prednisolone. After oral ingestion only 53.4 +/- 11.7% of labelled material was excreted in the urine of Crohn's patients compared with 82.5 +/- 3.6% in the normal subjects. The oral/intravenous availability ratio was 0.61 +/- 0.14 in Crohn's patients and 0.89 +/- 0.07 in the normal group. Areas under plasma concentration-time curves were lower in patients than normal subjects and the oral/intravenous ratios were 0.6 +/- 0.2 and 0.86 +/- 0.09 respectively. Faecal excretion of radioactivity after oral ingestion was greater in Crohn's patients (19.3 +/- 2.5%, n = 3) than in normal subjects (7 +/- 2.8%, n = 4). The range for each type of measurement was much wider in the patient group than in the normal subjects. These data suggest that patients with Crohn's disease do not absorb prednisolone normally and that absorption varies between patients.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Habet S., Rogers H. J. Pharmacokinetics of intravenous and oral prednisolone. Br J Clin Pharmacol. 1980 Nov;10(5):503–508. doi: 10.1111/j.1365-2125.1980.tb01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best W. R., Becktel J. M., Singleton J. W., Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976 Mar;70(3):439–444. [PubMed] [Google Scholar]
- Pickup M. E. Clinical pharmacokinetics of prednisone and prednisolone. Clin Pharmacokinet. 1979 Mar-Apr;4(2):111–128. doi: 10.2165/00003088-197904020-00004. [DOI] [PubMed] [Google Scholar]
- Rocci M. L., Jr, Johnson N. F., Jusko W. J. Serum protein binding of prednisolone in four species. J Pharm Sci. 1980 Aug;69(8):977–978. doi: 10.1002/jps.2600690831. [DOI] [PubMed] [Google Scholar]
- Rocci M. L., Jr, Szefler S. J., Acara M., Jusko W. J. Prednisolone metabolism and excretion in the isolated perfused rat kidney. Drug Metab Dispos. 1981 May-Jun;9(3):177–182. [PubMed] [Google Scholar]
- Swartz S. L., Dluhy R. G. Corticosteroids: clinical pharmacology and therapeutic use. Drugs. 1978 Sep;16(3):238–255. doi: 10.2165/00003495-197816030-00006. [DOI] [PubMed] [Google Scholar]
- Tanner A. R., Halliday J. W., Powell L. W. Serum prednisolone levels in Crohn's disease and coeliac disease following oral prednisolone administration. Digestion. 1981;21(6):310–315. doi: 10.1159/000198583. [DOI] [PubMed] [Google Scholar]
- Tanner A., Bochner F., Caffin J., Halliday J., Powell L. Dose-dependent prednisolone kinetics. Clin Pharmacol Ther. 1979 May;25(5 Pt 1):571–578. doi: 10.1002/cpt1979255part1571. [DOI] [PubMed] [Google Scholar]


