Abstract
The purpose of this study was to examine how gonadal steroid hormones modulate basal nociception and morphine antinociception relative to regulating reproduction in the adult rat. Male and female Sprague–Dawley rats were either gonadectomized (GDX) or sham-gonadectomized (sham); GDX males were implanted subcutaneously with capsules containing testosterone (T), estradiol (E2), dihydrotestosterone (DHT), E2 and DHT, or nothing (0). GDX females received E2, T, or empty (0) capsules immediately after surgery, and vehicle or progesterone (P4) injections at 4-day intervals. Basal nociception and morphine antinociception were tested 28 days after surgery on 50°C and 54°C hotplate tests, and reproductive behavior and physiology were assessed shortly thereafter. There were no significant differences in baseline hotplate latencies among the male treatment groups, but morphine was significantly more potent in sham and GDX + T males than in GDX + 0 males. The ability of T to increase morphine's potency was approximated by its major metabolites E2 and DHT, given together but not alone. Baseline hotplate latencies were higher in sham females tested during diestrus than in those tested during estrus. Morphine was significantly more potent in sham females tested during proestrus and diestrus than in those tested during estrus. Baseline hotplate latencies were significantly higher, and morphine was significantly less potent in GDX + E2, GDX + E2/P4 and GDX + T females than in GDX + 0 females. All group differences in basal nociception and morphine antinociception observed on the 50°C hotplate test were smaller and generally non-significant on the 54°C hotplate test. Steroid manipulations produced the expected changes in reproductive behaviors and steroid-sensitive organs. These results demonstrate that in adult rats, gonadal steroid manipulations that are physiologically relevant, modulate (1) basal nociception in females but not males, and (2) morphine's antinociceptive potency in both males and females.
Keywords: Estrogen, Testosterone, Progesterone, Pain, Analgesia, Gender
1. Introduction
Numerous studies report sex differences in the analgesic effects of opioids. In humans, the mu agonist morphine (Sarton et al., 2000) and the mixed-action opioids pentazocine, butorphanol and nalbuphine (Gear et al., 1996a,b, 1999, 2000) produced greater or more prolonged analgesia in women than in men. Sex differences in opioid antinociception also are widely reported in rodents, although they tend to be in the opposite direction to those observed in humans. For example, morphine was significantly more potent or produced a greater effect in male than in female mice (Kavaliers and Innes, 1987; Lipa and Kavaliers, 1990; Candido et al., 1992) and rats (Baamonde et al., 1989; Cicero et al., 1996; Craft et al., 1999; Cook et al., 2000). Variables that may influence the magnitude of sex differences in opioid antinociception in rodents include opioid efficacy/selectivity, intensity of the noxious stimulus used in the pain test, and subject genotype (i.e. rodent strain) (Kest et al., 1999; Mogil et al., 2000; Cook et al., 2000; Craft and Bernal, 2001; Barrett et al., 2002). Sex differences in opioid analgesia have also been reported in the rhesus monkey (Negus and Mello, 1999, 2002); these sex differences are similar to those observed in rodent studies, using similar tests of acute thermal nociception. It is not yet known whether the opposite sex differences observed in human vs. animal studies reflect a true genotypic difference or are due to the different conditions under which opioid analgesia has been examined in human vs. animal subjects.
Sex differences in opioid antinociception are presumably due to the modulatory influence of gonadal steroid hormones, organizationally and/or activationally. Two recent studies have demonstrated organizational effects of gonadal steroids, such that neonatal gonadal steroid manipulations – orchidectomy in males and androgenization in females – eliminate sex differences in morphine's antinociceptive potency (Cicero et al., 2002; Krzanowska et al., 2002). The activational role of gonadal steroids has been examined by many laboratories, but with inconsistent results. For example, gonadectomy of adult male rats or mice has been shown to increase (Chatterjee et al., 1982; Ali et al., 1995), decrease (Rao and Saifi, 1985; Terner et al., 2002), or not affect (Kepler et al., 1989, 1991; Candido et al., 1992; Islam et al., 1993; Cicero et al., 1996, 2002; Krzanowska and Bodnar, 1999) opioid antinociception. Gonadectomy in adult female rats also has been shown to increase (Kepler et al., 1989; Ali et al., 1995; Krzanowska and Bodnar, 1999; Terner et al., 2002), decrease (Banerjee et al., 1983), or not affect (Kepler et al., 1991; Cicero et al., 1996, 2002; Terner et al., 2002) opioid antinociception. The effect of adult gonadectomy on opioid antinociception has been shown to depend on subject age and opioid dose (Islam et al., 1993), as well as rodent genotype and opioid efficacy (Terner et al., 2002).
To further investigate the modulatory role of gonadal steroids in opioid antinociception in adult animals, several studies have followed gonadectomy with steroid replacement. These studies have also produced variable results. For example, whereas some studies showed that estradiol (E2), progesterone (P4), or both produced no significant change in morphine antinociception in ovariectomized female rats (Banerjee et al., 1983) or rhesus monkeys (Negus and Mello, 1999, 2002), other studies showed that E2, P4, or both steroids increased (Nomikos et al., 1987) or decreased (Nomikos et al., 1987; Berglund et al., 1988; Ratka and Simpkins, 1991) morphine sensitivity in ovariectomized female rats. Long-term testosterone (T) replacement did not significantly alter the antinociceptive potency of several opioid agonists in ovariectomized rhesus monkeys (Negus and Mello, 2002). The effect of acute T replacement on antinociceptive sensitivity to morphine also has been examined, but the effects of T's primary metabolites, E2 and dihyrotestosterone (DHT), have not. In the brain, T is converted to E2 by aromatase, and to DHT by 5-alpha-reductase (Martini et al., 1993). Because these metabolites are active, and sometimes more potent than T itself (Roy and Chatterjee, 1995), examining their influence on morphine antinociception may clarify the role of T in sex differences in morphine antinociception.
Gonadal steroids also have been shown to modulate basal nociception. For example, in gonadectomized (GDX) male rats, T replacement returned basal nociceptive sensitivity to that seen in sham-gonadectomized (sham) males (Forman et al., 1989; Pednekar and Mulgaonker, 1995). In GDX female rats, E2 replacement returned nociceptive sensitivity to that of gonadally intact controls (Forman et al., 1989; Pednekar and Mulgaonker, 1995). In contrast, numerous studies reported no difference in nociceptive threshold between GDX rats compared to their sham-operated controls (Beatty and Beatty, 1970; Nomikos et al., 1987; Kepler et al., 1989; Martinez-Gomez et al., 1994; Ali et al., 1995; Cicero et al., 1996). Gonadal steroid modulation of pain also appears to occur in human subjects. For example, for most stimulus modalities, women had higher pain thresholds and pain tolerance during the follicular phase than during the luteal phase of the menstrual cycle (for review, see Riley et al., 1999). Although the literature is inconsistent regarding the modulatory effects of gonadal steroids on nociceptive thresholds, potential differences in nociceptive sensitivity must be taken into account when comparing opioid antinociception among treatment groups.
The variability across studies involving gonadectomy and steroid hormone replacement in adult animals is likely due to methodological differences across studies. First, recovery time between gonadectomy and antinociceptive testing may be important. Although levels of circulating hormones drop significantly within 24 h after gonadectomy (Keating and Tcholakian, 1983), the reproductive behavioral effects of these hormones may persist for several weeks post-gonadectomy in rodents (Davidson, 1966; Madlafousek et al., 1976). A second important factor, steroid replacement regimen (dose, duration and interval between steroid administration and nociceptive testing), also varies among studies. Gonadal steroids are traditionally described as lipid soluble signals that activate nuclear receptors and modulate transcription in target cells (Williams and Stancel, 1996; Wilson, 1996). These genomic effects are slow: it can be several hours to days before functional changes can be measured in the cell. However, it is now known that gonadal steroids may also have rapid effects at the target cell membrane. For example, E2 and T can bind to membrane-associated receptors and rapidly (within seconds) induce intracellular signaling changes (Lagrange et al., 1997; Moore and Evans, 1999). These changes appear too rapidly to be associated with transcriptional changes within the cell, which usually occur several hours after intracellular receptor activation (Moore and Evans, 1999). Thus, measuring responses minutes to hours after steroid treatment could yield different results than measuring responses after days or weeks of steroid treatment (e.g. Nomikos et al., 1987; Ryan and Maier, 1988; Alonso and Lopez-Coviella, 1998).
In addition to the particular gonadectomy and steroid replacement regimen used, aspects of the nociceptive testing procedure may affect the outcome of studies examining steroid influence on basal nociception and morphine antinociception. For example, some researchers tested only one dose of morphine (Rao and Saifi, 1985; Ratka and Simpkins, 1991; Ali et al., 1995), whereas others tested a range of doses (e.g. Chatterjee et al., 1982; Banerjee et al., 1983; Nomikos et al., 1987; Berglund et al., 1988; Kepler et al., 1989; Candido et al., 1992; Islam et al., 1993; Cicero et al., 1996; Krzanowska and Bodnar, 1999). Testing multiple doses of drug is preferable as it provides important information about how gonadal steroids affect drug potency and efficacy, which cannot be deduced from single-dose studies. Intensity of the noxious stimulus is another procedural variable that differs among studies of gonadal steroid modulation of nociception. Group differences in basal nociception and opioid antinociception have been shown to vary in size (and significance) with the intensity of the noxious stimulus used in the nociceptive test (Morgan et al., 1999; Negus and Mello, 1999; Cook et al., 2000; Craft and Bernal, 2001; Barrett et al., 2002; Terner et al., 2002).
To begin to investigate the physiological relevance of gonadal steroid-related changes in nociception and opioid antinociception, we examined the ability of gonadal steroids to modulate nociception and morphine antinociception relative to their well-known ability to modulate reproduction. Thus, gonadectomy-nociceptive test interval and steroid replacement regimens were chosen based on their documented ability to modify endogenous steroid levels and associated reproductive behavior and physiology of adult male and female rats (Young, 1961; Davidson, 1966; Davidson et al., 1968; Damassa et al., 1977). Because gonadal steroids fluctuate in gonadally intact female rats, we employed two strategies for examining the modulatory effects of gonadal steroids in females. First, we examined gonadally intact (sham-operated) females tested at different phases of the estrous cycle. Second, to isolate the effects of the two major gonadal steroids, female rats were gonadectomized and replaced with E2, P4 or both steroids together. T and E2 were given chronically because single-dose acute administration of these steroids will not fully restore reproductive behavior. P4 was given intermittently to approximate the cyclic changes in progestins observed in intact cycling females, and females were tested approximately 4–6 h after P4 injection because that is the peak time of P4's effect on reproductive behavior and physiology (Fadem et al., 1979; Feder, 1981).
Morphine antinociception was assessed using a cumulative dosing procedure so that a complete dose–effect curve was obtained in each rat, allowing for potency comparisons among treatment groups. Additionally, rats were tested on the hotplate at two different temperatures, to determine whether steroid hormone effects were dependent on the intensity of the noxious stimulus used in the pain test.
2. Materials and methods
2.1. Subjects
Male and female Sprague–Dawley rats (bred in-house from Taconic stock, Germantown, NY) were used. Rats were housed in same-sex pairs, according to gonadal steroid status after surgery (e.g. an orchidectomized rat with T replacement was housed with another male in the same treatment group). Males and females were housed in separate rooms maintained at 21.5 ± 2°C with a 12:12 h light:-dark cycle (lights on at 0600 hours). Access to food and water was ad libitum except during testing.
2.2. Apparatus
For nociceptive testing, one Hot Plate Analgesia Meter (Columbus Instruments, Columbus, OH) set at 50 (±0.1)°C and another set at 54 (±0.1)°C were used. The surface of each plate measured 25.3 cm2, and was surrounded by 30-cm high Plexiglas® walls. Reproductive behavior testing was conducted in a cylindrical clear Lexan® arena (45 cm high, 36 cm diameter) with standard cage bedding covering the floor.
2.3. Procedure
The experimental timeline is shown in Table 1. Surgery (gonadectomy or sham-gonadectomy) was performed in rats that were approximately 3 months old. In all rats except sham females, basal nociception and morphine antinociception were tested 28 days after surgery, when endogenous hormone levels and associated reproductive behaviors have been shown to be minimal in GDX rats (Young, 1961; Davidson, 1966; Davidson et al., 1968; Damassa et al., 1977). Sham females were tested between 21 and 35 days post-surgery so that females in diestrus, proestrus and estrus could be identified and tested in approximately equal numbers. To verify that gonadectomy and the various steroid replacement regimens were behaviorally effective, most rats were tested for sex-appropriate reproductive behavior during the 12-day period following nociceptive testing. Four days after reproductive behavior testing, organs sensitive to circulating levels of gonadal steroids were harvested.
Table 1.
Experimental timeline
| Day 0 | Days 4, 8, 12, etc. | Day 28 | Days 32, 36, 40 | Day 44 |
|---|---|---|---|---|
| Surgery + steroid capsule implantation | P4 or vehicle injections | Nociceptive testing | Reproductive behavior testing | Reproductive organ harvest |
| Vaginal smears (day 15 through day 44) |
2.3.1. Surgery
For gonadectomy and sham-gonadectomy, rats were anesthetized with Equithesin intraperitoneally (i.p.) (active ingredients: sodium pentobarbital, 28.2 mg/kg and chloral hydrate, 123.3 mg/kg). In males, the scrotal area was shaved and scrubbed with betadyne. A 1-cm transverse incision was made at midline scrotum. Both testes were exteriorized, epididymis first, with the associated fat pad. The tubules were tied tightly with 4.0 silk suture. Tissue below the knot (testes, epididymis and fat pad) was cut and discarded. For sham orchidectomy, the testes were exposed but not exteriorized. The incision was closed with wound clips.
In females, the ovaries were located by palpation. Both sides of the back (in the lumbar region) were shaved and scrubbed with betadyne. A 1-cm incision was made through the skin, connective tissue and muscle layer. The ovary, associated fat pad, Fallopian tube and upper uterine horn was exteriorized. The blood supply to the ovary was restricted with 3.0 silk suture. The ovary was cut and discarded, leaving the uterus intact. For sham ovariectomy, the ovary, associated fat pad, Fallopian tube and upper uterine horn were exteriorized and then returned to the peritoneum. The muscle wall was closed with 3.0 chromic suture, and the skin was closed with wound clips.
2.3.2. Steroid hormone replacement
Chronic steroid replacement was achieved through Silastic® capsules implanted subcutaneously (s.c.), immediately following gonadectomy. A small incision was made between the shoulder blades, capsules were inserted in the subcutaneous space and the skin was closed with wound clips. Capsules containing T or DHT were initially prepared in 5- and 10-mm lengths; one 5-mm T capsule was implanted in females, and one 10-mm T or DHT capsule/100 g of body weight was implanted in males. Capsules containing E2 were prepared in 10-mm (male) and 1-mm (female) lengths; one 10-mm capsule/100 g of body weight was implanted in males and one 1-mm capsule was implanted in females. To compare the effects of different doses of T and E2, a separate group of males was implanted with T at a lower dose of one 2-mm capsule/100 g body weight, and a separate group of females was implanted with E2 at a higher dose of one 5-mm capsule/rat. To control for possible effects of capsule implantation, sham-operated and GDX control rats were implanted with empty capsules (blanks): males received two 10-mm blank capsules and females received one 1-mm blank capsule.
Some females also received P4 treatment. Beginning 4 days after surgery, injections of P4 (500 μg/rat in 0.1 ml volume, s.c.) were given every 4 days at approximately 1000 hours, to mimic the pre-ovulatory P4 surge observed in normal-cycle females (Feder, 1981). All rats (females and males) not receiving P4 treatment received safflower oil vehicle injections (0.1 ml/rat, s.c.) on the same schedule, so that all rats would be handled/injected the same number of times. Table 2 summarizes the 14 different treatment groups in the study.
Table 2.
Treatment groups
| Group | Surgery | Steroid hormone capsules | Steroid hormone injection (4-day cycle) | Treatment abbreviation |
|---|---|---|---|---|
| Males | ||||
| 1 | Sham-orchidectomy | Blank | Vehicle | Sham |
| 2 | Orchidectomy | Blank | Vehicle | GDX + 0 |
| 3 | Orchidectomy | Ta | Vehicle | GDX + T |
| 4 | Orchidectomy | E2 | Vehicle | GDX + E2 |
| 5 | Orchidectomy | DHT | Vehicle | GDX + DHT |
| 6 | Orchidectomy | E2 + DHT | Vehicle | GDX + E2/DHT |
| Intact femalesb | ||||
| 7 | Sham-ovariectomy | Blank | Vehicle | Sham-diestrus-1 (D) |
| 8 | Sham-ovariectomy | Blank | Vehicle | Sham-proestrus (P) |
| 9 | Sham-ovariectomy | Blank | Vehicle | Sham-estrus (E) |
| GDX females | ||||
| 10 | Ovariectomy | Blank | Vehicle | GDX + 0 |
| 11 | Ovariectomy | E2c | Vehicle | GDX + E2 |
| 12 | Ovariectomy | E2c | P4 | GDX + E2/P4 |
| 13 | Ovariectomy | Blank | P4 | GDX + P4 |
| 14 | Ovariectomy | T | Vehicle | GDX + T |
In GDX males, two doses of T were tested: ‘low’, one 2-mm capsule/100 g body weight; ‘high’, one 10-mm capsule/100 g body weight.
No sham females were tested during diestrus-2 because, in terms of gonadal steroid levels, reproductive behavior and reproductive physiology, there are few differences between diestrus-1 and -2 (Freeman, 1988).
In GDX females, two doses of E2 were tested: ‘low’, one 1-mm capsule/rat; ‘high’, one 5-mm capsule/rat.
2.3.3. Nociceptive testing
Nociceptive testing was conducted in the afternoon of the 28th day post-surgery. Testing began at approximately 1400 hours, to occur within the period of P4's peak reproductive effect, 4–6 h post-injection (e.g. Fadem et al., 1979). To obtain non-drug response latencies (to lick a hindpaw or jump off the plate), each rat was tested on the 50°C and then 54°C hotplates, three times: first at time 0 (baseline 1), then again 15 min later (baseline 2); immediately following this second baseline test, saline was administered (1 ml/kg, s.c.) and rats were tested a third time on each hotplate 30 min later (saline test). Immediately following the third baseline test, increasing doses of morphine (in 1/4-log unit increments) were administered every 30 min, with hotplate latencies obtained 30 min after each injection. Thus, 3.2 mg/kg morphine was administered, and hotplate latency was tested 30 min later; immediately following testing, an additional 2.4 mg/kg morphine (total 5.6 mg/kg) was administered and hotplate latency was tested 30 min later. Testing continued with increasing doses of morphine until the rat reached the cutoff latency on both hotplates. Once the rat reached cutoff on one hotplate, subsequent testing was conducted only on the other hotplate.
We have previously observed that baseline response latencies on the hotplate are significantly higher in intact female than male rats (Bartok and Craft, 1997; Craft and Bernal, 2001). To normalize the test range in seconds across sexes, cutoff latencies of 60 and 80 s were used for male and female rats, respectively, on the 50°C hotplate test. On the 54°C hotplate, cutoff latencies of 15 and 20 s were used for male and female rats, respectively. If the rat did not respond by the cutoff latency, it was removed from the hotplate and the cutoff latency was recorded.
2.3.4. Reproductive behavior testing
Reproductive behavior testing was conducted 4, 8 and 12 days after nociceptive testing for all GDX rats and for sham males, to coincide with the 4-day schedule of P4/vehicle injections (see Table 1). Sham females were tested for reproductive behavior only once within the 14 days following their nociceptive test, so that their reproductive behavior test could be conducted when they were in the same estrous stage as they were for nociceptive testing. Because of the difficulty of efficiently obtaining both nociceptive and reproductive data in the same female at the same stage, however, different sham females sometimes were used for nociceptive testing vs. reproductive behavior testing.
Experimental rats were exposed to stimulus rats of the opposite sex, and the reproductive behaviors of the experimental rats were scored. Before testing with experimental male rats, ovariectomized stimulus female rats received an E2 injection (25 μg/rat) between 1800 and 2000 hours, on each of two consecutive days before testing. Stimulus females then received P4 injections (500 μg/rat) at 1500 hours on the day of testing. This treatment produced highly proceptive and receptive stimulus females. At least 1 week before testing with experimental females, stimulus male rats were trained by pairing with receptive stimulus female rats in the testing apparatus. This training created sexually experienced stimulus males that would vigorously mount experimental females.
On the fourth day after testing antinociception, experimental females were injected with P4 or vehicle at approximately 1500 hours; males were injected with vehicle. At approximately 1800 hours, stimulus and experimental rats were moved from the vivarium to the testing room. To avoid disrupting the light:dark cycle, the testing room was illuminated only with red light (two 25-W bulbs). Testing began at 2000 hours. Testing of male reproductive behavior began by placing the experimental male in an arena. After 5 min, a stimulus female was placed in the arena. If the male rat did not show an intromission pattern (i.e. successfully mount the female and show one intromission) after 15 min exposure to the stimulus female, a new stimulus female was introduced, and the test continued. The male was given a total of 60 min (four females) to show an intromission pattern. If the experimental male failed to show an intromission pattern during the 60 min exposure to the stimulus females, the test was terminated. If the experimental male did show an intromission pattern during any 15-min period, the time of the intromission was recorded and the male was observed for an additional 30 min with that stimulus female. If the experimental male showed an ejaculatory pattern during that 30 min, the time was recorded and the male was observed for an additional 15 min to record whether another intromission pattern occurred. When an intromission pattern occurred or when 15 min elapsed, the test was terminated. If the experimental male did not show an ejaculatory pattern during the 30 min, he was exposed to a new stimulus female and observed for an additional 30 min. If he failed to show an ejaculation pattern in the second 30 min, the test was terminated. If the experimental male showed an ejaculatory pattern during the second 30 min, the time was recorded and he was observed for an additional 15 min to record whether an additional intromission pattern occurred, as noted above.
Testing for reproductive behavior in females began by placing a stimulus male in the arena. After 5 min, the experimental female was placed in the arena. The rats remained together until the stimulus male mounted the female ten times. The number of times the female displayed lordosis (dorsoventral flattening of the body, with the head and tail raised) in response to the stimulus male's mounts was recorded. After ten mounts, the test was terminated.
Reproductive behavior testing was repeated 4 and 8 days later for both experimental male and female rats (see Table 1), with the exception of sham females, as noted above.
2.3.5. Vaginal cytology
To further characterize gonadal steroid hormone state in female rats, daily vaginal smears were taken beginning 2 weeks before nociceptive testing through the end of the experiment. Proestrus was identified by the prevalence (approximately 90% or more of total cells in sample) of nucleated epithelial cells; estrus was identified by the prevalence of dense sheets of cornified epithelial cells; diestrus-1 was identified by the presence of leukocytes and scattered nucleated and/or cornified epithelial cells; diestrus-2 was identified by the relative absence of any cells (Freeman, 1988). To keep the stages highly distinct, sham females whose vaginal cytology indicated that they were in transition from one estrous stage to another were not tested; however, smears that consisted of approximately 50% nucleated and 50% cornified epithelial cells, which were common in some groups, were designated ‘proestrus/estrus’ for purposes of estrous cycle comparison (Table 4). In order to represent a ‘normal’ sample of females, testing in sham females was not limited to 4-day cyclers. Thus, the designations of diestrus-1 and diestrus-2 are based on vaginal cytology alone, and do not necessarily indicate the first and second day after estrus.
Table 4.
Estimated percent time (mean ± 1 SEM) that female rats were in each phase of the estrous cycle, and the number of rats that were in each stage on the day of nociceptive testing (in parentheses)a
| % Time in each phase of estrous cycleb |
|||||
|---|---|---|---|---|---|
| Treatment group | Proestrus | Proestrus/estrus | Estrus | Diestrus-1 | Diestrus-2 |
| Sham | 14.0 ± 2.4 (7) | 7.4 ± 1.7 (0) | 17.0 ± 2.7 (10) | 41.9 ± 4.0 (9) | 20.6 ± 3.2 (0) |
| GDX + 0 | 0 ± 0 (0) | 0 ± 0 (0) | 0 ± 0 (0) | 80.0 ± 12.3 (10) | 20.0 ± 12.3 (1) |
| GDX + E2 (lowc) | 0 ± 0 (0) | 22.5 ± 10.1 (2) | 77.5 ± 10.0 (7) | 0 ± 0 (0) | 0 ± 0 (0) |
| GDX + E2 (highc) | 2.5 ± 2.7 (0) | 75.5 ± 12.9 (2) | 11.5 ± 6.0 (9) | 0 ± 0 (0) | 0 ± 0 (0) |
| GDX + E2 (low)/P4 | 18.3 ± 7.7 (2) | 10.0 ± 2.8 (2) | 50.0 ± 10.6 (4) | 21.7 ± 11.5 (0) | 0 ± 0 (0) |
| GDX + E2 (high)/P4 | 15.4 ± 4.5 (1) | 10.1 ± 2.4 (2) | 60.9 ± 10.2 (7) | 6.4 ± 2.7 (0) | 0 ± 0 (0) |
| GDX + P4 | 0 ± 0 (0) | 0 ± 0 (0) | 0 ± 0 (0) | 77.1 ± 7.7 (8) | 22.8 ± 7.7 (3) |
| GDX + T | 0 ± 0 (0) | 0 ± 0 (0) | 0 ± 0 (0) | 72.0 ± 6.1 (7) | 28.0 ± 6.1 (3) |
For treatment group abbreviations see Table 2.
Based on daily vaginal cytology sampled on 14 consecutive days, beginning on day 15 post-surgery (except sham females, in which the average sample was based on 10 days, because some females were tested before day 28).
Low, 1-mm capsule/rat; high, 5-mm capsule/rat.
Smears also were obtained in all sham female rats immediately following the morphine antinociception test. Only data from females that remained in the same stage from before to after testing were included in the analyses. To control for possible effects of handling, sham male rats also were brought to the laboratory and handled on the same days and times when vaginal smears were obtained in females.
2.3.6. Steroid-sensitive tissue harvest
Sixteen days after nociceptive testing, rats were euthanized. Capsules implanted following surgery were removed and counted to confirm treatment. To determine the reproductive physiological effects of gonadectomy and gonadal steroid treatment, organs sensitive to E2, T or DHT were removed. In male rats, the seminal vesicles and bulbocavernosus/levator ani (BC/LA) muscle complex were removed and fixed (seminal vesicles in Bouin's solution; BC/LA complex in 4% paraformaldehyde) for at least 2 weeks. Seminal vesicles were later trimmed and separated for weighing; excess moisture was blotted before weighing. Similarly, after fixing, the BC/LA muscle complex was trimmed and the two muscles separated. After blotting excess moisture, BC and LA muscles were weighed separately and their weights were summed. In female rats, the uterus was removed, fixed (4% paraformaldehyde), trimmed, blotted and weighed. Because it was difficult to obtain organs in all sham females at the same stage that they had been in during behavioral testing, 13 sham females (three diestrus, five proestrus, and five estrus) did not undergo nociceptive or reproductive behavior testing but were euthanized on day 28 to harvest organs.
2.4. Data analysis
Latency to respond in seconds was recorded for each hotplate test. Baseline response latency was calculated for each rat as the mean of the second baseline test and the saline test. Baseline response latencies were compared within the male and within the GDX-female groups using Dunnett's test, because the primary comparisons of interest were between sham or steroid-replaced rats and their same-sex GDX controls (GDX + 0). Dunnett's test was also used to compare sham female groups to the sham male group, and to compare sham female groups to the GDX + 0 female group. Analysis of variance (ANOVA) with Student–Newman–Keuls post-hoc tests were used to compare baselines among sham female groups, as there were no a priori comparisons between particular groups. Because there were significant differences in baseline response latencies among treatment groups, individual response latencies obtained following each dose of morphine were converted to % maximum possible effect (%MPE): ((drug latency − baseline latency)/(cutoff latency − baseline latency)) × 100. Cutoff latency was chosen so that the difference between the mean baseline and cutoff value was the same in all groups (i.e. cutoff was set at 31.4 and 10.9 s over baseline for the 50°C and 54°C hotplates, respectively); these cutoff adjustments equalized the %MPE scale among all treatment groups within each stimulus intensity, so that group comparisons of morphine's potency at each stimulus intensity were based on the same 0–100% scale. From these %MPE data, the estimated dose at which antinociception was 50% (ED50) was calculated for each rat by linear interpolation, generally using one point above 50% and one point below 50%. ED50 values were compared among the male groups and among the GDX-female groups using Dunnett's test, because the primary comparisons of interest were between sham or steroid-replaced rats and their same-sex GDX controls (GDX + 0). ANOVA with Student–Newman–Keuls post-hoc tests were used to compare ED50 values among sham female groups, as there were no a priori comparisons between particular groups.
During male reproductive behavior testing, the following data were recorded: latency to first mount, total number of mounts, latency to first intromission, total number of intro-missions, latency to ejaculation, and latency from ejaculation to next intromission (post-ejaculatory interval, PEI). If no intromission patterns occurred, latency to mount and latency to intromission each were 60 min. If no sexual behavior occurred in the 15 min following ejaculation, the PEI was 15 min.
From these data, copulatory rate and copulatory efficiency were calculated. Copulatory rate was calculated as: number of mounts + number of intromission patterns + the ejaculatory pattern/total test length (min), excluding the PEI. Copulatory efficiency was calculated as: number of intromission patterns + the ejaculatory pattern/number of mounts + number of intromission patterns + the ejaculatory pattern (not including events occurring during the PEI). If a male mounted but did not display an intromission pattern, copulatory efficiency was zero. If a male did not mount, copulatory efficiency was not computed. Copulatory rate and copulatory efficiency for each male were averaged over the three test sessions. Latency to ejaculate was calculated as the duration (minutes) between the first mount and the ejaculation. Latency to ejaculate was averaged over the three test sessions. For female reproductive behavior, the lordosis quotient was calculated as: (number of lordosis behaviors/10) × 100. Because many sham females had only one reproductive behavior test, group comparisons of lordosis quotients were made using only data obtained in the first test in all female rats. Group differences in copulatory rate, copulatory efficiency, lordosis quotient and organ weights were analyzed as described above for baseline and ED50 data. The significance level for all statistical tests was P ≤ 0.05.
To compare estrous cycling among the female groups, the number of days spent in each phase of the cycle, based on vaginal cytology (proestrus, proestrus/estrus, estrus, diestrus-1 and diestrus-2), was counted for each female. The number of days spent in a given stage was divided by the total number of days in the sample (maximum 14, days 15–28), to obtain an estimate of percent time spent in each phase of the estrous cycle (see Table 4). Smear samples after the nociceptive test day were not included because of the possible suppressive effect of morphine on estrous cyclicity (Pfeiffer and Herz, 1984).
2.5. Drugs
Morphine sulfate (National Institute on Drug Abuse, Rockville, MD) was dissolved in 0.9% physiological saline; saline served as the control vehicle for morphine. Saline and morphine injections were administered s.c. in a volume of 1.0 ml/kg. When injected, E2 and P4 (Steraloids, Wilton, NH) were dissolved in safflower oil; safflower oil served as the control vehicle for these steroids. Steroid injections were given s.c. in a volume of 0.1 ml/rat. To administer steroid hormones by constant-release s.c. capsule, Silastic® tubing (0.062 inch i.d./0.125 inch o.d.) was filled with crystallized E2, T, DHT or nothing. Capsules were sealed with Silastic® adhesive and allowed to dry for a minimum of 24 h. They were then soaked in 70% ethanol for a minimum of 24 h before use, to test the integrity of the seal (Smith et al., 1977).
3. Results
3.1. Effects of gonadal steroid manipulations in male rats
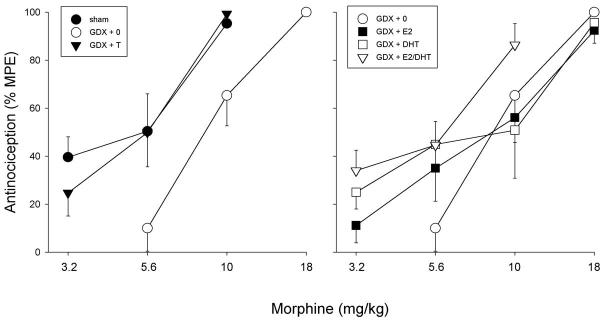
In male rats, gonadectomy and steroid hormone replacement did not significantly affect basal nociception on the 50°C hotplate test (Fig. 1); although the GDX + DHT group appeared to be lower than the GDX + 0 group, this difference was not statistically significant (P = 0.096). In contrast, gonadectomy and steroid replacement did influence morphine's antinociceptive potency in male rats (Fig. 2): on the 50°C hotplate test, the morphine dose–effect curve in sham and GDX + T males lies to the left of that in GDX + 0 controls (left panel). The ability of T to increase morphine's potency was not mimicked by its major metabolites E2 and DHT, given alone or together (right panel). Statistical comparison of ED50 values derived from the morphine dose–effect curves indicates that morphine was significantly more potent in the sham and GDX + T groups relative to GDX + 0 controls (Table 3); the difference in ED50 values between the GDX + E2/DHT group and the GDX + 0 control group was not significant (P = 0.16). There were no significant group differences in baseline nociception or in morphine's potency on the 54°C hotplate test (not shown).
Fig. 1.
Males: effects of GDX and steroid hormone replacement on basal nociception on the 50°C hotplate test. Each bar is the mean + 1 SEM of seven to eleven rats. For treatment group abbreviations see Table 2; only high-dose T group is shown.
Fig. 2.
Males: effects of GDX and steroid hormone replacement on morphine antinociception on the 50°C hotplate test. Each point is the mean ± 1 SEM of seven to eleven rats. For treatment group abbreviations see Table 2; only high dose T group is shown.
Table 3.
ED50 values (mean ± 1 SEM) for morphine antinociception on the 50°C hotplatea
| Males |
Females |
||||
|---|---|---|---|---|---|
| Treatment group | ED50 | N | Treatment group | ED50 | N |
| Sham-D | 5.65 ± 0.60 | 9 | |||
| Sham-P | 6.82 ± 1.26 | 7 | |||
| Sham | 5.61 ± 0.88*,** | 8 | Sham-E | 10.46 ± 0.93*,*** | 10 |
| GDX + 0 | 9.49 ± 0.93 | 11 | GDX + 0 | 6.43 ± 1.05 | 11 |
| GDX + T (low) | 5.30 ± 0.64* | 8 | GDX + E2 (low) | 15.61 ± 1.26* | 11 |
| GDX + T (high) | 5.34 ± 0.90* | 8 | GDX + E2 (high) | 10.07 ± 1.35 | 9 |
| GDX + E2 | 10.35 ± 1.43 | 11 | GDX + E2 (low)/P4 | 13.10 ± 1.05* | 8 |
| GDX + DHT | 10.91 ± 1.93 | 7 | GDX + E2 (high)/P4 | 11.60 ± 1.00* | 10 |
| GDX + E2/DHT | 6.80 ± 0.89 | 10 | GDX + P4 | 6.31 ± 1.18 | 11 |
| GDX + T | 14.03 ± 1.81* | 10 | |||
For treatment group abbreviations see Table 2.
Significantly different from GDX + 0 rats of the same sex, P ≤ 0.05, Dunnett's.
Significantly different from sham-E females, P ≤ 0.05, t-test
Significantly different from sham-D and sham-P female rats, P ≤ 0.05, Student–Newman–Keuls.
Fig. 3 shows that gonadectomy and steroid replacement also significantly influenced measures of reproductive behavior (top panel) and reproductive physiology (bottom panel) in male rats. Sham males, and GDX males replaced with T, E2 or E2/DHT showed significantly greater rates of copulation and greater copulatory efficiency than did GDX males without steroid replacement (GDX + 0 controls). An additional measure of copulatory efficiency, latency to ejaculate, was also significantly shorter in sham, GDX + T, GDX + E2 and GDX + E2/DHT males than in GDX + 0 controls (P < 0.001, P = 0.003, P = 0.047, P < 0.001, respectively; data not shown). As expected, replacement with DHT alone did not restore reproductive behavior in GDX male rats. Effects of gonadectomy and steroid replacement in the same rats were also reflected in the weights of steroid-sensitive reproductive organs: the BC/LA muscle complex and seminal vesicles were significantly heavier in sham, GDX + T, GDX + DHT and GDX + E2/DHT males than in GDX + 0 controls (Fig. 3, bottom panel). As expected, replacement with E2 alone did not restore reproductive organ weights in GDX male rats.
Fig. 3.
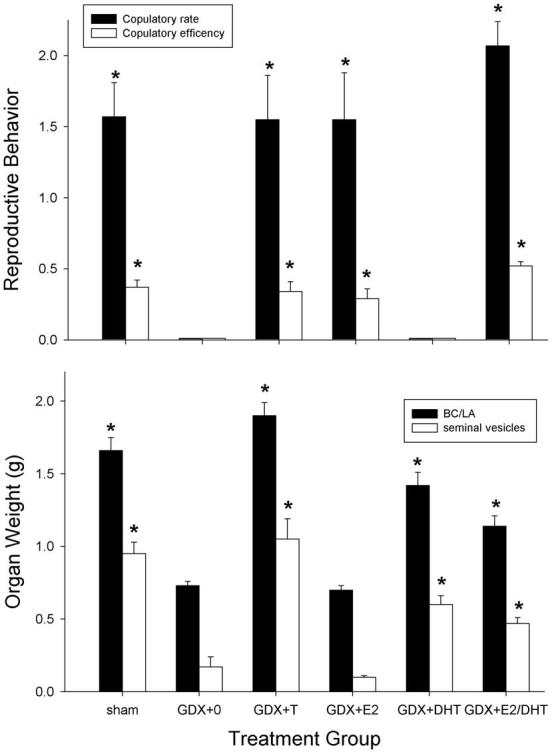
Males: effects of GDX and steroid hormone replacement on reproductive behavior (top panel) and physiology (bottom panel). Top panel: copulatory rate = male reproductive behaviors (mounts, intromissions, ejaculation)/min; copulatory efficiency = number of intromissions and the ejaculation relative to total reproductive behaviors performed. Each bar is the mean + 1 SEM of seven to ten rats. Bottom panel: weight of steroid-sensitive organs; each bar is the mean + 1 SEM of five to 11 organs. For treatment group abbreviations see Table 2; only high dose T group is shown. *Significantly different from GDX + 0, P ≤ 0.05, Dunnett's.
To determine whether a lower dose of T would also significantly influence morphine's potency in GDX males, a separate group of GDX males was replaced with a lower dose of T (2-mm capsule/100 g body weight rather than 10-mm capsule/100 g body weight). Results in GDX males replaced with the lower dose of T did not differ significantly from those in GDX males replaced with the higher dose of T, on basal nociception (data not shown) or morphine ED50 (Table 3). In GDX males that received the lower dose of T, copulatory rate and BC/LA complex weight were slightly lower, and the number of mounts (P = 0.009) and seminal vesicle weight (P = 0.022) were significantly lower than in GDX males that received the higher dose of T, although all reproductive behavior and organ weight measures were still significantly higher than in GDX + 0 controls (GDX + low dose T: copulatory rate = 1.01 ± 0.13; copulatory efficiency = 0.5 ± 0.08; BC/LA weight = 1.65 ± 0.07 g; seminal vesicle weight = 0.72 ± 0.06 g).
3.2. Nociception, antinociception and reproductive behavior in intact (sham) female rats
Fig. 4 shows that sham female rats tested in different phases of the estrous cycle showed significantly different baseline latencies on the 50°C hotplate test (F(2,23) = 3.55, P = 0.045). Sham females tested in estrus had significantly lower baselines than sham females tested in diestrus-1. Fig. 5 shows that morphine was more potent in sham females tested during diestrus-1 and proestrus than in those tested during estrus on the 50°C hotplate test. Comparison of ED50 values derived from these morphine dose–effect curves indicates that these potency differences were statistically significant (F(2, 23) = 8.02, P = 0.002), with morphine being significantly more potent in diestrus-1 and proestrus females than in estrus females (Table 3). Group differences in baseline nociception and in morphine's antinociceptive potency were not significant on the 54°C hotplate test (not shown).
Fig. 4.
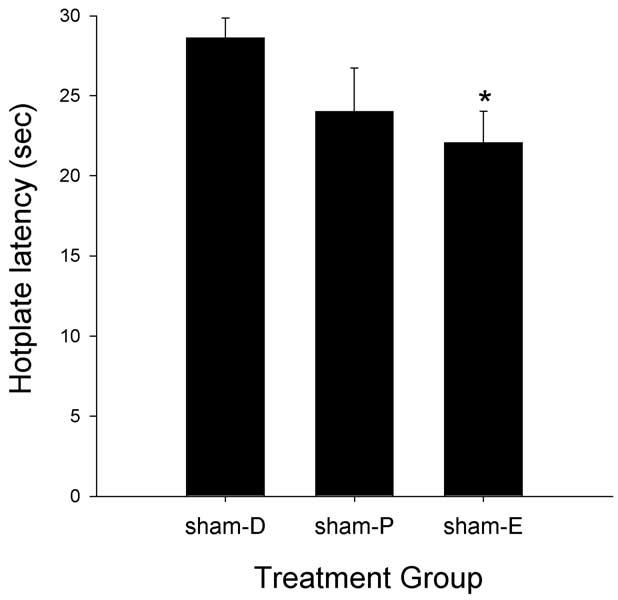
Intact females: effects of estrous stage on basal nociception on the 50°C hotplate test in sham-GDX females (D, diestrus-1; P, proestrus; E, estrus). Each bar is the mean + 1 SEM of seven to ten rats. *Significantly different from sham-D, P ≤ 0.05, Student–Newman–Keuls.
Fig. 5.
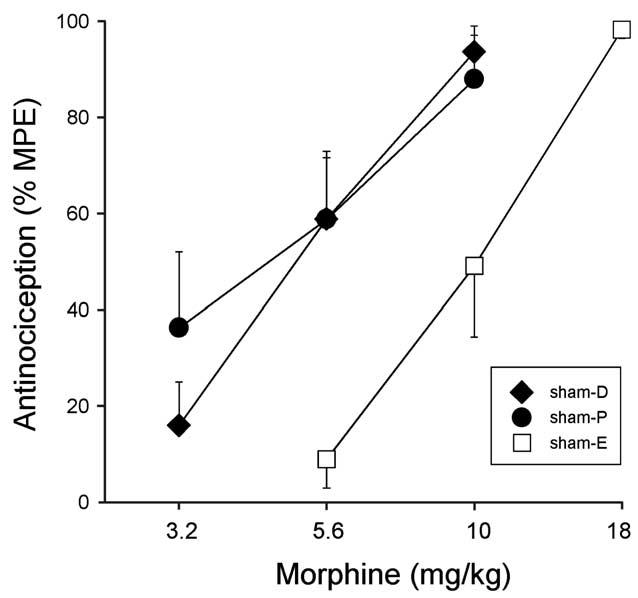
Intact females: effects of estrous stage on morphine antinociception on the 50°C hotplate test in sham-GDX females (D, diestrus-1; P, proestrus; E, estrus). Each point is the mean ± 1 SEM of seven to eleven rats.
Fig. 6 shows that measures of reproductive behavior (top panel) and reproductive physiology (bottom panel) also differed, in the expected manner, among sham females tested in different phases of the estrous cycle. Only females in vaginal estrus showed a high level of lordosis behavior (F(2, 21) = 44.60, P < 0.001), whereas females in proestrus showed the greatest uterine weights (F(2, 17) = 62.46, P < 0.001).
Fig. 6.
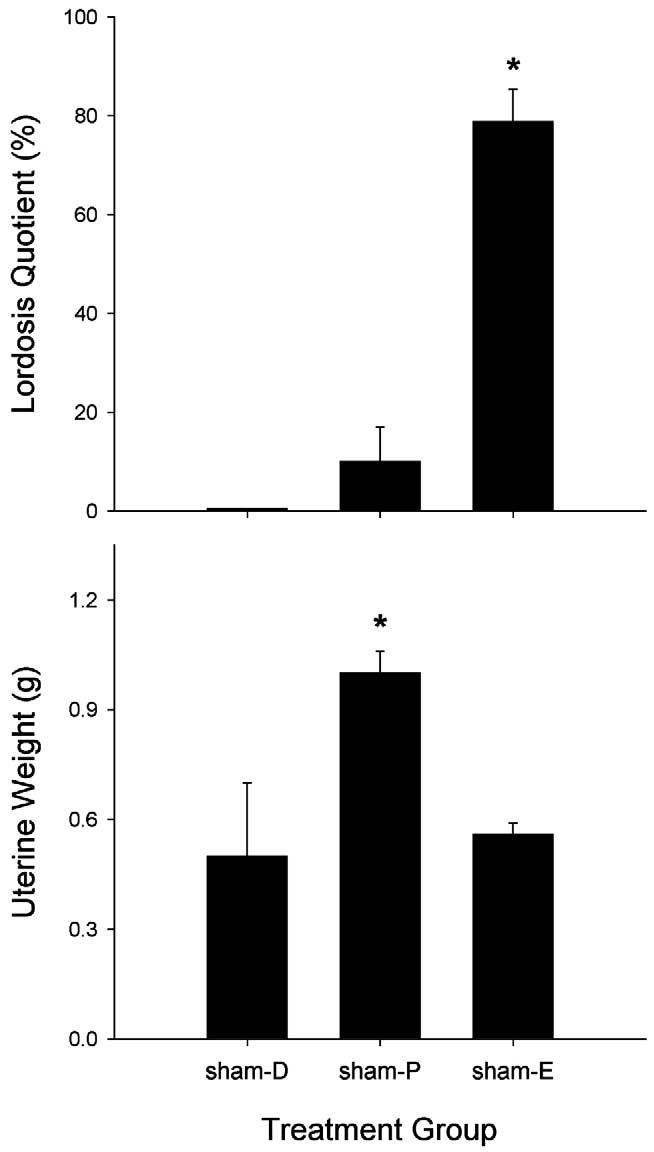
Intact females: effects of estrous stage on reproductive behavior (top panel) and physiology (bottom panel) in sham-GDX females (D, diestrus-1; P, proestrus; E, estrus). Top panel: lordosis quotient, frequency of lordosis behavior shown by females when mounted by a male; each bar is the mean + 1 SEM of six to ten rats. Bottom panel: weight of the uterus, a steroid-sensitive organ; each bar is the mean + 1 SEM of six to eight organs. *Significantly different from sham-D, P ≤ 0.05, Student–Newman–Keuls.
When sham females were compared to sham males, sex differences were found to depend on the estrous stage in which females were tested. First, only sham females in diestrus had higher nociceptive thresholds than sham males, on the 50°C hotplate test (P = 0.02) (compare Figs. 1 and 4). Second, morphine was significantly more potent in its antinociceptive effect in sham males only compared to sham-estrus females on the 50°C hotplate test (P = 0.001) (Table 3). Morphine was significantly more potent in sham males than in sham-estrus (P = 0.001) and sham-diestrus (P = 0.018) females on the 54°C hotplate test (not shown).
3.3. Effects of gonadal steroid hormone manipulations in female rats
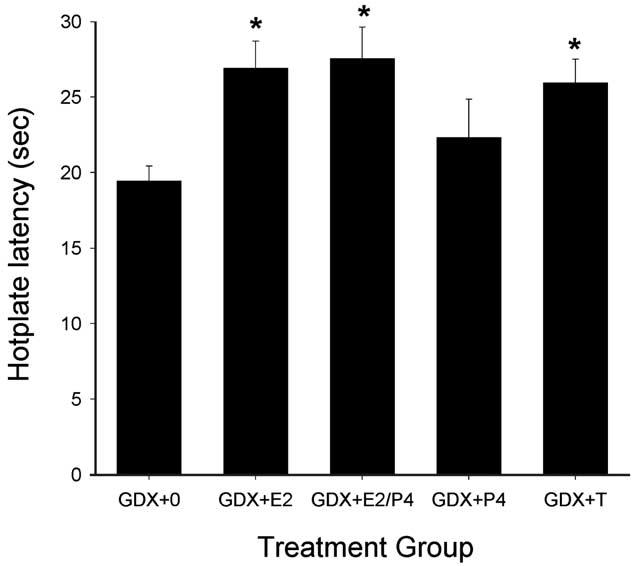
Gonadectomy in female rats significantly altered basal nociception and morphine's antinociceptive potency, but differences between the GDX + 0 and sham female groups were estrous stage-dependent. On the 50°C hotplate test, gonadectomy decreased hotplate latencies compared to sham females, but this difference was statistically significant only for GDX + 0 females compared to sham females tested during diestrus (P < 0.001) (compare Figs. 4 and 7). Gonadectomy also increased morphine's antinociceptive potency, but only when compared to sham females tested in estrus (P = 0.006) (Table 3). The latter group difference was also significant on the 54°C hotplate test (P = 0.033) (not shown).
Fig. 7.
GDX females: effects of steroid hormone replacement on basal nociception on the 50°C hotplate test. Each bar is the mean + 1 SEM of eight to eleven rats. For treatment group abbreviations see Table 2; only low dose E2 group is shown. *Significantly different from GDX + 0, P < 0.05, Dunnett's.
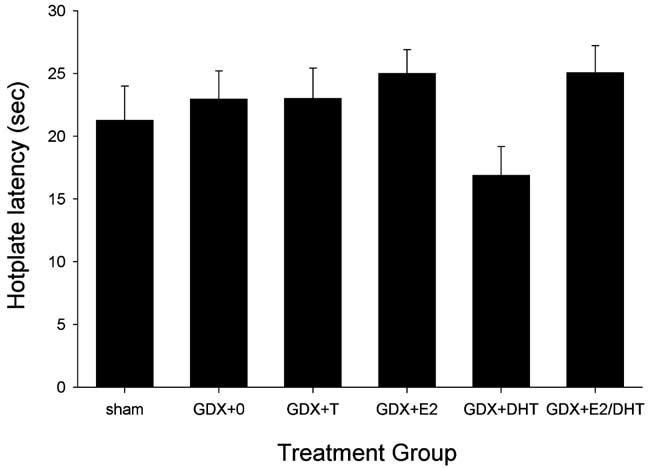
Fig. 7 shows that in GDX female rats, steroid hormone replacement significantly altered basal nociception on the 50°C hotplate test. GDX females with E2, E2/P4 or T replacement had significantly longer response latencies than GDX + 0 controls (P = 0.012, P = 0.009, P = 0.026, respectively). There were no group differences on the 54°C hotplate test (not shown).
Fig. 8 shows that on the 50°C hotplate test, steroid hormone replacement also influenced morphine's antinociceptive potency. Based on ED50 values derived from these morphine dose–effect curves (Table 3), morphine was significantly less potent in GDX + E2, GDX + E2/P4, and GDX + T females relative to GDX + 0 control females (P < 0.001, P = 0.002, P < 0.001, respectively), whereas morphine's potency did not differ between GDX + P4 and GDX + 0 females. Some group differences were significant on the 54°C hotplate as well: morphine was significantly less potent in GDX + E2 (P = 0.05) and GDX + E2/P4 (P = 0.016) but not in GDX + T (P = 0.069) females vs. GDX + 0 controls (not shown). Thus, morphine's antinociceptive potency was similar among GDX females with no steroid or only P4 replacement and sham females tested in diestrus or proestrus, whereas sham females tested in estrus, or GDX females replaced with E2, E2/P4 or T were considerably less sensitive to morphine (see Table 3).
Fig. 8.
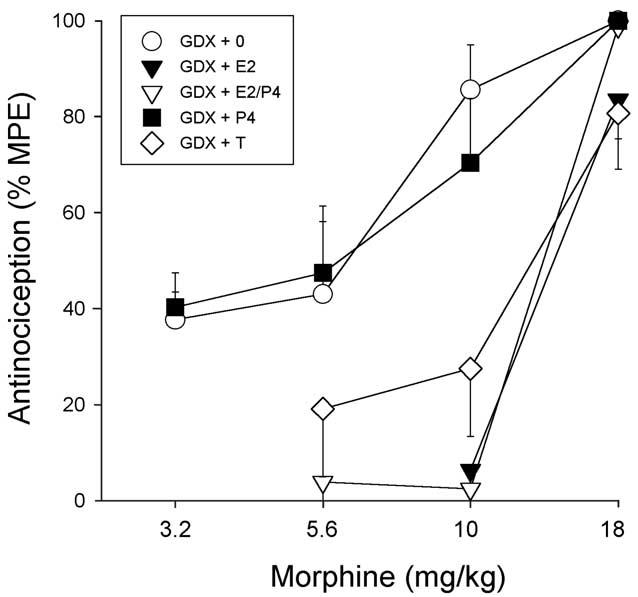
GDX females: effects of steroid hormone replacement on morphine antinociception on the 50°C hotplate test. Each point is the mean ± 1 SEM of eight to eleven rats. For treatment group abbreviations see Table 2; only low dose E2 group is shown.
Fig. 9 shows that gonadectomy and steroid replacement produced the expected changes in reproductive behavior and reproductive physiology in female rats. GDX + 0 and GDX + P4 females showed no lordosis behavior (top panel) and minimal uterine weights (bottom panel). Thus, GDX + 0 and GDX + P4 females resembled sham-diestrus females in their lack of reproductive behavior, although uterine weights were approximately 50% lower in GDX + 0 and GDX + P4 females than those in the sham-diestrus group (compare Figs. 6 and 9). GDX females replaced with E2 or E2/P4 showed robust lordosis behavior (Fig. 9, top panel) and large increases in uterine weight (Fig. 9, bottom panel) relative to GDX + 0 controls; these high values were comparable to those obtained in sham females tested during estrus (lordosis) or proestrus (uterine weight) (compare Figs. 6 and 9). Finally, Fig. 9 shows that GDX females replaced with T showed reproductive behavior and uterine weights that were intermediate to that of GDX + 0 and GDX + E2 females: T-replaced females showed lordosis behavior approximately half as often as did E2-replaced females, and had uterine weights that were slightly but not significantly greater than those in GDX + 0 females (in the range of sham-diestrus females). Lordosis behavior in T-replaced females was also accompanied by aggressive behavior towards the male (kicking), which was not observed in any other females.
Fig. 9.
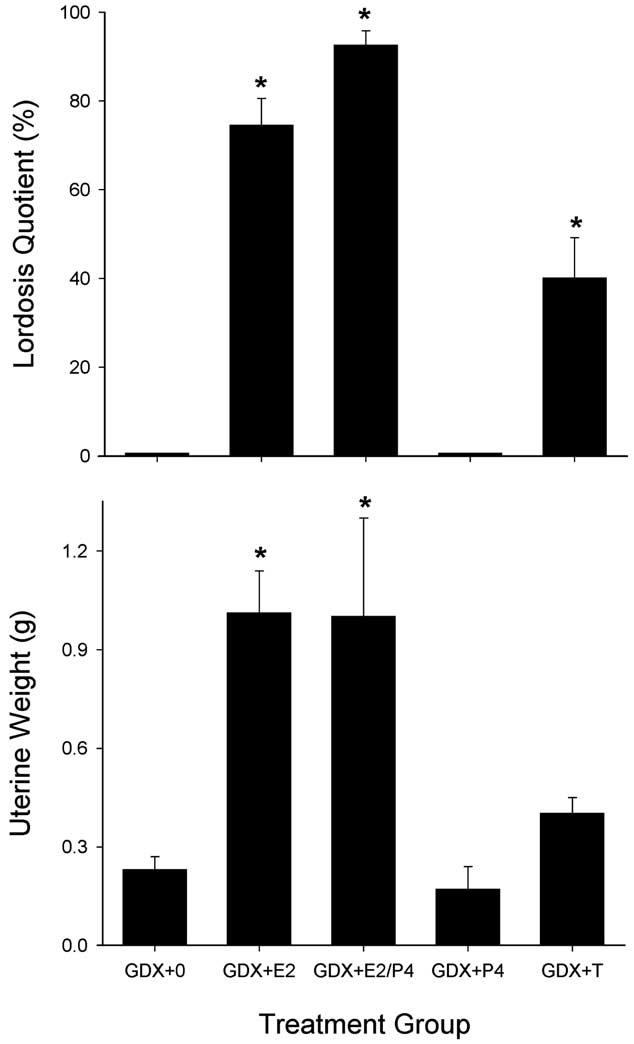
GDX females: effects of steroid hormone replacement on reproductive behavior (top panel) and physiology (bottom panel) in female rats. Top panel: lordosis quotient, the frequency of lordosis behavior shown by females when mounted by a male; each bar is the mean + 1 SEM of eight to eleven rats. Bottom panel: weight of the uterus, a steroid-sensitive organ; each bar is the mean + 1 SEM of eight to ten organs. For treatment group abbreviations see Table 2; only low dose E2 group is shown. *Significantly different from GDX + 0, P ≤ 0.05, Dunnett's.
Separate groups of GDX females replaced with a higher dose of E2 (5-mm capsule/rat rather than 1-mm capsule/rat), but given the same vehicle or P4 injections at 4-day intervals, were also tested. Results in GDX females replaced with the higher dose of E2 did not differ significantly from those in GDX females replaced with the lower dose of E2, on basal nociception, reproductive behavior or organ weight (data not shown). However, morphine's potency was slightly lower in GDX females replaced with the higher dose of E2 than in GDX females replaced with the lower dose of E2 (Table 3). Thus, the higher dose of E2 was less effective than the lower dose of E2 in decreasing morphine's potency relative to GDX + 0 controls, although ED50 values in high-dose-E2/P4-replaced females were still significantly higher than those in GDX + 0 controls.
3.4. Vaginal cytology
To provide additional information about relative gonadal steroid hormone states in female rats, vaginal cytology was assessed daily beginning on day 15 post-surgery until the day of nociceptive testing. Table 4 shows estimates of the percent time during this period spent in each phase of the estrous cycle in each of the female treatment groups. Sham females showed the expected estrous cyclicity, with approximately 14% of smear samples being proestrus, 24% proestrus–estrus or estrus, 42% diestrus-1, and 20% diestrus-2. In contrast, GDX + 0, GDX + P4 and GDX + T females showed no evidence of proestrus or estrus stages and GDX + E2 females appeared to be in proestrus/estrus or estrus most of the time. GDX females replaced with both E2 and P4 showed cycling that was most similar to that of sham females, although GDX + E2/P4 females given either ‘low’ or ‘high’ dose E2 spent more time in estrus and less time in diestrus than did sham females.
4. Discussion
The main finding of the present study is that gonadal steroid hormone manipulations that alter reproductive physiology and behavior also alter basal nociception in adult female rats, and morphine's antinociceptive potency in adult rats of both sexes. These findings demonstrate that reproductively meaningful changes in gonadal steroids modulate pain and opioid analgesia in adult rats.
4.1. Effects of gonadal steroid manipulations in males
The long-term (28-day) gonadectomy and steroid replacement procedures used in the present study produced expected changes in reproductive behavior and organ weights in male rats (Davidson, 1966; Damassa et al., 1977), indicating that the steroid manipulations were reproductively relevant. The failure of these gonadal steroid manipulations to significantly alter basal nociception in male rats is consistent with several previous studies (Beatty and Beatty, 1970; Kepler et al., 1989; Ali et al., 1995; Cicero et al., 1996; Krzanowska and Bodnar, 1999), although three studies reported decreased nociceptive thresholds in GDX male rats when compared to sham-operated controls (Marks and Hobbs, 1972; Forman et al., 1989; Pednekar and Mulgaonker, 1995), and this effect was reversed by T replacement in two cases (Forman et al., 1989; Pednekar and Mulgaonker, 1995).
In contrast to the null effect of gonadal steroid manipulations on basal nociception, morphine's antinociceptive potency was significantly decreased by gonadectomy in males. Furthermore, T replacement (at either 2- or 10-mm capsule/100 g body weight) restored morphine's potency to that seen in sham males. A previous study reported the same result (Rao and Saifi, 1985). In the only other study in which T was administered to adult GDX males – 30 min before testing – T did not restore morphine sensitivity (Chatterjee et al., 1982). Previous studies in which only gonadectomy was performed in adult male rats reported that sensitivity to morphine either increased (Ali et al., 1995; Chatterjee et al., 1982; Terner et al., 2002) or did not change (Kepler et al., 1989; Candido et al., 1992; Islam et al., 1993; Cicero et al., 1996, 2002; Krzanowska and Bodnar, 1999) relative to controls. Some of these studies varied considerably from the present study in their methodology (e.g. gonadectomy-test interval). T removal via gonadectomy does not completely eliminate reproductive behavior in males until 2–3 weeks later (Davidson, 1966; Madlafousek et al., 1976). Gonadal steroids, including T, are known to act primarily as transcription factors and thus may require hours to produce detectable changes in tissues (Wilson, 1996). Although it is possible that failure to observe effects of gonadectomy and/or T replacement in some previous studies can be attributed to using shorter-term gonadal steroid manipulations than those used in the present study, testing this hypothesis will require systematic examination of various gonadectomy-test intervals and durations of steroid replacement.
Chronic administration of either of T's major metabolites, E2 or DHT, to GDX males did not significantly increase morphine's antinociceptive potency compared to GDX + 0 controls, although the mean ED50 in the GDX males that received both E2 and DHT was not different from that in GDX + T males. As expected from previous work (Baum and Vreeburg, 1973; McDonald et al., 1970), long-term exposure to E2 but not DHT restored male reproductive behavior, and DHT but not E2 restored reproductive organ weights. These results indicate that the E2 and DHT replacement regimens used in the present study were physiologically relevant. The fact that E2 and DHT given together approximated the effect of T on morphine potency suggests that both of T's metabolites, and/or T itself, must be present to maintain sensitivity to morphine's antinociceptive effect in male rats. Administering a non-aromatizable androgen – or blocking T metabolism with an aromatase inhibitor – could be used to confirm whether the effects of T on morphine sensitivity are due to T itself, or to the combination of its major metabolites.
4.2. Effects of gonadal steroid fluctuations in intact (cycling) females
Nociceptive threshold on the 50°C hotplate test was significantly altered by estrous phase in sham females, suggesting that gonadal steroids modulate pain sensitivity in gonadally intact female rats. Nociceptive threshold was significantly higher in sham females tested during diestrus than in those tested during estrus. Previous studies also have demonstrated significant changes in nociceptive thresholds across the estrous cycle in rats: the stages at which threshold was highest were proestrus (Kepler et al., 1989; Molina et al., 1990), diestrus-2/proestrus (Martinez-Gomez et al., 1994), and diestrus-2 (Frye et al., 1993) using tailflick tests, and diestrus-1/diestrus-2 in paw and tail pressure tests (Kayser et al., 1996), and in hotplate and tail withdrawal tests (Craft and Bernal, 2001). Thus, there is some consistency among the present and previous studies in terms of which estrous stages females tend to be most and least sensitive to nociceptive stimuli, although there are some reports of no significant differences in nociceptive threshold among female rodents in different estrous stages (e.g. Ryan and Maier, 1988; Kepler et al., 1989; Mogil et al., 2000).
In the present study, sensitivity to morphine antinociception also differed among sham females tested at different stages of the estrous cycle: morphine was significantly less potent in estrus females than in proestrus or diestrus-1 females. A previous study also reported that female rats in estrus were less sensitive than females in diestrus to systemically administered morphine (Banerjee et al., 1983). Similarly, Ryan and Maier (1988) found that estrus females showed significantly less opioid receptor-mediated stress-induced analgesia compared to diestrus females. In contrast, there was no significant difference in the potency of intracerebroventricular (i.c.v.) morphine between female rats in estrus vs. diestrus (Kepler et al., 1989), or in the effect of a single dose of s.c. morphine in diestrus, estrus or proestrus CD-1 mice (Mogil et al., 2000). Finally, two studies reported that diestrus rats (Berglund and Simpkins, 1988) and Swiss–Webster mice (Mogil et al., 2000) were more sensitive than those tested in proestrus to the antinociceptive effects of morphine, which contrasts the present study in which proestrus and diestrus-1 females had similar sensitivity to morphine. However, it should be noted that in one of these previous studies, proestrus was divided into two stages: late (afternoon) proestrus rats were less sensitive to morphine than early (morning) proestrus rats (Berglund and Simpkins, 1988); it is likely that early vs. late proestrus in this study were comparable to our proestrus vs. proestrus/estrus categorizations, respectively – in which case the results between our study and this previous study may actually agree. Although we did not specifically test intact females during the proestrus/estrus stage (when cornified epithelial cells are evident but not yet predominant), the few we have inadvertently tested were similar, in terms of their sensitivity to morphine, to females that were completely in estrus. Thus, inconsistencies among studies involving cycling females could be due to how each stage is defined, the precise timing of nociceptive testing relative to determining stage of cycle, and perhaps whether testing was limited to 4-day cyclers. These aspects of the methods are not generally specified. In most studies, estrous phase is determined from vaginal cytology alone, and the nociceptive test may be completed up to several hours after the smear is obtained. In the present study, a second smear was obtained post-test, and data from any females that were not still in the same stage were omitted. The lordosis and uterine weight data confirm that reproductively, females characterized as proestrus, estrus and diestrus-1 in the present study were highly distinct from each other. The reproductive indices further suggest that despite the fact that we did not limit our sample to 4-day cyclers, females characterized as being in proestrus, estrus or diestrus-1 according to vaginal cytology alone appeared to be similar to actual 4-day cyclers, and our designation of proestrus is likely “early” rather than ‘late’ proestrus, similar to the ‘ProAM’ designation previously used by Berglund and Simpkins (1988).
4.3. Effects of gonadal steroid manipulations in females
In the present study, ovariectomy lowered nociceptive threshold relative to sham controls, and replacement with E2 (low or high dose), E2 + P4, or T, but not P4, increased thresholds significantly above those in GDX control females (at 50°C). These results are consistent with those of a previous study that employed a similar, long-term gonadectomy/steroid replacement regimen and hotplate test (Forman et al., 1989). It has been suggested that changes in basal nociception may be produced by ovarian steroid-dependent fluctuations in endogenous opioid levels (Romano et al., 1989; Dawson-Basoa and Gintzler, 1993; Aloisi et al., 1995). However, there are a number of studies reporting no significant change in basal nociception following ovariectomy (Beatty and Beatty, 1970; Nomikos et al., 1987; Martinez-Gomez et al., 1994; Ali et al., 1995; Cicero et al., 1996; Krzanowska and Bodnar, 1999). Systematic study will be required to determine how potentially relevant variables such as gonadectomy-test interval, steroid replacement regimen, subject genotype, and parameters of the pain test influence basal nociception in female rodents.
In the present study, gonadal steroid manipulations also affected morphine's antinociceptive potency in female rats. E2 (low dose), E2 (low or high dose) + P4, and T, but not P4 alone, significantly decreased morphine's potency relative to GDX controls without steroid replacement (at 50°C). As expected, E2, E2 + P4, and T, but not P4 alone, also increased reproductive behavior and uterine weight, although to varying extents. As shown previously in studies of reproductive behavior, E2 by itself almost fully restored lordosis behavior and uterine weight to that observed in sham-estrus females, and P4 by itself had no effect on lordosis (Davidson et al., 1968; Young, 1961). In contrast, T only partially restored lordosis, and slightly, but not significantly, increased uterine weight above that in GDX control females. The similar effects of T and E2 on basal nociception and morphine antinociception may be due to the aromatization of T to E2. Like male rats, females convert T to E2 via aromatase in the brain, though to a lesser extent than do males (Wagner and Morrell, 1996; Roselli and Resko, 1997). However, if the effects of T on basal nociception and morphine antinociception were actually due to E2, then the limited lordosis and uterine weights, and failure to show any proestrus or estrus stages based on vaginal cytology suggest that considerably less E2 is necessary to produce changes in nociception and morphine antinociception than is necessary to fully reinstate reproductive capabilities. As in males, it would be useful to repeat the T experiment in females using an aromatase inhibitor, to determine whether any of the effects of T are due to T itself, or solely to E2.
Several other studies have reported changes in morphine antinociception in female rats following gonadectomy that are relatively consistent with the findings in the present study (Kepler et al., 1989; Ali et al., 1995; Krzanowska and Bodnar, 1999). In contrast, other studies report decreased (Banerjee et al., 1983) or unchanged (Cicero et al., 1996, 2002) morphine sensitivity following gonadectomy in female rats. Differences across studies may be due to differences in the characterization of gonadally intact females. The present study, as well as several others (Kepler et al., 1989; Krzanowska and Bodnar, 1999; Krzanowska et al., 2002) report increases in morphine sensitivity primarily when comparing GDX females to intact females in estrus. Many studies do not differentiate between sham females in different stages of the estrous cycle (e.g. Ali et al., 1995; Cicero et al., 1996, 2002; Terner et al., 2002). Because levels of endogenous gonadal steroids (e.g. Feder, 1981) and both basal nociception and morphine antinociception have been shown to fluctuate with the estrous cycle (Banerjee et al., 1983; Ryan and Maier, 1988; Berglund and Simpkins, 1988; present study), conclusions about the effect of GDX in females may vary across studies due to the estrous stage the intact, comparison females are in at the time of testing. In the present study, there were significant differences in morphine sensitivity between GDX + 0 females and sham-estrus females, but not between GDX + 0 females and sham-diestrus or -proestrus females. Given that even 4- to 5-day cyclers are in diestrus approximately 60% of the time, and some females cycle at longer intervals (Freeman, 1988), random selection of intact females on the nociceptive test day would result in a majority of females being tested in diestrus. According to the present results, comparison of GDX females – or gonadally intact males – to a random selection of intact females may yield no significant difference. These results suggest that future research using intact females will require considerably more careful characterization of their hormonal state at the time of behavioral testing than has typically been done in the past (including by this laboratory).
Several previous studies have shown that E2 (but not P4, in most cases) changes sensitivity to opioid antinociception in GDX female rodents, although again, agreement among studies is not perfect (e.g. see Banerjee et al., 1983; Nomikos et al., 1987; Ryan and Maier, 1988; Ratka and Simpkins, 1991). The effect of T in adult female rodents has been examined in only one previous study: Banerjee et al. (1983) reported that an injection of T 2 h pre-test increased sensitivity to morphine antinociception – opposite to the result obtained in the present study, in which 28-day T exposure was used. As noted above, there is some evidence that factors such as duration of steroid treatment and interval between steroid treatment and nociceptive testing may be important; moreover, steroid effects on nociception and morphine antinociception may be dose-dependent (Nomikos et al., 1987; Ratka and Simpkins, 1991). Short-term T treatment could produce different levels of E2 and DHT than those produced by long-term T exposure. In the present study, the low E2 dose was slightly more effective than the high E2 dose in decreasing morphine sensitivity. In future studies we plan to examine additional doses of E2 and T that are closer to the threshold for reinstating reproductive behavior and organ weight, to determine the lower limit at which opioid sensitivity is altered.
4.4. Comparison of intact cycling females with steroid-replaced females
Although gonadectomy and steroid replacement are frequently used to examine the role of gonadal steroids in nociception and antinociception, it is important to note that steroid replacement does not actually mimic the hormonal milieu of the intact female. Thus, the roles of various steroid hormones deduced from studies of steroid-replaced females do not necessarily tell us what these hormones do in a normal, gonadally intact female whose steroid levels are constantly changing. Although there were some similarities in results between intact cycling females and steroid-replaced females in the present study, there were also some differences. For example, GDX females replaced with E2 chronically and P4 on a 4-day cycle should have been similar to sham females – and they were, in terms of vaginal cytology, lordosis behavior (similar to sham-estrus), uterine weight (similar to sham-proestrus), and morphine sensitivity (similar to sham-estrus). Therefore the role of E2 in morphine sensitivity appears to be consistent between the steroid-replaced and intact females: GDX rats that were given E2 chronically, and sham females tested during estrus – approximately 24 h after E2 peaks (Feder, 1981) – were the least sensitive to morphine. It has been shown previously that even a single injection of E2 in GDX females decreases opioid sensitivity, with peak effects at approximately 1–2 days post-injection (Ryan and Maier, 1988). These results suggest that E2's effect on systems mediating sensitivity to morphine antinociception is a slow (presumably genomic) process that takes some hours to manifest, and subsides some hours thereafter if E2 declines, as in acutely injected rats or those that are normally cycling. In contrast, it cannot readily be concluded from the present study that E2 in intact females increases pain threshold: although hotplate baseline latencies were highest in E2 (and T)-replaced GDX females, it was sham females tested during diestrus-1 – when plasma E2 has been relatively low for at least 48 h – that had the highest baselines among the intact females. Taken together, results of the present and previous studies suggest that although steroid replacement experiments are useful for characterizing the effects of single steroids on nociception and opioid antinociception in females, the results may not agree with those obtained in intact cycling females if the duration/chronicity of treatment and/or the interval between steroid treatment and nociceptive testing are not taken into account.
4.5. Comparison of gonadal steroid effects in males vs. females
The present study demonstrates that T and E2 significantly affect morphine's antinociceptive potency; however, these steroids had different effects in male vs. female rats. In males, T increased morphine sensitivity and E2 had no effect, whereas in females, both E2 and T decreased morphine sensitivity. As mentioned previously, the similarity between the effects of T and E2 replacement on morphine sensitivity in female rats suggests that in females, T may be changing morphine sensitivity via its metabolite E2. The fact that E2 had different effects on morphine sensitivity in adult male and female rats suggests that the mechanism by which E2 affects opioid systems is already organized by the time rats reach maturity, presumably by neonatal, sexually dimorphic gonadal steroid exposure. In fact, Cicero et al. (2002) recently demonstrated that neonatal treatment of female rats with T or neonatal gonadectomy of males altered their sensitivity to systemically administered morphine as adults: neonatally androgenized females were significantly more sensitive to morphine (like adult males) and neonatally gonadectomized males were significantly less sensitive to morphine (like adult females) compared to their respective, same-sex controls. Similar organizational effects of gonadal steroids were observed when morphine was administered to the ventrolateral periaqueductal gray (PAG) (Krzanowska et al., 2002). These findings plus the differential effects of E2 and T in adult males vs. females in the present study demonstrate an important organizational role for gonadal steroids in opioid sensitivity. It should be noted that the doses of T and E2 that we used in males were generally higher than what we used in females, so the possibility of a dose-dependent explanation for the apparent sexual dimorphism in the effects of T and E2 cannot be discounted.
4.6. Effect of nociceptive stimulus intensity
Previous studies have shown that the intensity of the noxious stimulus used in the pain test can influence the magnitude of sex differences in opioid antinociception (Negus and Mello, 1999; Cook et al., 2000; Craft and Bernal, 2001; Barrett et al., 2002), and in nociceptive thresholds (e.g. Aloisi et al., 1995). In the present study, group differences in basal nociception and morphine antinociception observed on the 50°C hotplate test were generally smaller and non-significant on the 54°C hotplate test. These results suggest that intensity of the noxious stimulus used in the pain test may also influence the conclusions drawn from studies examining gonadal steroid modulation of nociception and opioid antinociception. A significant limitation of the present study is the potential confound of order in which the two stimulus intensities were tested: rats were always tested on the lower stimulus intensity first. However, it should be noted that another recent study in rodents – using a counter-balanced presentation of stimulus intensities – also demonstrated that the effects of gonadal steroid manipulations on opioid analgesia may be stimulus intensity-dependent (Terner et al., 2002).
4.7. Mechanisms of steroid modulation of nociception and opioid antinociception
The mechanisms underlying hormone-related changes in basal nociception and morphine antinociception are not fully understood. Brain areas such as the PAG, which are involved in descending pain inhibition and morphine antinociception, contain androgen receptors (Murphy et al., 1999) and estrogen (alpha) receptors (Murphy et al., 1999; VanderHorst et al., 1998). In the lumbosacral spinal cord, estrogen receptors have also been described in the small cells of lamina I, II, V and VII (VanderHorst et al., 1997) and virtually all motor neurons contain androgen receptors (Matsumoto, 1997). The areas of the PAG and spinal cord that express gonadal steroid hormone receptors closely overlap the areas known to contain opioid receptors (Murphy et al., 1999; VanderHorst et al., 1997). These studies suggest possible sites in the central nervous system, specifically in the descending pain inhibitory pathway, where gonadal steroids could modulate basal nociception as well as opioid antinociception.
Gonadal steroids have been shown to influence opioidergic systems in several ways. For example, E2 has been shown to increase spinal cord enkephalin mRNA (Amandusson et al., 1999) and hypothalamic pre-proenkephalin gene expression (Zhu et al., 2001) in ovariectomized female rats; E2 also differentially regulates hypothalamic pre-proenkephalin mRNA in male vs. female rats (Segarra et al., 1998). Kappa opioid receptor density in lumbosacral spinal cord has been observed to change across the estrous cycle in female rats (Chang et al., 2000). In hypothalamic neurons, E2 has been shown to attenuate the ability of the mu opioid receptor to activate intracellular signaling pathways (Kelly et al., 1999). All of these mechanisms may contribute to the behavioral observation of sex-dependent gonadal steroid modulation of pain and analgesia. Finally, there is some evidence to suggest that gonadal steroids modulate metabolism of morphine by the liver (South et al., 2001; Baker and Ratka, 2002). Thus, both pharmacodynamic and pharmacokinetic explanations must be considered.
5. Conclusions
The present study demonstrates that gonadal steroid hormones, manipulated in ways that are reproductively relevant, significantly influence adult rats' sensitivity to pain and opioid analgesia. The specific effects of each steroid agree with some but not all previous studies, suggesting that procedural or subject variables such as steroid manipulation regimen, type/intensity of pain, and subject genotype may be important modulators of steroid effect. Gonadal steroids also modulate stress-induced analgesia in adult male and female rodents (Romero et al., 1987; Ryan and Maier, 1988; Mogil et al., 1993), and pregnancy (or comparable ovarian steroid elevations) alter females' analgesic state (Gintzler and Liu, 2000). Taken together, these data indicate that reproductive state and analgesic state – either drug- or environmentally induced – are closely tied and commonly controlled by gonadal steroid hormones.
Acknowledgements
This work was supported by DA10284 from the National Institute on Drug Abuse, and by funds provided for medical and biological research by the State of Washington Initiative Measure No. 171.
References
- Ali BH, Sharif SI, Elkadi A. Sex differences and the effect of gonadectomy on morphine-induced antinociception and dependence in rats and mice. Clin Exp Pharmacol Physiol. 1995;22:342–344. doi: 10.1111/j.1440-1681.1995.tb02012.x. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Sacerdote P, Albonetti ME, Carli G. Sex-related effects on behaviour and -endorphin of different intensities of formalin pain in rats. Brain Res. 1995;699:242–249. doi: 10.1016/0006-8993(95)00912-a. [DOI] [PubMed] [Google Scholar]
- Alonso R, Lopez-Coviella I. Gonadal steroids and neuronal function. Neurochem Res. 1998;23(5):675–688. doi: 10.1023/a:1022442922931. [DOI] [PubMed] [Google Scholar]
- Amandusson Å, Hallbeck M, Hallbeck A-L, Hermanson O, Blomqvist A. Estrogen-induced alterations of spinal cord enkephalin gene expression. Pain. 1999;83:243–248. doi: 10.1016/s0304-3959(99)00109-8. [DOI] [PubMed] [Google Scholar]
- Baamonde AI, Hidalgo A, Andres-Trelles F. Sex-related differences in the effects of morphine and stress on visceral pain. Neuropharmacology. 1989;28(9):967–970. doi: 10.1016/0028-3908(89)90197-4. [DOI] [PubMed] [Google Scholar]
- Baker L, Ratka A. Sex-specific differences in levels of morphine, morphine-3-glucuronide, and morphine analgesia in rats. Pain. 2002;95:65–74. doi: 10.1016/s0304-3959(01)00376-1. [DOI] [PubMed] [Google Scholar]
- Banerjee P, Chatterjee TK, Ghosh JJ. Ovarian steroids and modulation of morphine-induced analgesia and catalepsy in female rats. Eur J Pharmacol. 1983;96:291–294. doi: 10.1016/0014-2999(83)90319-9. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Cook CD, Terner JM, Roach EL, Syvanthong C, Picker MJ. Sex and rat strain determine sensitivity to opioid-induced antinociception. Psychopharmacology. 2002;160:170–181. doi: 10.1007/s00213-001-0949-2. [DOI] [PubMed] [Google Scholar]
- Bartok RE, Craft RM. Sex differences in opioid antinociception. J Pharmacol Exp Ther. 1997;282(2):769–778. [PubMed] [Google Scholar]
- Baum MJ, Vreeburg JTM. Copulation in castrated male rats following combined treatment with estradiol and dihydrotestosterone. Science. 1973;182:283–285. doi: 10.1126/science.182.4109.283. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Beatty PA. Hormonal determinants of sex differences in avoidance behavior and reactivity to electric shock in the rat. J Comp Physiol Psychol. 1970;73(3):446–455. doi: 10.1037/h0030216. [DOI] [PubMed] [Google Scholar]
- Berglund LA, Derendorf H, Simpkins JW. Desensitization of brain opiate receptor mechanisms by gonadal steroid treatments that stimulate luteinizing hormone secretion. Endocrinology. 1988;122(6):2718–2726. doi: 10.1210/endo-122-6-2718. [DOI] [PubMed] [Google Scholar]
- Berglund LA, Simpkins JW. Alterations in brain opiate receptor mechanisms on proestrous afternoon. Neuroendocrinology. 1988;48:394–400. doi: 10.1159/000125040. [DOI] [PubMed] [Google Scholar]
- Candido J, Lutfy K, Billings B, Sierra V, Duttaroy A, Intrurrisi CE, Yoburn BC. Effect of adrenal and sex hormones on opioid analgesia and opioid receptor regulation. Pharmacol Biochem Behav. 1992;42:685–692. doi: 10.1016/0091-3057(92)90015-8. [DOI] [PubMed] [Google Scholar]
- Chang PC, Aicher SA, Drake CT. Kappa opioid receptors in spinal cord vary across the estrous cycle. Brain Res. 2000;861:168–172. doi: 10.1016/s0006-8993(99)02461-0. [DOI] [PubMed] [Google Scholar]
- Chatterjee TK, Das S, Banerjee P, Ghosh JJ. Possible physiological role of adrenal and gonadal steroids in morphine analgesia. Eur J Pharmacol. 1982;77:119–121. doi: 10.1016/0014-2999(82)90005-x. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER. Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther. 1996;279(2):767–773. [PubMed] [Google Scholar]
- Cicero TJ, Nock B, O'Connor L, Meyer ER. Role of steroids in sex differences in morphine-induced analgesia: activational and organizational effects. J Pharmacol Exp Ther. 2002;300:695–701. doi: 10.1124/jpet.300.2.695. [DOI] [PubMed] [Google Scholar]
- Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ. Sex-related differences in the antinociceptive effects of opioids: importance of rat genotype, nociceptive stimulus intensity, and efficacy at the opioid receptor. Psychopharmacology. 2000;150:430–442. doi: 10.1007/s002130000453. [DOI] [PubMed] [Google Scholar]
- Craft RM, Bernal SA. Sex differences in opioid antinociception: kappa and mixed action agonists. Drug Alcohol Depend. 2001;63:215–228. doi: 10.1016/s0376-8716(00)00209-x. [DOI] [PubMed] [Google Scholar]
- Craft RM, Stratmann JA, Bartok RE, Walpole TI, King SJ. Sex differences in development of morphine tolerance and dependence in the rat. Psychopharmacology. 1999;143:1–7. doi: 10.1007/s002130050911. [DOI] [PubMed] [Google Scholar]
- Damassa DA, Smith ER, Tennent B, Davidson JM. The relationship between circulating testosterone levels and male sexual behavior in rats. Horm Behav. 1977;8:275–286. doi: 10.1016/0018-506x(77)90002-2. [DOI] [PubMed] [Google Scholar]
- Davidson JM. Characteristics of sex behavior in male rats following castration. Anim Behav. 1966;14:266–272. doi: 10.1016/s0003-3472(66)80082-9. [DOI] [PubMed] [Google Scholar]
- Davidson JM, Rodgers CH, Smith ER, Bloch GJ. Stimulation of female sex behavior in adrenalectomized rats with estrogen alone. Endocrinology. 1968;82:193–195. doi: 10.1210/endo-82-1-193. [DOI] [PubMed] [Google Scholar]
- Dawson-Basoa MB, Gintzler AR. 17β-Estradiol and progesterone modulate an intrinsic opioid analgesic system. Brain Res. 1993;601:241–245. doi: 10.1016/0006-8993(93)91716-6. [DOI] [PubMed] [Google Scholar]
- Fadem BH, Barfield RJ, Whalen RE. Dose–response and time–response relationships between progesterone and the display of patterns of receptive and proceptive behavior in the female rat. Horm Behav. 1979;13:40–48. doi: 10.1016/0018-506x(79)90033-3. [DOI] [PubMed] [Google Scholar]
- Feder HH. Estrous cyclicity in mammals. In: Adler NT, editor. Neuroendocrinology of reproduction. Plenum Press; New York, NY: 1981. pp. 279–348. [Google Scholar]
- Forman LJ, Tingle V, Estilow S, Cater J. The response to analgesia testing is affected by gonadal steroids in the rat. Life Sci. 1989;45:447–454. doi: 10.1016/0024-3205(89)90631-0. [DOI] [PubMed] [Google Scholar]
- Freeman ME. The ovarian cycle of the rat. In: Knobil E, Neill J, editors. The physiology of reproduction. Raven Press; New York, NY: 1988. pp. 1893–1928. [Google Scholar]
- Frye CA, Cuevas CA, Kanarek RB. Diet and estrous cycle influence pain sensitivity in rats. Pharmacol Biochem Behav. 1993;45:255–260. doi: 10.1016/0091-3057(93)90116-b. [DOI] [PubMed] [Google Scholar]
- Gear RW, Gordon NC, Heller PH, Paul S, Miaskowski C, Levine JD. Gender difference in analgesic response to the kappa-opioid pentazocine. Neurosci Lett. 1996a;205:207–209. doi: 10.1016/0304-3940(96)12402-2. [DOI] [PubMed] [Google Scholar]
- Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med. 1996b;2(11):1248–1249. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. The kappa opioid nalbuphine produces gender- and dose-dependent analgesia and antianalgesia in patients with postoperative pain. Pain. 1999;83:339–345. doi: 10.1016/s0304-3959(99)00119-0. [DOI] [PubMed] [Google Scholar]
- Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Action of naloxone on gender-dependent analgesic and antianalgesic effects of nalbuphine in humans. J Pain. 2000;1(2):122–127. [Google Scholar]
- Gintzler AR, Liu N-J. Ovarian sex steroids activate antinociceptive systems and reveal gender-specific mechanisms. In: Fillingim RB, editor. Sex, gender, and pain. IASP Press; Seattle, WA: 2000. [Google Scholar]
- Islam AK, Cooper ML, Bodnar RJ. Interactions among aging, gender, and gonadectomy effects upon morphine antinociception in rats. Physiol Behav. 1993;54:45–53. doi: 10.1016/0031-9384(93)90042-e. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Innes DGL. Sex and day–night differences in opiate-induced responses of insular wild deer mice, Peromyscus maniculatus triangularis. Pharmacol Biochem Behav. 1987;27:477–482. doi: 10.1016/0091-3057(87)90351-0. [DOI] [PubMed] [Google Scholar]
- Kayser V, Berkley KJ, Keita H, Gautron M, Guilbaud G. Estrous and sex variations in vocalization thresholds to hindpaw and tail pressure stimulation in the rat. Brain Res. 1996;742:352–354. doi: 10.1016/s0006-8993(96)01108-0. [DOI] [PubMed] [Google Scholar]
- Keating RJ, Tcholakian RK. In vivo patterns of circulating testosterone following castration and intramuscular testosterone proprionate injections of adult male rats. Steroids. 1983;42(1):63–76. doi: 10.1016/s0039-128x(83)90096-x. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Lagrange AH, Wagner EJ, Rønnekleiv OK. Rapid effects of estrogen to modulate G protein-coupled receptors via activation of protein kinase A and protein kinase C pathways. Steroids. 1999;64:64–75. doi: 10.1016/s0039-128x(98)00095-6. [DOI] [PubMed] [Google Scholar]
- Kepler KL, Kest B, Kiefel JM, Cooper ML, Bodnar RJ. Roles of gender, gonadectomy and estrous phase in the analgesic effects of intracerebroventricular morphine in rats. Pharmacol Biochem Behav. 1989;34:119–127. doi: 10.1016/0091-3057(89)90363-8. [DOI] [PubMed] [Google Scholar]
- Kepler KL, Standifer KM, Paul D, Kest B, Pasternak GW, Bodnar RJ. Gender effects and central opioid analgesia. Pain. 1991;45:87–94. doi: 10.1016/0304-3959(91)90168-W. [DOI] [PubMed] [Google Scholar]
- Kest B, Wilson SG, Mogil JS. Sex differences in supraspinal morphine analgesia are dependent on genotype. J Pharmacol Exp Ther. 1999;289:1370–1375. [PubMed] [Google Scholar]
- Krzanowska EK, Bodnar RJ. Morphine antinociception elicited from the ventrolateral periaqueductal gray is sensitive to sex and gonadectomy differences in rats. Brain Res. 1999;821:224–230. doi: 10.1016/s0006-8993(98)01364-x. [DOI] [PubMed] [Google Scholar]
- Krzanowska EK, Ogawa S, Pfaff DW, Bodnar RJ. Reversal of sex differences in morphine analgesia elicited from the ventrolateral periaqueductal gray in rats by neonatal hormone manipulations. Brain Res. 2002;929:1–9. doi: 10.1016/s0006-8993(01)03350-9. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ. Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- Lipa SM, Kavaliers M. Sex differences in the inhibitory effects of the NMDA antagonist, MK-801, on morphine and stress-induced analgesia. Brain Res Bull. 1990;24:627–630. doi: 10.1016/0361-9230(90)90169-z. [DOI] [PubMed] [Google Scholar]
- Madlafousek J, Hlinak Z, Beran J. Decline of sexual behavior in castrated male rats: effects of female precopulatory behavior. Horm Behav. 1976;7:245–252. doi: 10.1016/0018-506x(76)90051-9. [DOI] [PubMed] [Google Scholar]
- Marks HE, Hobbs SH. Changes in stimulus reactivity following gonadectomy in male and female rats of different ages. Physiol Behav. 1972;8:1113–1119. doi: 10.1016/0031-9384(72)90206-5. [DOI] [PubMed] [Google Scholar]
- Martinez-Gomez M, Cruz Y, Salas M, Hudson R, Pacheco P. Assessing pain threshold in the rat: changes with estrus and time of day. Physiol Behav. 1994;55:651–657. doi: 10.1016/0031-9384(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Martini L, Melcangi RC, Maggi R. Androgen and progesterone metabolism in the central and peripheral nervous system. J Steroid Biochem Mol Biol. 1993;47:195–205. doi: 10.1016/0960-0760(93)90075-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto A. Hormonally induced neuronal plasticity in the adult moto-neurons. Brain Res Bull. 1997;44:539–547. doi: 10.1016/s0361-9230(97)00240-2. [DOI] [PubMed] [Google Scholar]
- McDonald P, Beyer C, Newton F, Brien B, Baker R, Tan HS, Sampsom C, Kitching P, Greenhill R, Pritchard D. Failure of 5-dihydrotestosterone to initiate sexual behaviour in the castrated male rat. Nature. 1970;227:964–965. doi: 10.1038/227964a0. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev. 2000;24:375–389. doi: 10.1016/s0149-7634(00)00015-4. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Sternberg WF, Kest B, Marek P, Liebeskind JC. Sex differences in the antagonism of swim stress-induced analgesia: effects of gonadectomy and estrogen replacement. Pain. 1993;53:17–25. doi: 10.1016/0304-3959(93)90050-Y. [DOI] [PubMed] [Google Scholar]
- Molina N, Bedran-De-Castro MTB, Bedran-De-Castro JC. The role of opioids in the analgesic response of rats during the estrous cycle. Braz J Med Biol Res. 1990;23:1157–1159. [PubMed] [Google Scholar]
- Moore FL, Evans SJ. Steroid hormones use non-genomic mechanisms to control brain functions and behaviors: a review of evidence. Brain Behav Evol. 1999;54:41–50. doi: 10.1159/000006610. [DOI] [PubMed] [Google Scholar]
- Morgan D, Cook CD, Smith MA, Picker MJ. An examination of the interactions between the antinociceptive effects of morphine and various μ-opioids: the role of intrinsic efficacy and stimulus intensity. Anesth Analg. 1999;88:407–413. doi: 10.1097/00000539-199902000-00035. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Shupnik MA, Hoffman GE. Androgen and estrogen (α) receptor distribution in the periaqueductal gray of the male rat. Horm Behav. 1999;36:98–108. doi: 10.1006/hbeh.1999.1528. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Opioid antinociception in ovariectomized monkeys: comparison with antinociception in males and effects of estradiol replacement. J Pharmacol Exp Ther. 1999;290:1132–1140. [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of gonadal steroid hormone treatment on opioid antinociception in ovarietomized rhesus monkeys. Psychopharmacology. 2002;159:275–283. doi: 10.1007/s002130100912. [DOI] [PubMed] [Google Scholar]
- Nomikos G, Spyraki C, Kazandijan A, Sfikakis A. Estrogen treatment to ovariectomized rats modifies morphine-induced behavior. Pharmacol Biochem Behav. 1987;27:611–617. doi: 10.1016/0091-3057(87)90182-1. [DOI] [PubMed] [Google Scholar]
- Pednekar JR, Mulgaonker VK. Role of testosterone on pain threshold in rats. Indian J Physiol Pharmacol. 1995;39:423–424. [PubMed] [Google Scholar]
- Pfeiffer A, Herz A. Endocrine actions of opioids. Horm Metab Res. 1984;16:386–397. doi: 10.1055/s-2007-1014801. [DOI] [PubMed] [Google Scholar]
- Rao SS, Saifi AQ. Influence of testosterone on morphine analgesia in albino rats. Indian J Physiol Pharmacol. 1985;29:103–106. [PubMed] [Google Scholar]
- Ratka A, Simpkins JW. Effects of estradiol and progesterone on the sensitivity to pain and on morphine-induced antinociception in female rats. Horm Behav. 1991;25:217–228. doi: 10.1016/0018-506x(91)90052-j. [DOI] [PubMed] [Google Scholar]
- Riley JL, Robinson ME, Wise EA, Price DD. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81:225–235. doi: 10.1016/S0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- Romano GJ, Mobbs CV, Howels RD, Pfaff DW. Estrogen regulation of proenkephalin gene expression in the ventromedial hypothalamus of the rat: temporal qualities and synergism with progesterone. Mol Brain Res. 1989;5:51–58. doi: 10.1016/0169-328x(89)90017-x. [DOI] [PubMed] [Google Scholar]
- Romero M-T, Kepler KL, Cooper ML, Komisaruk BR, Bodnar RJ. Modulation of gender-specific effects upon swim analgesia in gonadectomized rats. Physiol Behav. 1987;40:39–45. doi: 10.1016/0031-9384(87)90183-1. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA. Sex differences in androgen-regulated expression of cytochrome P450 aromatase in the rat brain. J Steroid Biochem Mol Biol. 1997;61(3–6):365–374. [PubMed] [Google Scholar]
- Roy AK, Chatterjee B. Androgen action. Crit Rev Eukaryot Gene Expr. 1995;5(2):157–176. doi: 10.1615/critreveukargeneexpr.v5.i2.30. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Maier SF. The estrous cycle and estrogen modulate stress-induced analgesia. Behav Neurosci. 1988;102:371–380. doi: 10.1037//0735-7044.102.3.371. [DOI] [PubMed] [Google Scholar]
- Sarton E, Olofsen E, Romberg R, den Hartigh J, Kest B, Nieuwenhuijs D, Burm A, Peppema L, Dahan A. Sex differences in morphine analgesia. Anesthesiology. 2000;93:1245–1254. doi: 10.1097/00000542-200011000-00018. [DOI] [PubMed] [Google Scholar]
- Segarra AC, Acosta AM, Gonzalez JL, Angulo JA, McEwen BS. Sex differences in estrogenic regulation of preproenkephalin mRNA levels in the medial preoptic area of prepubertal rats. Mol Brain Res. 1998;60:133–139. doi: 10.1016/s0169-328x(98)00160-0. [DOI] [PubMed] [Google Scholar]
- Smith ER, Damassa EA, Davidson JM. Hormone administration: peripheral and intracranial implants. In: Myers RD, editor. Methods in psychobiology. Vol. 3. Academic Press; New York, NY: 1977. pp. 259–279. [Google Scholar]
- South SM, Wright AWE, Lau M, Mather LE, Smith MT. Sex-related differences in antinociception and tolerance development following chronic intravenous infusion of morphine in the rat: modulatory role of testosterone via morphine clearance. J Pharmacol Exp Ther. 2001;297:446–457. [PubMed] [Google Scholar]
- Terner JM, Barrett AC, Grossell E, Picker MJ. Influence of gonadectomy on the antinociceptive effects of opioids in male and female rats. Psycho-pharmacology. 2002;163:183–193. doi: 10.1007/s00213-002-1143-x. [DOI] [PubMed] [Google Scholar]
- VanderHorst VGJM, Meijer E, Schasfoort FC, Leeuwen FWV, Holstege G. Estrogen receptor-immunoreactive neurons in the lumbosacral cord projecting to the periaqueductal gray in the ovariectomized female cat. Neurosci Lett. 1997;236:25–28. doi: 10.1016/s0304-3940(97)00750-7. [DOI] [PubMed] [Google Scholar]
- VanderHorst VGJM, Schasfoort FC, Meijer E, Leeuwen FWV, Holstege G. Estrogen receptor-alpha-immunoreactive neurons in the periaqueductal gray of the adult ovariectomized female cat. Neurosci Lett. 1998;240:13–16. doi: 10.1016/s0304-3940(97)00900-2. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Morrell JI. Distribution and steroid hormone regulation of aromatase mRNA expression in the forebrain of adult male and female rats: a cellular-level analysis using in situ hybridization. J Comp Neurol. 1996;370:71–84. doi: 10.1002/(SICI)1096-9861(19960617)370:1<71::AID-CNE7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Williams CL, Stancel GM. Estrogens and progestins. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman and Gilman's the pharmacological basis of therapeutics. 9th McGraw-Hill; New York, NY: 1996. pp. 1411–1440. [Google Scholar]
- Wilson JD. Androgens. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman and Gilman's the pharmacological basis of therapeutics. 9th McGraw-Hill; New York, NY: 1996. pp. 1441–1457. [Google Scholar]
- Young WC. Hormones and mating behavior. In: Young WC, editor. Sex and internal secretions. Vol. 2. Waverly Press; Baltimore, MD: 1961. pp. 1173–1239. [Google Scholar]
- Zhu Y-S, Cai L-Q, You X, Duan Y, Imperato-McGinley J, Chin W, Pfaff DW. Molecular analysis of estrogen induction of preproenkephalin gene expression and its modulation by thyroid hormones. Mol Brain Res. 2001;91:23–33. doi: 10.1016/s0169-328x(01)00109-7. [DOI] [PubMed] [Google Scholar]