Abstract
Recent studies by our group and others have demonstrated that growth hormone (GH) is produced endogenously within the hippocampal formation, a brain structure associated with learning and aspects of emotional experience. Here, we demonstrate that this endogenously produced GH is modulated by age and sex differences and the presence of estrogen. GH mRNA levels were higher in females than males, especially during proestrus, a stage of estrus when estrogen levels are elevated. Moreover, GH expression was increased in ovariectomized females that were treated with estradiol. This increase in GH mRNA in response to estrogen was followed by the appearance of GH protein and was negatively correlated with the expression levels of insulin-like growth factor-I mRNA, suggesting a feedback relationship between insulin-like growth factor-I and GH in the brain. GH mRNA levels were also elevated in primary neuronal cultures exposed to 17-β-estradiol in vitro, further confirming the direct influence of estrogen on GH expression. Finally, exposure to an acute stressful event increased the expression and production of GH in both males and females. However, the stress-induced increase of GH in females depended on the stage of the estrous cycle in which they were exposed to the stressful event. Together, these data further demonstrate that GH is endogenously produced in the adult hippocampal formation, where it is regulated by age, estrogen, and exposure to environmental stimuli. These results suggest that GH may be involved in functions ascribed to the hippocampus, such as learning and the response to stressful experience.
Keywords: estrogen, growth factor, learning, somatotropin, insulin
Growth hormone (GH) is a 19- to 21-kDa cytokine polypeptide that is produced primarily in the anterior pituitary, where it is most often associated with postnatal longitudinal growth in target tissues, including liver, muscle, adipose, and bone and cellular metabolism (1). GH exerts its effects in these tissues either directly or indirectly through its downstream effector, insulin-like growth factor-I (IGF-I). Recent studies suggest that the GH/IGF-I axis may also play an important role in central nervous system functions, including those associated with neuronal growth, development, and protection (2, 3). Furthermore, several studies suggest the GH/IGF axis may play a role in influencing aspects of mood and cognition (4, 5). GH-binding sites have been identified in several areas of the brain, including the choroid plexus, putamen, thalamus, pituitary, hippocampus, and cortex (6), suggesting the presence of GH receptors (GHRs) in these tissues. Furthermore, studies by our group and others have provided evidence for endogenous expression of GH in the hippocampus (7, 8). Additionally, receptors for IGF-I, a known mediator of GH signaling, are prominent in the hippocampus and parahippocampal regions and are detectable in the amygdala, cerebellum, and cortex, as are mRNAs for these receptors (9). Several studies have established a role for IGF-I in neuronal development and growth (10) and neurogenesis in the adult hippocampus (11–13).
In a previous study, we used transcriptional profiling to identify genes that were associated with learning. Surprisingly, we found that acquisition of a hippocampal-dependent learning task increased the expression of GH in the male hippocampus (8). This increase occurred in animals that had learned, whereas naïve animals and those exposed to other types of training experiences expressed very low or undetectable levels of GH. These results were unexpected because endogenous expression of GH had not yet been localized to the hippocampus.
GH is also associated with sexual maturation because levels increase during puberty and decrease with age in both men and women (14, 15). Moreover, the sex hormone estrogen stimulates the release of GH, which in turn can induce ovulation (15, 16). Given the known effects of GH on sexual development, we characterized the expression of endogenous GH in the hippocampus as a function of age and sex. First, we tested whether GH expression changed as animals reached sexual maturity and whether there were sex differences and corresponding changes across the estrous cycle of female rats. The effects of ovariectomy (OVX) and replacement with estrogen in vivo and the effects of estradiol in vitro on expression of GH were also determined. In addition, we examined the effects of estrogen, age, and sex differences on mRNA for the GHR and IGF-I. Finally, the effects of an acute stressful experience on the GH response in males and females during different stages of the estrous cycle were examined.
Results
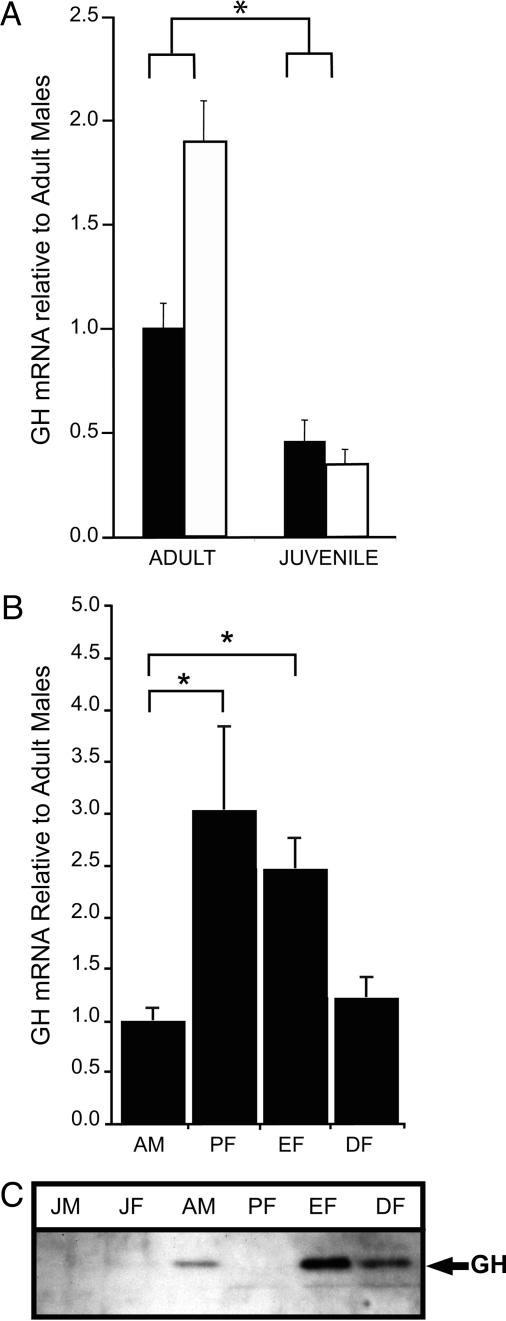
Using real-time PCR, we measured GH mRNA levels from the left hippocampus in juvenile male and female rats and adult males and females. The results are presented relative to expression in adult males and were analyzed by ANOVA and planned comparisons of the animal means (Fig. 1A). Expression of GH mRNA was more than twice as high in adult than in juvenile males [ F(1,9) = 9.6; P < 0.05] and more than three times higher in adult than juvenile females [F(1,9) = 5.4; P = 0.05]. Additionally, adult females expressed nearly twice as much GH mRNA as adult males [F(1,22) = 3.9; P = 0.07]. There were no differences in expression between male and female juvenile rats (P > 0.05).
Fig. 1.
Age and sex differences in GH expression. (A) GH mRNA levels are depicted as a function of age (adult vs. juvenile) and sex [males (filled bars) vs. females (open bars)]. Levels were increased in adults, especially in females. (B) The expression of GH mRNA in females is depicted during stages of estrus, including proestrus (PF), estrus (EF), and diestrus (DF), and compared with expression in adult males (AM). They were increased in females during proestrus and estrus. (C) Examples of a Western blot are shown, illustrating the presence of GH protein in the hippocampus, especially in females during estrus. JM, juvenile males; JF, juvenile females.
These results suggested that estrogen may influence the expression of GH in the hippocampus. To examine this possibility, we measured GH in females during proestrus, estrus, and diestrus. Females in proestrus expressed the most GH mRNA, nearly three times that in males [F(1,8) = 6.6; P < 0.05] (Fig. 1B). Females in estrus produced slightly less GH mRNA, although still more than males [F(1,12) = 8.9; P < 0.05]. Females in diestrus expressed little GH and at levels similar to males [F(1,10) = 0.26; P < 0.62].
We used the other hippocampus to determine whether protein levels of GH changed as a function of age and sex. GH protein was undetectable in juvenile animals but evident in adult males (Fig. 1C). In adult females, GH was not detected in proestrus, but was present in estrus and to a lesser degree in diestrus. Given that proestrus persists for a very short period, the stage differences between levels of mRNA and protein most likely reflect the time required for translation.
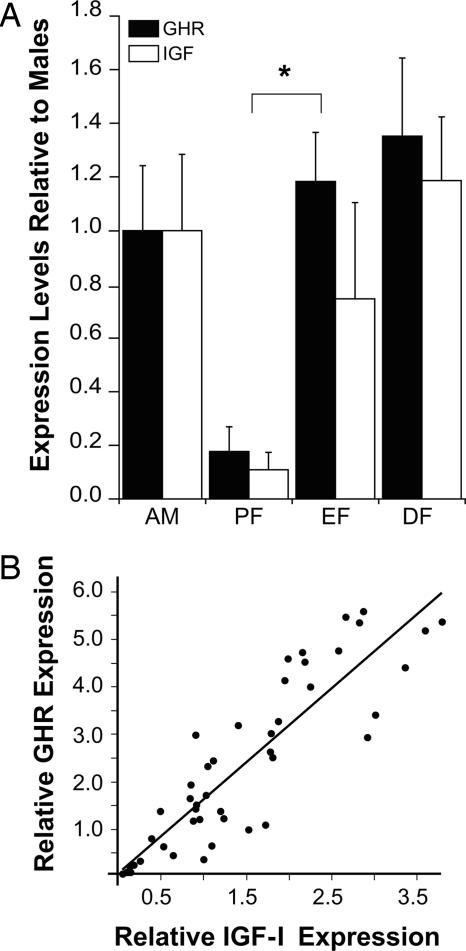
Next, we measured the mRNA for GHR and IGF-I. Both were expressed in males and females during estrus and diestrus (Fig. 2A), but barely detectable during proestrus. In fact, females in estrus expressed seven times as much IGF-I mRNA as those in proestrus [F(1,11) = 19.1; P < 0.01]. These data suggested that IGF-I and GHR are coregulated in the hippocampus. The relative expression levels of IGF-I and GHR mRNA levels for all of the animals in the study were correlated and revealed a strong positive correlation (r2 = 0.78, P < 0.0001), as illustrated in Fig. 2B. A relationship between IGF-I and GHR has been reported in other studies (17) and along with the present results would indicate that estrogen inhibits both the expression of GHR and IGF-I in the hippocampus, which then results in the expression of GH.
Fig. 2.
GHRs and expression of IGF-I. (A) GHR (filled bars) and IGF-I (open bars) mRNA levels are presented as a function of age and sex differences. mRNAs for both were decreased in females during proestrus (PF), relative to females in estrus (EF) and diestrus (DF), as well as in adult males (AM). (B) A scatterplot analysis shows the expression of GHR vs. IGF-I for all animals. The data suggest a strong positive correlation between the expression of the GHR and IGF-I.
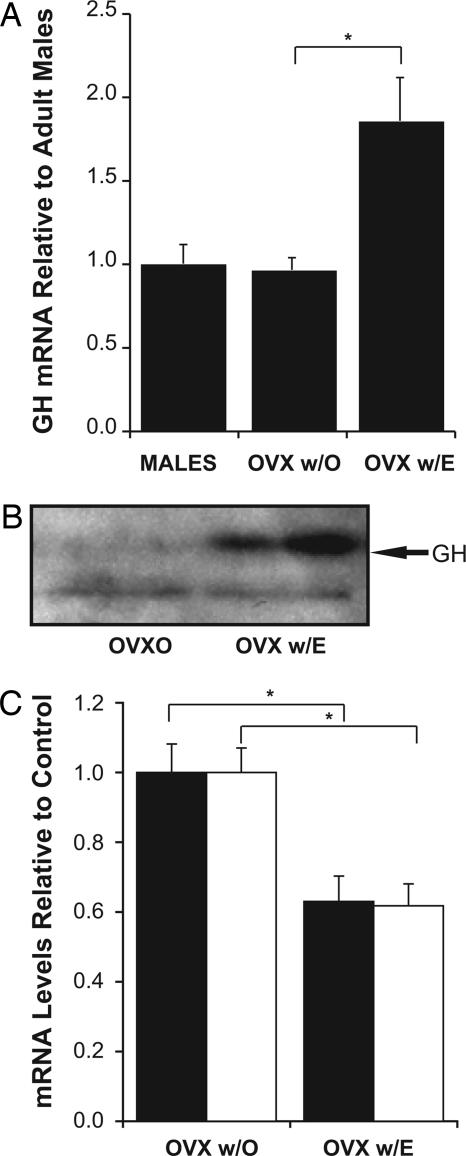
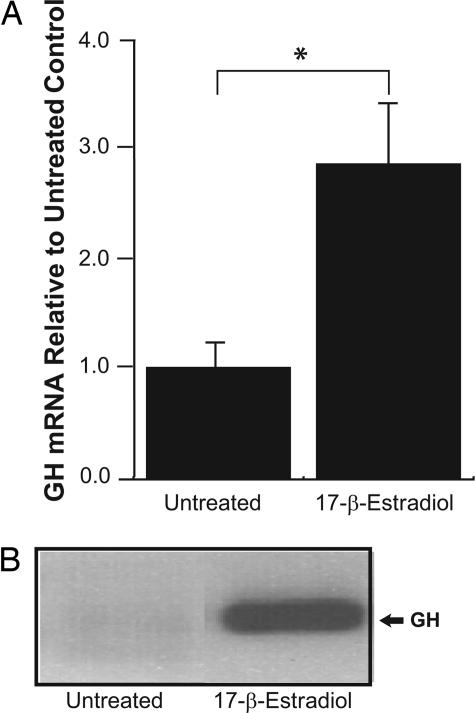
We then examined the direct effects of estradiol treatment on GH expression. As expected, concentrations of estradiol were higher in blood from ovariectomized females treated with estradiol (63.85 ± 11.49 pg/ml) than those treated with vehicle (4.82 ± 2.15 pg/ml) [F(1,8) = 25.95; P < 0.001]. mRNA for GH was similar in ovariectomized females to that in males and was greatly increased by estradiol (Fig. 3A), as was the protein (Fig. 3B). In addition to levels of GH mRNA, we measured levels of IGF-I and GHR mRNA. It had previously been reported that estradiol treatment decreases GHR mRNA levels in the hippocampus of ovariectomized females (17). Similarly, here we found that estrogen treatment significantly decreased the expression of GHR in ovariectomized females [F(1,9) = 5.11; P < 0.03]. We also found estrogen decreased the expression of IGF-I [F(1,9) = 5.11; P < 0.05]. These results are consistent with the data showing that estrogen represses the expression of IGF-I and GHR while inducing the expression of GH in females with a normal estrous cycle. To confirm that estrogen was directly affecting the expression of GH, we exposed 1-week-old primary neurons in culture to estradiol (10 nM 17-β-estradiol for 24 h). GH mRNA was three times higher in cultures treated with estradiol than those that were untreated [F(1,10) = 12.5; P < 0.001] (Fig. 4). The primary neurons were cultured in the absence of serum to limit the proliferation of glia and to address more directly the effect of estrogen on neurons. These data indicate that estradiol increases the expression of GH mRNA in primary neuronal cultures and thus add to the data, suggesting that estrogen increases the expression of GH in neurons.
Fig. 3.
Estrogen stimulates GH production. (A) Ovariectomized females injected with an oil vehicle (OVX w/O) produced similar levels of GH as adult males but twice as much GH upon exposure to 17-β-estradiol (OVX w/E). (B) A Western blot shows the production of GH protein in response to estrogen treatment (OVX w/E) after OVX (OVX w/O). (C) IGF-I (filled bars) and GHR (open bars) mRNA levels were decreased in the hippocampus of estrogen-treated ovariectomized females (OVX w/E) relative to those injected with oil (OVX w/O).
Fig. 4.
Estrogen stimulates GH production. (A) GH mRNA levels were increased in cortical neurons upon exposure to 10 nM 17-β-estradiol relative to untreated controls. (B) RT-PCR products from amplification of cDNA generated from the total RNA of untreated 1-week-old primary neuronal cultures and from the same cultures treated with 10 nM 17-β-estradiol for 24 h.
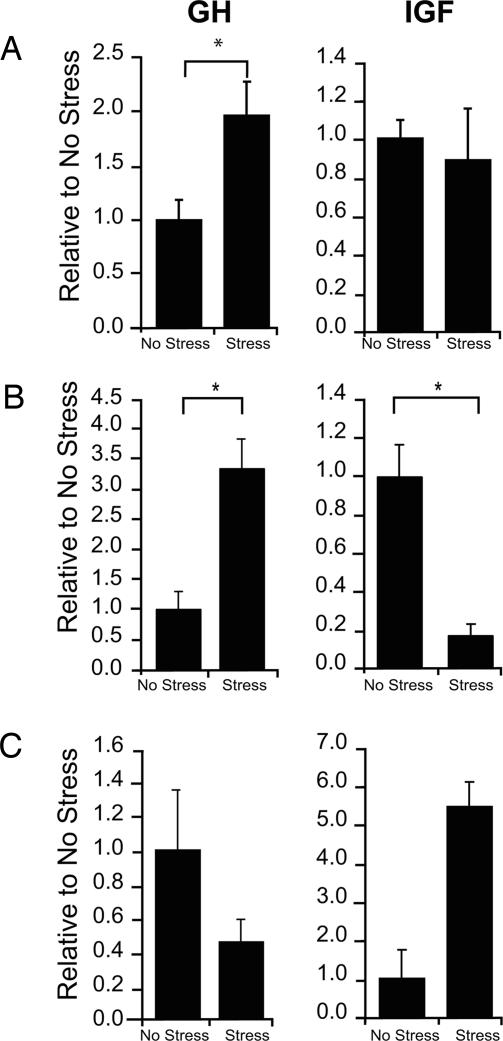
As shown here, the expression of GH mRNA in the hippocampus is sexually dimorphic and much greater in females than in males. GH in the hippocampus also responds to learning experiences that depend on the hippocampus (8). These results indicate that GH can respond to environmental events and suggest that it may play a role in cognitive or emotional responses that are different between males and females. It has been shown that male and female rats respond differently to an acute stressful event, and indeed males learn better whereas females are learning impaired (18). From this study we hypothesized that exposure to a stressful event would affect the expression of GH differently in males than it would in females. To test this hypothesis, we measured the effect of an acute stressful event on the expression of GH in the hippocampal formation in both males and females. One day after exposure to 30 brief intermittent tail shocks (1 mA, 1 s), the male hippocampus produced nearly twice as much GH mRNA [F(1,10) = 12.5; P < 0.001] (Fig. 5A). In females, the effect was different and depended on the stage of estrus in which they were exposed to the stressor. Those that were stressed during diestrus produced significantly more mRNA for GH as measured 24 h after the stressor had occurred [F(1,14) = 5.79; P = 0.03], whereas those that were stressed during either estrus or proestrus were unaffected (P > 0.05) (Fig. 5B and C). Together, the data indicate that stress affects GH expression differently in females depending on the stage of estrus in which the event occurs. Because females in estrus and proestrus express more GH when they are unstressed than do females in diestrus, the absence of a stress effect in these females could reflect a “ceiling” effect; in other words, they may already be expressing the maximum amount of GH and stress could not further enhance its expression. Another possibility is that exposure to the stressful event increases GH expression in males but only in females when their estrogen levels are relatively low, as they are in males.
Fig. 5.
Stress induces GH production. (A) Males exposed to an acute stressful event produced twice as much GH mRNA as males that received no stress, although IGF levels were unchanged. (B) Females exposed to a stressor during diestrus produced more than three times as much GH mRNA and 20% of IGF mRNA. (C) GH and IGF mRNA in those stressed during estrus were unaffected.
IGF-I mRNA levels were quantified in animals that were exposed to stress to determine whether the induction in GH mRNA correlated with a repression in IGF-I mRNA. Interestingly, even though GH mRNA levels were increased in stressed males, there was no apparent effect of the stressful event on IGF-I mRNA levels in the male hippocampus [F(1,10) = 0.09; P = 0.77] (Fig. 5A). In contrast, exposure to the stressor decreased the expression of IGF-I by 3.2-fold in females that were in diestrus [F(1,14) = 6.17; P = 0.03] (Fig. 5B). This decrease in expression was persistent because it was evident even 24 h after the stressful event had ceased, indicating a relatively long-lasting effect of an environmental event on IGF-I mRNA in the hippocampus. Overall, these data indicate that GH expression and presumably its production are affected by stressful experience but that the pathways that are involved are different, one modulated by changes in IGF-I and the other not. Also, the response in females changes across the estrous cycle and thus appears to be a more dynamic response in females than it is in males.
Discussion
Age and Sex Differences in GH Expression.
GH is well known for its role in body growth and development, but its role in brain function is less understood. In fact, until recently, it was not clear whether GH was even produced in the brain. Here, we show that the mRNA and the protein are produced in the hippocampal formation and that they respond, along with IGF-I to age and sex differences. Specifically, adult rats expressed more than twice as much GH in their hippocampus when compared with the hippocampus of juveniles that had yet to enter puberty. Also, the expression of GH was produced more in females than males and was especially sensitive to the presence of estrogen. The highest degree of GH expression occurred in the hippocampus of females while they were in proestrus, a stage associated with ovulation and high concentrations of estrogen in the blood. Because hormones other than estrogen change across the estrous cycle, we examined the direct effect of estrogen on GH expression in females without ovaries. OVX alone reduced GH mRNA levels in the hippocampus of females to levels similar to those measured in adult males. Moreover, exposure to exogenous estradiol increased the expression of hippocampal GH mRNA and protein levels significantly more than did exposure to the oil vehicle alone. The increase in GH expression in response to estradiol appears to occur in neurons, at least to the extent that it was enhanced in primary neurons in culture that were exposed to 17-β-estradiol. Together, these data indicate that GH in the hippocampus is regulated by sex differences and that these differences are influenced by estrogen and changes that occur as animals establish reproductive maturity.
Relationship Between GH mRNA and Its Receptor and IGF-I.
In most biological systems, GH, IGF-I and their respective receptors are intimately related. We demonstrate here that such a relationship also exists in the hippocampus. There was a very strong correlation between the expression of the receptor for GH and IGF-I (Fig. 2C) and an inverse relationship between the presence of GH mRNA and that of IGF-I. We also found evidence that IGF-I is regulated by estrogen in the hippocampus. The hippocampus of ovariectomized females expressed less GH than that of females with ovaries and expressed levels similar to those found in the male hippocampus. However, the hippocampus of ovariectomized females that had been injected with estradiol had increased expression of GH mRNA and reduced mRNA levels for IGF-I and GHR. These data are consistent with reports that estrogen down-regulates the expression of GHR mRNA in the hippocampus of female rats (17). The relationship between hippocampal GH and IGF-I was also apparent in the intact females as they transitioned through different stages of the estrous cycle. Levels of IGF-I mRNA were barely detectable in the hippocampus of females that were in proestrus, whereas levels of GH mRNA were relatively high. Because estrogen concentrations in the blood are elevated during this stage of estrus, these data would suggest that estrogen regulates the GH/IGF-I axis in the hippocampus as females ovulate. A similar relationship reportedly occurs within the liver; estrogen inhibits the expression of IGF-I, which then results in the stimulation of GH synthesis through the negative feedback of the GH/IGF-I axis (19). That said, given the dynamic nature of the estrous cycle, it is difficult to describe the exact relationship between GH and IGF-I in the female hippocampus. However, in all of the experiments presented here, when estrogen levels were elevated, IGF-I mRNA levels were significantly reduced and GH mRNA levels were up-regulated. These results suggest that in the female hippocampus the induction of GH mRNA by estrogen is likely the result of negative feedback of the GH/IGF-I axis.
Stressful Experience Alters the Expression of GH in the Hippocampus.
The results presented so far suggest that GH in the hippocampus along with its receptor and IGF-I are strongly regulated by sex and age. However, these data do not address the functional significance of GH in the hippocampus. Because the hippocampus is necessary for many types of learning, it seems reasonable to propose that hippocampal GH might also be involved in processes of learning. Consistent with this idea, we previously reported that hippocampal-dependent learning increases the expression of GH in the hippocampus in male rats (8). Several studies have demonstrated that males and females learn at different rates and that their learning abilities are affected very differently after exposure to an acute stressful experience (20, 21). Here, we report that exposure to an acute stressful experience of intermittent tail shocks increases the expression and production of GH in the hippocampus of males. The stress-induced increase was evident 24 h after the event had ceased, indicating a relatively persistent effect of the stressful event on GH expression. Exposure to the acute stressor increased GH expression in females, but only those that were in diestrus when the stressful event occurred. In these same females, there was also a decrease in IGF-I expression, suggesting that stressful experience, similar to estrogen exposure, inhibits the expression of IGF-I and thereby increases the expression of GH mRNA level. Most interestingly, the increase in GH mRNA in males was not accompanied by a decrease in IGF-I. Thus, it would appear that the induction of GH after stress in males is regulated by a different mechanism than it is in females and one that does not involve changes in IGF-I. In summary, these data indicate that GH is not only sensitive to age and sex differences, but also that it interacts with two functions ascribed to the hippocampus, one being learning and the other its regulation of the stress response. Exactly how GH contributes to these processes is unknown and will likely remain unknown until techniques are developed that can block GH expression in discrete brain regions.
The Potential Role of GH in the Hippocampal Formation.
Overall, these data in combination with our previous study demonstrate that GH production in the hippocampus is sensitive to stressful experience, learning a hippocampal-dependent memory task, and levels of estrogen. Thus, one might propose that it plays a role in neuronal processes related to mood and cognition, as has been proposed (4, 5). If so, these effects would likely be mediated by its effects on neuronal growth and development. Interestingly, the manipulations shown here that induce hippocampal GH are also associated with events that increase the growth of anatomical structures within the brain. Females possess more dendritic spines in the hippocampus than do males, especially during proestrus, when GH levels are high (22–25). They also produce more new neurons in the hippocampus during proestrus (26). Additionally, stressful experience changes the density of these structures differently in males than in females, depending on the stage of estrus, as it does GH (20, 21, 23, 24). Finally, and perhaps not coincidentally, all three measures, GH, spines and neurogenesis, increase with learning (8, 27, 28). It is thus possible that features of mood and cognition are controlled by the production of GH within the brain itself.
Materials and Methods
Subjects.
Adult male and virgin female Sprague–Dawley rats (250–300 g) were obtained from Zivic Laboratories and housed individually before and after surgery in Rutgers University's Department of Psychology animal facility. Rats had unlimited access to water and Purina Lab Chow (Ralston-Purina) and were maintained on a 12:12 light-dark cycle with light onset at 7 a.m.
Vaginal Cytology.
Vaginal cytology was monitored through daily vaginal smears obtained between 10 a.m. and 12 p.m (18). Sterile cotton swabs were immersed in physiological saline and gently inserted into the vaginal tract, and loose epithelial cells were removed, then rolled onto a slide. Cells were dried, fixed in 95% EtOH, rinsed in buffered water, stained in 1% aqueous filtered toluidine blue, rinsed in 70% buffered EtOH, and fixed again in 95% EtOH. Based on their vaginal cytology, rats were classified into four stages of estrous: proestrus was marked by purple staining epithelial cells with dark nuclei, estrus by masses of dark blue staining cornified cells, diestrus 1 by numerous leukocytes and epilthelial cells, and diestrus 2 by a similar morphology but reduced numbers. Only females that had standard 4- to 5-day estrous cycles were included.
OVX and Estrogen Replacement.
Animals were anesthetized with 30 mg/kg pentobarbital injected i.p. supplemented with Isoflurane inhalant and bilaterally ovariectomized. The ovaries were removed through a small midline incision on the ventrum. After removal of the ovaries, the muscle wall and skin were closed with absorbable suture. After surgery, 0.3 ml of penicillin (250,000 units per ml) was administered intramuscularly, and the rat was kept warm until recovery from anesthesia. Postoperatively, rats were provided with 24-h access to acetaminophen (32 mg/ml; IDE Interstate, Amityville, NY) diluted 1:100 in drinking water for 2 days. After at least 5 days of recovery, rats were injected s.c. with 10 μg of 17-β-estradiol benzoate (Sigma) dissolved in sesame oil. Each group of estrogen-treated rats was tested with a separate group of OVX rats injected with sesame oil vehicle. All rats were injected, and then 24 h later, they were injected again with either the same dose of estradiol or vehicle. Twenty-five hours later, all animals were given a lethal dose of sodium pentobarbital, and blood samples were collected via cardiac puncture for RIA of hormone levels. Blood was added to test tubes containing 0.1 ml heparin and centrifuged for 20 min at 402 × g. The brain was dissected, and tissue was kept on dry ice until storage at −80°C. Plasma aliquots were stored at −20°C and thawed before analysis. Circulating levels of estradiol were measured by using a solid-phase RIA system (Coat-A-Count; Diagnostic Products, Los Angeles). Assay sensitivity for estradiol was 8 pg/ml, and interassay variability was <7%.
Stressor Exposure.
Male rats were removed from their home cage and taken into another room. They were placed in a restraining tube that was located within a dark sound-attenuating chamber. Electrodes were attached to the tail to deliver 30 shocks (1 s, 1 mA) at a rate of 1 per min. Immediately after, they were returned to their home cage. Twenty-four hours later, they and a group of unstressed controls were injected with a lethal dose of sodium pentobarbital. Under deep anesthesia, the brain was removed, dissected, kept on ice, and eventually stored at −80°C.
RNA Isolation and Real-Time PCR.
Total RNA was isolated from frozen hippocampal tissue with TRI Reagent (Sigma). First-strand cDNA was synthesized by using 500 ng of total RNA primed with random hexamers (Promega) as a template for avian myeloblastosis virus-reverse transcriptase (Promega). Real-time PCR was performed in the PE Biosystems Gene Amp 7700 sequence detection system (Applied Biosystems) using 5% of cDNA, primers specific for GH (5′-AAGAGTTCGAGCGTGCCTACATTCC-3′, 5′-AGTTCTCTGCTGGGCCTCCTCCTT-3′) IGF-I (5′-TCTGAGGAGGCTGGAGATGT-3′, 5′-TGACGTGGCATTTTCTGTTC-3′), or GHR (5′-TGGACACACTGGCAGCAT-3′, 5′-TCCTTTGCTGCTTTGAAAATATAACTA-3′), cDNA, and the SYBR green PCR reagents as recommended by the manufacturer (Applied Biosystems). PCR products for GH, IGF-I, and GHR were 125, 194, and 119 bp long, respectively. Individual primer pairs were tested at concentrations of 50, 250, and 500 nM to optimize reactions and ensure no primer dimer formation occurred within the number of cycles required for quantification.
Data from the real-time PCRs were analyzed by using Applied Biosystems sds 1.9 software. PCRs for each cDNA were performed simultaneously and normalized to the PCRs by using primers for RPL39 (5′-GATCCTCGCCATGTCTTCTC-3′, 5′-GCTTCGTTCTCCTCGAGTGT-3′, ribosomal protein L39 mRNA). To calculate fold change in gene expression a ΔCt (Ctsample − CtRPL39) value relative to RPL39 was first calculated for each cDNA and primer pair. Relative gene expression for each sample was calculated, 2ΔCt. This normalized value was then used to compare gene expression levels between samples.
Western Analysis.
Protein lysates were generated by homogenizing hippocampal tissue in lysis buffer consisting of 0.5% SDS, 0.5% Triton X-100, 1% Nonidet P-40, 50 mM Tris (pH 7.5), 150 mM NaCl, 2 mM EDTA, 1 mM PMSF, and protease inhibitors. Fifty micrograms of lysate was boiled in the presence of SDS sample buffer, and the sample was loaded onto 10–20% Tris/glycine-acrylamide gel and transferred to poly(vinylidene difluoride) membranes for immunoblot analysis using polyclonal goat-anti-rat GH antibodies obtained from Research Diagnostics (Flanders, NJ).
Neuronal Cultures.
Neuronal cultures were prepared as described (29). Briefly, pregnant embryonic day 18 Sprague–Dawley rats were killed by inhalation of CO2, and the embryos were removed immediately. Cortices were dissected and treated with 0.25% trypsin in Hepes-buffered Hanks' balanced salt solution (HBSS) without calcium or magnesium at 37°C for 15 min. The cortices were then washed with HBSS and manually dissociated with a fire-bored Pasteur pipette. Cells were plated at a concentration of 100,000 per cm2 on poly-l-lysine-coated 10-cm plates in plating medium containing DMEM and 10% FBS. After incubation overnight the medium was changed to Neurobasal medium containing B27 supplement and 0.5 mM glutamine. After 7–10 days in vitro primary neuronal cultures were treated with 100 nM 17-β-estradiol for 24 h.
Acknowledgments
This work was supported by National Institutes of Health Grant MH59970 (to T.J.S.).
Abbreviations
- GH
growth hormone
- GHR
GH receptor
- IGF
insulin-like growth factor
- OVX
ovariectomy.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kopchick J. J., Andry J. M. Mol. Genet. Metab. 2000;71:293–314. doi: 10.1006/mgme.2000.3068. [DOI] [PubMed] [Google Scholar]
- 2.Nyberg F. Front. Neuroendocrinol. 2000;21:330–348. doi: 10.1006/frne.2000.0200. [DOI] [PubMed] [Google Scholar]
- 3.Scheepens A., Williams C. E., Breier B. H., Guan J., Gluckman P. D. J. Pediatr. Endocrinol. Metab. 2000;13(Suppl. 6):1483–1491. doi: 10.1515/jpem-2000-s623. [DOI] [PubMed] [Google Scholar]
- 4.Sonntag W. E., Ramsey M., Carter C. S. Ageing Res. Rev. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 5.van Dam P. S., Aleman A. Eur. J. Pharmacol. 2004;490:87–95. doi: 10.1016/j.ejphar.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 6.Nyberg F., Burman P. Horm. Res. 1996;45:18–22. doi: 10.1159/000184753. [DOI] [PubMed] [Google Scholar]
- 7.Sun L. Y., Al-Regaiey K., Masternak M. M., Wang J., Bartke A. Neurobiol. Aging. 2005;26:929–937. doi: 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Donahue C. P., Jensen R. V., Ochiishi T., Eisenstein I., Zhao M., Shors T., Kosik K. S. Hippocampus. 2002;12:821–833. doi: 10.1002/hipo.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dam P. S., Aleman A., de Vries W. R., Deijen J. B., van der Veen E. A., de Haan E. H., Koppeschaar H. P. Growth Horm. IGF Res. 2000;10(Suppl. B):S69–S73. doi: 10.1016/s1096-6374(00)80013-1. [DOI] [PubMed] [Google Scholar]
- 10.Werther G. A., Russo V., Baker N., Butler G. Horm. Res. 1998;49(Suppl. 1):37–40. doi: 10.1159/000053066. [DOI] [PubMed] [Google Scholar]
- 11.Aberg M. A., Aberg N. D., Hedbacker H., Oscarsson J., Eriksson P. S. J. Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson M. F., Aberg M. A., Nilsson M., Eriksson P. S. Brain Res. Dev. Brain Res. 2002;134:115–122. doi: 10.1016/s0165-3806(02)00277-8. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenwalner R. J., Forbes M. E., Bennett S. A., Lynch C. D., Sonntag W. E., Riddle D. R. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 14.Hull K. L., Harvey S. Endocrine. 2000;13:243–250. doi: 10.1385/ENDO:13:3:243. [DOI] [PubMed] [Google Scholar]
- 15.Hull K. L., Harvey S. J. Endocrinol. 2001;168:1–23. doi: 10.1677/joe.0.1680001. [DOI] [PubMed] [Google Scholar]
- 16.Hull K. L., Harvey S. Rev. Reprod. 2000;5:175–182. doi: 10.1530/ror.0.0050175. [DOI] [PubMed] [Google Scholar]
- 17.Bennett P. A., Levy A., Carmignac D. F., Robinson I. C., Lightman S. L. Endocrinology. 1996;137:3891–3896. doi: 10.1210/endo.137.9.8756562. [DOI] [PubMed] [Google Scholar]
- 18.Wood G. E., Shors T. J. Proc. Natl. Acad. Sci. USA. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy L. J., Friesen H. G. Endocrinology. 1988;122:325–332. doi: 10.1210/endo-122-1-325. [DOI] [PubMed] [Google Scholar]
- 20.Leuner B., Mendolia-Loffredo S., Shors T. J. Biol. Psychiatry. 2004;56:964–970. doi: 10.1016/j.biopsych.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood G. E., Beylin A. V., Shors T. J. Behav. Neurosci. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- 22.Woolley C. S. Horm. Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- 23.Shors T. J., Chua C., Falduto J. J. Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shors T. J., Falduto J., Leuner B. Eur. J. Neurosci. 2004;19:145–150. doi: 10.1046/j.1460-9568.2003.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gould E., Tanapat P., Rydel T., Hastings N. Biol. Psychiatry. 2000;48:715–720. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- 26.Tanapat P., Hastings N. B., Reeves A. J., Gould E. J. Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leuner B., Falduto J., Shors T. J. J. Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gould E., Beylin A., Tanapat P., Reeves A., Shors T. J. Nat. Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 29.Banker G. A., Waxman A. B. In: Studies of the Intrinsic Determinants of Neuronal Form and Function. Lasek R. J., Black M. M., editors. New York: Liss; 1988. pp. 61–82. [Google Scholar]