Abstract
Dynamic extracellular matrix (ECM) synthesis is explored in a hypothesized engineered cartilage construct. Growth (α) and decay (β) rate parameters are developed from a previous engineered cartilage model. The presented mathematical model was constructed from the parameterized experimental data using a deterministic and stochastic examination of ECM synthesis based on a negative feedback control mechanism. A growth factor supplementation is incorporated in a probabilistic mathematical approach. The growth factor component modified an initial deterministic model through a Gaussian white noise fluctuation. As the primary constituents of ECM, the mathematical tool is intended to characterize the probable steady state distribution of glycosaminoglycan (GAG) and collagen molecules as well as mean mass accumulation at homeostasis. Computer simulation of the models is applied to reported data from four similar chondrocyte-polymer construct culture systems. The range in rate ratios reflect the differing nature of GAG and collagen synthesis (αGAG/βGAG = 4.2 to 148.6; αcollagen/βcollagen = 8.1 to 2590.4). This technique reduced the influencing synthesis factors to a few key descriptive parameters. Additional anabolic and catabolic factors may further be built into the models.
Keywords: glycosaminoglycan, collagen, extracellular matrix, growth factor, tissue engineering, gaussian white noise, probability density function, cartilage
Introduction
Functional characteristics of articular cartilage involve the response of active load bearing (compressive and shear forces) within diarthrodial joints. Damage to the articulating surface can occur due to adverse mechanical and biological perturbations. Despite being a living tissue it has a limited capacity for healing. Damage to the articular surface may spread, resulting in pain and loss of joint movement. With minimal regenerative potential, degenerative damage can lead to osteoarthritis. This joint disease is the most common form of arthritis in Australia where 5 to 10% of the total population is affected.1 In the United States, more than one million people require treatment per year for damaged articular cartilage.2
Native articular cartilage is a composite of cartilage cells (chondrocytes), extracellular matrix (ECM), and water. The three main biomolecular components of ECM are structural proteins, specialized proteins, and proteoglycans. Structural proteins consist of type II collagen and elastin, specialized proteins consist of fibrillin, fibronectin, and laminin, and lastly, proteoglycans are core proteins to which are attached long chains of repeating disaccharide units known as glycosaminoglycans (GAG). The mechanical behaviour of cartilage is strongly influenced by constituent content and the electrostatic forces between matrix charge groups.3 Quantifying collagen and GAG content over time provides an indicator of the state of ECM and the chondrocyte regenerative or degenerative potential.
Existing techniques to repair damaged articular cartilage with autograft/allograft transplants have had limited success. Tissue engineered cartilage offers a possible alternative. Cartilage tissue engineering typically utilizes the seeding of chondrogenic cells into biodegradable polymeric scaffolds.4–8 In this arrangement, ECM concentration in cell-polymer constructs is designed to gradually increase and form neo-tissue while scaffold concentration decreases with time.9 However, the role of ECM in maintaining and modulating cell function is not fully understood although recent reports have described the role of receptor molecules such as integrins in regulating chondrocytes.10–11
A relatively strong negative correlation has been observed between GAG molecule deposition within ECM and its synthesis rate in the study of native12 and engineered4 cartilage. This result is consistent with that reported from in vitro modulation of chondrocyte metabolism by extracellular proteoglycan.13 Based on this product inhibition mechanism, a mathematical formulation for GAG deposition was described by Hascall et al.12,14 and adapted for use in cartilage tissue engineering by Wilson et al.9 for a characterization of ECM mass or concentration:
| (1) |
where kECM is an ECM synthesis rate constant and the steady-state (ss) condition occurs at t → ∞ and d(ECM)/dt = 0. Thus ECM mass or concentration as a function of time follows the form:
| (2) |
Degradation of scaffold mass or concentration is also assumed to follow a first order decay equation:9
| (3) |
where ks is the scaffold decay rate constant. Again, this implies at time-dependent characterization as:
| (4) |
By combining the mathematical formulations (2) and (4), along with the cellular mass content, a total construct mass model is proposed and experimentally validated as a function of culture time.9 In the above approach, dependencies between chondrocyte function and ECM molecule production rates as well as the effect of local biomechanical or biochemical factors are not explicitly taken into account.
It is well established that growth factors and cytokines modulate cell function and cartilage development.15–18 The effects depend on specific combinations of factors, time sequences of their application, and interactions with the mechanical environment (hydrodynamic shear and compression). It has been observed experimentally by several investigators that growth factors play an important role in matrix production and inhibition, but there is limited knowledge about the functional mechanisms associated with these effects.16–22 This leads to the present objective which is to explore the influence of growth factors within engineered cartilage by modelling hypothetical characteristics of ECM synthesis.
In previous approaches, we presented predictive models which examined deterministic and stochastic mechanisms of engineered cartilage composition. These works examined cellular proliferation effects,23 a randomised influence of growth factors on synthesis rate,24 a deterministic approach with exponential synthesis terms,25 and a randomised influence of growth factors limited by a carrying capacity parameter with an initial growth factor state.25 Here, dynamic compositional models are again formulated albeit with concomitant growth and decay rate terms. These modelling iterations investigate possible influences in the modulation of ECM synthesis which could be manipulated to enhance the regenerative potential in cartilage therapies. Therefore, the specific aims of this study are to: a) develop a deterministic mathematical model based on published experimental results that describe the accumulation of matrix molecules using distinct rate terms; b) quantify a ‘growth factor effect’ on matrix molecule deposition through a stochastic influence on the synthesis rate term. Both of these modelling approaches are based on current cell-polymer constructs developed for tissue engineered cartilage.
Methods and materials
Deterministic control model
An alternative deterministic model is presented to describe the accumulation of ECM molecules in a cell-polymer construct based on a negative feedback control mechanism. This approach applies distinct growth and decay rate terms originally reported for GAG molecules.4,13 Here, the model is introduced to describe ECM mass or concentration:
| (5) |
where α0 and β0 are, respectively, the natural growth and decay rates of ECM molecules in a cell-polymer construct. The first term in equation (5) incorporates the feedback mechanism for the system. During variable manipulation, ECM is replaced by X and restated as:
| (6) |
of which the general solution is:
| (7) |
where:
| (8) |
At the initial condition, t = 0, when chondrocytes are seeded into a polymeric scaffold, the ECM concentration is zero and:
| (9) |
In the steady state condition (t → ∞), Z → Ω and:
| (10) |
By assuming that both GAG and collagen molecules follow the same growth kinetics within ECM, the Deterministic Control Model describes steady state (ss) content:
| (11) |
where, αGAG and βGAG are the natural growth and decay rates, respectively, of GAG molecules and:
| (12) |
where, αcollagen and βcollagen are the natural growth and decay rates, respectively, of collagen molecules. By combining equations (11), (12), scaffold degradation of (4), and the cellular mass content, , the mean mass of the total engineered cartilage construct, , in the steady state can then be described:
| (13) |
Stochastic growth hormone treated model
ECM molecule dynamics are further explored through the inclusion of hypothetical growth factor effects. As the functional characteristics of the growth factors are not exactly known, their influence is treated here as random fluctuations. This influence is incorporated into the modelled system as Gaussian white noise. The presence of growth factor is assumed to manipulate the growth rate constant such that:
| (14) |
where, ρ0ξ(t) is the time-dependent, randomised contribution to the synthesis rate. Equation (14) can be rewritten as:
| (15) |
where the random noise function, ξ(t), has the following mean and covariance conditions:
| (16) |
A replacement term can be utilized, based on the interpretation of the integral of ξ(t) :26–27
| (17) |
and dW (t) ~ N(0, dt)
where N(μ, σ) is a normal distribution with mean, μ, and standard deviation, σ. Equation (15) then becomes:
| (18) |
The corresponding Fokker-Planck equation can be applied with the intent of describing the probabilistic distribution of the matrix molecules:26–28
| (19) |
where, P(ECM ,t) is the probability density function (PDF) of ECM deposition at time t. In the steady state, as t → ∞ and d(ECM)/dt = 0, the PDF of P(ECM, ∞) or P∞(ECM) satisfies:
| (20) |
Therefore, the steady state PDF of ECM deposition, Pss(ECM) , is proportional to:
| (21) |
and thus,
| (22) |
where the constant of proportionality,C0, can be obtained by solving:
| (23) |
Thus, equation (22) represents the generalized PDF for the matrix molecules. The steady state PDF for the primary ECM molecules are assumed to be independent of each other and controlled by their own growth and decay rates.
Thus the PDF of GAG molecule deposition is given by:
| (24) |
and the PDF for collagen molecule deposition:
| (25) |
For the overall mass of the engineered construct, it is assumed that growth factor stimulation and accumulated ECM have no effect on scaffold degradation. The scaffold concentration follows a simple first order decay.9 It is also assumed that cellular mass, , reaches a constant level after proliferating to a concentration level specific to the culture environment.29 Here, this cellular concentration is assumed to be independent of the growth factor-influenced ECM synthesis rates. The mean total engineered cartilage construct mass in the steady state, , can thus be described as:
| (26) |
where the integral terms define the ECM mass associated with mean distribution, .
Computational analysis
Application of the Deterministic Control Model and the Stochastic Treated Model require quantified parameters which characterize engineered cartilage content. Reports of direct and indirect assays for cell, ECM, and scaffold content through periods of culture are available for parameterization.9 In addition, the intrinsic synthesis rate, k, or one over the characteristic time constant, τ, as defined in the initial model,9 is applied here as a second equation to determine the two unknown rates of growth and decay for model input:
| (27) |
Thus, the steady state values of ECM molecules, GAGss and collagenss, as well as the characteristic time constants which have been previously determined from experimental data are incorporated into the presented models.9,29–31 Solutions from equations (11) and (12) combined with relationship (27) defined the rate parameters for each biomolecule: αGAG, βGAG, αcollagen, and βcollagen (Table 1). Representative experimentally derived characteristic time constants and kinetic rates of scaffold degradation have been included for comparison (Table 2). All variable manipulation and computational analysis were completed on commercially available software (MathCAD v12, Mathsoft Engineering and Education, Inc., Cambridge, MA).
Table 1.
Representative ECM synthesis parameters previously derived from a cartilage tissue engineering model applied to published experimental data.9 Growth (α) and decay (β) rates are further extracted in this work from the Deterministic Control Model. Note: PGA = polyglycolic acid scaffold; BAC = bovine articular cartilage;τECM = matrix molecule characteristic time constants; dw = dry weight; ww = wet weight.
| Growth (α), Decay (β) Rates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Scaffold | Cell type | Ref | GAGss | τGAG | Collagenss | τcollagen | αGAG βGAG | αcollagen βcollagen |
| PGA | BAC | 29 | 6.8%
ww |
31.3
days |
3.7%
ww |
15.9
days |
3.256 x 10−2
6.139 x 10−4 |
6.672 x 10−2
3.837 x 10−3 |
| PGA | BAC | 30 | 11.7%
dw |
18.6
days |
50.4%
dw |
131.5
days |
5.441 x 10−2
3.662 x 10−4 |
7.607 x 10−3
2.937 x 10−6 |
| PGA | BAC | 9 | 6.1%
dw |
187.0
days |
6.5%
dw |
18.9
days |
5.474 x 10−3
1.264 x 10−4 |
5.400 x 10−2
1.108 x 10−3 |
| PGA | BAC | 31 | 1.6%
ww |
22.2
days |
2.4%
ww |
16.7
days |
5.900 x 10−2
1.400 x 10−2 |
6.800 x 10−2
8.363 x 10−3 |
Table 2.
Representative polymeric scaffold degradation parameters derived from a previous cartilage tissue engineering model applied to published experimental data.9 Note: τScaffold = scaffold degradation characteristic time constant.
| Scaffold material | Initial scaffold porosity | Reference | τScaffold | Scaffold decay rate |
|---|---|---|---|---|
| PGA | 97% | 32 | 56.1 days | 1.70 x 10−2 |
| PGA | 97% | 29 | 24.3 days | 4.11 x 10−2 |
Results
The specific aims of this work were satisfied through t h e formulation and application of two mathematical models characterizing ECM synthesis within generalized engineered cartilage constructs. Previously parameterized data from four published reports were used to define the growth and decay rates for application in the current models (Table 1). The computer simulations using the Deterministic Control Model reflect the variety of GAG and collagen accumulation growth patterns found in engineered cartilage studies (Figure 1). The curves describe matrix molecule synthesis based on the numerical analysis of experimentally observed engineered cartilage steady state values and intrinsic growth rates as input. The resulting curves were not ‘fitted’ to the previous data but utilize parameters previously defined from that data. The mathematical model was based on two input variables and therefore does not consider the multitude of constituent interactions within ECM which may or may not account for differences between constituents.
Figure 1.
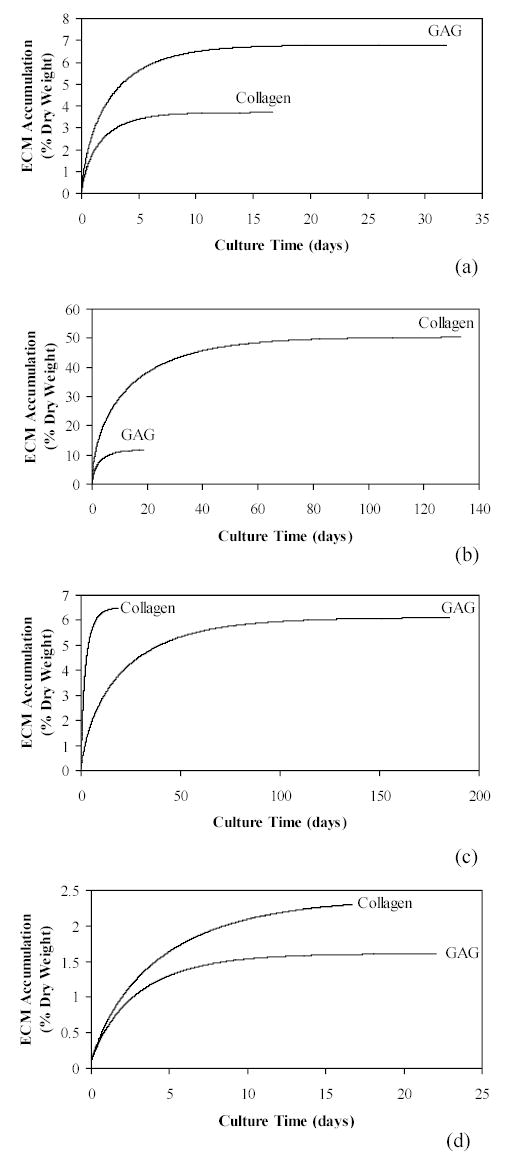
Simulated time-dependent ECM accumulation leading to steady state levels using the Deterministic Control Model, equations (7) through (12). Input parameter values were chosen from reported cartilage engineering experimental data (Table 1): a) Freed et al.29; b) Vunjak-Novakovic et al.30; c) Wilson et al.9; and d) Vunjak-Novakovic et al.31.
The Stochastic Growth Hormone Treated Model resulted in distribution frequencies of ECM accumulation based on the PDF for each molecule. The randomised growth hormone affect on synthesis rate again produced a variety of accumulation patterns (Figure 2). These distribution patterns for each of the four studies describe probabilistic accumulation as influenced from a single initial relative growth hormone level (ρGAG = ρcollagen = 2.0). Again, the model does not account for a myriad of possible influences in accumulation which could be causing the dramatic differences between GAG and collagen content. Finally, mean steady state accumulations were compared between Control and Treated Models as simulated for both GAG and collagen (Figure 3). Although the expected increase in accumulation due to a stimulated growth rate is apparent, the diverse effect within the four sets of parameters is noticeable.
Figure 2.
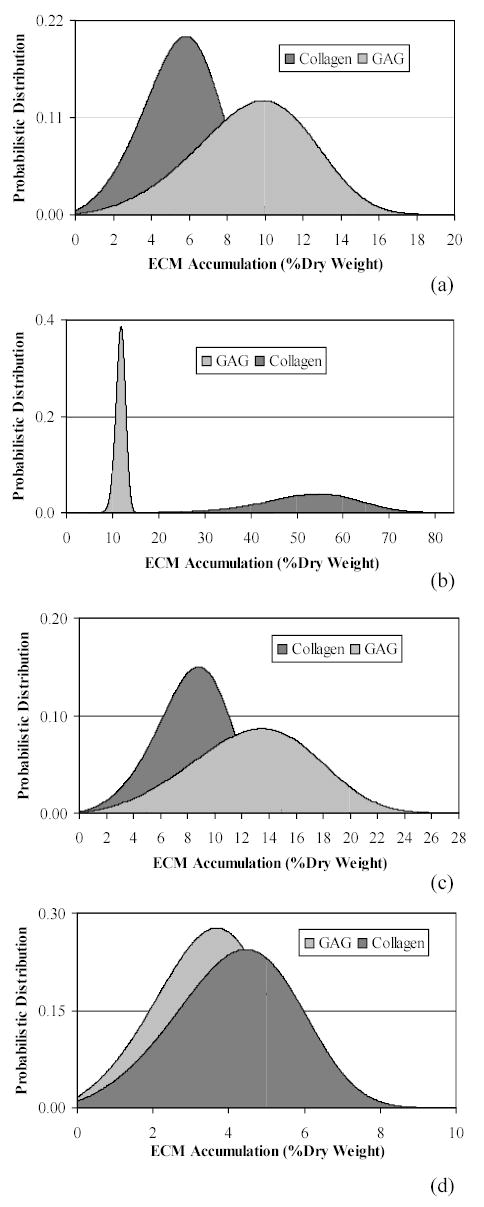
Distribution plots produced from the Probability Density Function describing mean ECM accumulation at the steady state condition for each of the modelled constituents, equations (24) and (25). Here, the growth and density rates for both GAG and collagen were incorporated from representative published cartilage engineering experiments (Table 1): a) Freed et al.29; b) Vunjak – Novakovic et al.30; c) Wilson et al.9; and d) Vunjak - Novakovic et al.31. For all distributions, the relative growth factor effect is the same, ρGAG = ρcollagen = 2.0.
Figure 3.
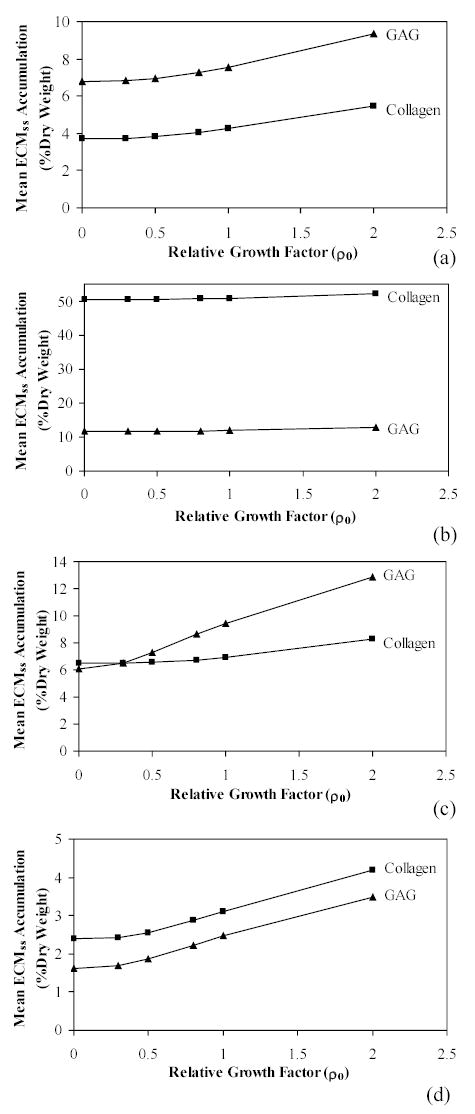
A comparison of the mean steady state ECM accumulation with supplemented growth factor effects. Steady state values of growth factor-influenced accumulation are calculated from the means of the probability functions, , applied to equations (24) and (25) and shown for each constituent in equation (26). ECM growth and density rates were incorporated from representative published cartilage engineering experiments (Table 1): A) Freed et al.29; B) Vunjak-Novakovic et al.30; C) Wilson et al.9; and D) Vunjak-Novakovic et al.31. For each ECM constituent, mean accumulation values are determined at distinct relative growth factor levels within the Stochastic Treated Model, ρGAG = ρcollagen = 0.3, 0.5, 0.8, 1.0, and 2.0. Accumulation at ρ0 = 0 represents the mean steady state level from the Deterministic Control Model.
Discussion
In this work, generalized mathematical models of cartilage matrix molecule accumulation in engineered cell-polymer constructs have been constructed based on parameters derived from previous experimental results. The models incorporated a product inhibition mechanism via a negative feedback control network where the rate of synthesis decreases during matrix production.
The presented models provide an analytical tool from which to identify synthesis-influencing variables and parametrically compare empirical data. A new technique is formulated to explicitly determine growth and decay rates of matrix molecules at the steady state condition. In the deterministic model, these two rate parameters characterize the time-dependency of growth leading to the steady state condition. The generalized mathematical model is further studied in an environment where growth factors hypothetically influence the dynamics of matrix molecule accumulation within the cell-polymer construct. The biophysical knowledge of growth factors in cell-polymer constructs is generally insufficient for full analytical prediction. Researchers are investigating the modulatory mechanisms of these growth factors through computational33 and experimental34 efforts to define explicit physical laws. The presented approach represents a modelling continuation which explores ECM synthesis through deterministic descriptions and stochastic fluctuations within the system dynamics in order to identify the action potentials of anabolic and catabolic factors within the engineered constructs.23–25 In each of the previous models, published data describing ECM was used to construct models which, in turn attempted to characterize the results based on: cellular proliferation effects,23 deterministic steady state boundaries,25 and stochastic contributions of growth factors on mathematical synthesis terms.24,25
The presented deterministic model is again built from parameters defined by cultured experimental constructs. The stochastic modification proposes that the ‘growth factor effect’ acts only on the growth rates of the ECM molecules as distinguished from a decay rate. As a basis for comparison with other growth factor studies, the model shows that an increase in the relative level of growth factor will create more random fluctuations within the system and thus the overall (mean) production of a matrix molecule will be higher. This result is similar to what has been observed experimentally by several researchers describing anabolic manipulation.10,18,35–37 These investigations reported on positive response in synthesis due to sequential application of various factors. Specifically, recombinant osteogenic protein-1 (OP-1), also known as bone morphogenetic protein-7 (BMP-7), stimulates ECM production. It has been reported that type II collagen and proteoglycan synthesis increases in bovine and human articular chondrocytes. It has also been reported that insulin-like growth factor (IGF-1) and transforming growth factor (TGF-2) influence proteoglycan synthesis and accumulation in the cell associated matrix.37 These growth factors increase the concentration of proteoglycans although rate effects are not clear.
The models represent a new method to estimate the homeostasis of each matrix molecule species within the construct through incorporation of growth and decay rates. As with any model, limitations exist. Here interactions between molecules or between ECM and the scaffold material are not explored. Although the parameterization process reduces the many possible synthesis influencing factors or variables into a few terms, it does not provide the subtle modification with known active or inactive states of ECM, growth factors, chrondrocytes, etc. which comprise the overall composite. These additional influences may be the cause of the variety of differences shown between GAG and collagen synthesis states (Figures 1 to 3). The reduction of influencing terms and a clear mathematical model does, however, provide a starting point for comparison between the various studies. Additionally, the functional nature of the resulting constructs, although often analysed, are not incorporated into a mechanical result here. Interactions between constituents and the mechanical implications are being explored in ongoing modelling efforts.38
The models use a simplified approach to investigate matrix molecule biosynthesis under the influence of growth factors. Model outcomes can be used to parametrically compare experimental results as well as to explore matrix concentration in the steady state due to additional anabolic and catabolic factors. As a predictive tool, the models may be able to assess culture times necessary to produce a functionally viable construct. This potential application will require a transient analysis of the proposed model, which is currently underway.
Acknowledgments
The authors recognize valuable technical comments from Drs. Gordana Vunjak-Novakovic and Lisa E. Freed, Division of Health Sciences and Technology, Massachusetts Institute of Technology, USA. Partial support for this work was provided by NIH grant DE014288 from the National Institute for Dental and Craniofacial Research to SSK and Kohles Bioengineering, USA.
References
- 1.Patterson, K., Media Release: Innovative Community Projects to Combat Bone and Joint Diseases, Australian Department of Health and Ageing Website, 2003. www.health.gov.au/internet/wcms/Publishing.nsf/Content/health-mediarel-yr2003-kp-kp03123.htm
- 2.Langer R, Vacanti JP. Tissue Engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 3.Lee RC, Frank EH, Grodzinsky AJ, Roylance DK. Oscillatory Compressional Behaviour of Articular Cartilage and its Associated Electromechanical Properties. Journal of Biomechanical Engineering. 1981;103(4):280–292. doi: 10.1115/1.3138294. [DOI] [PubMed] [Google Scholar]
- 4.Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB. Chondrocytes in Agarose Culture Synthesize a Mechanically Functional Extracellular Matrix, Journal of Orthopaedic Research. 1992;10:745–758. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 5.Freed LE, Marquis JC, Nohria A, Emmanual J, Mikos AG, Langer R. Neocartilage Formation in vitro and in vivo using Cells Cultured on Synthetic Biodegradable Polymers. Journal of Biomedical Materials Research. 1993;27:11–23. doi: 10.1002/jbm.820270104. [DOI] [PubMed] [Google Scholar]
- 6.Ragan PM, Chin VI, Hung HK, Masuda K, Thonar EJ, Arner EC, Grodzinsky AJ, Sandy JD. Chondrocyte Extracellular Matrix Synthesis and Turnover Are Influenced by Static Compression in a New Alginate Disk Culture System. Archives of Biochemistry and Biophysics. 2000;383:256–264. doi: 10.1006/abbi.2000.2060. [DOI] [PubMed] [Google Scholar]
- 7.Lee CR, Grodzinsky AJ, Spector M. The Effects of Cross-Linking of Collagen-Glycosaminoglycan Scaffolds on Compressive Stiffness, Chondrocyte-Mediated Contraction, Proliferation and Biosynthesis. Biomaterials. 2001;22:3145–3154. doi: 10.1016/s0142-9612(01)00067-9. [DOI] [PubMed] [Google Scholar]
- 8.Mooney DJ, Mazzoni CL, Breuer C, McNamara K, Hern D, Vacanti JP, Langer R. Stabilized Polyglycolic Acid Fibre-Based Tubes for Tissue Engineering. Biomaterials. 1996;17:115–124. doi: 10.1016/0142-9612(96)85756-5. [DOI] [PubMed] [Google Scholar]
- 9.Wilson CG, Bonassar LJ, Kohles SS. Modelling the Dynamic Composition of Engineered Cartilage. Archives of Biochemistry and Biophysics. 2002;408:246–254. doi: 10.1016/s0003-9861(02)00562-3. [DOI] [PubMed] [Google Scholar]
- 10.van der Kraan PM, Buma P, van Kuppevelt T, van den Berg WB. Interaction of Chondrocytes, Extracellular Matrix and Growth Factors: Relevance for Articular Cartilage Tissue Engineering. Osteoarthritis and Cartilage. 2002;10:631–637. doi: 10.1053/joca.2002.0806. [DOI] [PubMed] [Google Scholar]
- 11.Leisner TM, Wencel-Drake JD, Wang W, Lam SC. Bidirectional Transmembrane Modulation of Integrin αIIbβ3Conformations. Journal of Biological Chemistry. 1999;274:12945–12949. doi: 10.1074/jbc.274.18.12945. [DOI] [PubMed] [Google Scholar]
- 12.Hascall, V. C., Luyten, F. P., Plaas, A. H. K., Sandy, J. D., Steady-State Metabolism of Proteoglycans in Bovine Articular Cartilage. In: Methods in Cartilage Research (Eds: A. Maroudas and K. Kuettner), Academic Press, San Diego, 108–112, 1990.
- 13.Handley S, Lowther DA. Extracellular Matrix Metabolism by Chondrocytes III. Modulation of Proteoglycan Synthesis by Extracellular Levels of Proteoglycan in Cartilage Cells in Culture. Biochimica et Biophysica Acta. 1977;500:132–139. doi: 10.1016/0304-4165(77)90053-8. [DOI] [PubMed] [Google Scholar]
- 14.Hascall, V. C., Sandy, J. D., Handley, C. J., Regulation of Proteoglycan Metabolism in Articular Cartilage. In: The Biology of the Synovial Joint (Eds: C. W. Archer, B. Caterson, M. Benjamin, and J. R. Ralphs), Hardwood Academic Publishers, The Netherlands, 101–120, 1999.
- 15.Cuevas P, Burgos J, Baird A. Basic Fibroblast Growth Factor (FGF) Promotes Cartilage Repair in vivo. Biochemical and Biophysical Research Communications. 1988;156:611–618. doi: 10.1016/s0006-291x(88)80887-8. [DOI] [PubMed] [Google Scholar]
- 16.Sah RL, Chen AC, Grodzinsky AJ, Trippel SB. Differential Effects of bFGF and IGF-1 on Matrix Metabolism in Calf and Adult Bovine Cartilage Explants. Archives of Biochemistry and Biophysics. 1994;308:137–147. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- 17.Sah RL, Trippel SB, Grodzinsky AJ. Differential Effects of Serum Insulin-Like Growth Factor-1, and Fibroblast Growth Factor-2 on the Maintenance of Cartilage Physical Properties During Long Term Culture. Journal of Orthopaedic Research. 1996;14:44–52. doi: 10.1002/jor.1100140109. [DOI] [PubMed] [Google Scholar]
- 18.Pei M, Seidel J, Vunjak-Novakovic G, Freed LE. Growth Factors for Sequential Cellular de- and re-Differentiation in Tissue Engineering. Biochemical and Biophysical Research Communications. 2002;294:149–154. doi: 10.1016/S0006-291X(02)00439-4. [DOI] [PubMed] [Google Scholar]
- 19.Blunk T, Sieminski AL, Gooch KJ, Courter DL, Hollander AP, Nahir AM, Langer R, Vunjak-Novakovic G, Freed LE. Differential Effects of Growth Factors on Tissue-Engineered Cartilage. Tissue Engineering. 2002;8:73–84. doi: 10.1089/107632702753503072. [DOI] [PubMed] [Google Scholar]
- 20.Fortier LA, Lust G, Mohammed HO, Nixon AJ. Coordinate Up Regulation of Cartilage Matrix Synthesis in Fibrin Cultures Supplemented with Exogenous Insulin-Like Growth Factor-1. Journal of Orthopaedic Research. 1999;17:467–474. doi: 10.1002/jor.1100170403. [DOI] [PubMed] [Google Scholar]
- 21.Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-Like Growth Factor-1 Enhances Cell-Based Repair of Articular Cartilage. Journal of Bone and Joint Surgery. 2002;84B:276–288. doi: 10.1302/0301-620x.84b2.11167. [DOI] [PubMed] [Google Scholar]
- 22.Nixon AJ, Saxer RA, Brower-Toland BD. Exogenous Insulin-Like Growth Factor-1 Stimulates an Autoinductive IGF-1 Autocrine/Paracrine Response in Chondrocytes. Journal of Orthopaedic Research. 2001;19:26–32. doi: 10.1016/S0736-0266(00)00013-9. [DOI] [PubMed] [Google Scholar]
- 23.Saha AK, Mazumdar JN. Dynamics of the Cell and its Extracellular Matrix: A Simple Mathematical Approach. IEEE Transactions in Nanobioscience. 2003;2(2):89–93. doi: 10.1109/tnb.2003.813921. [DOI] [PubMed] [Google Scholar]
- 24.Saha AK, Mazumdar JN, Morsi YS. Effects of Environmental Fluctuations on the Dynamic Composition of Engineered Cartilage: A Deterministic Model in Stochastic Environment. IEEE Transactions in Nanobioscience. 2003;2:158–162. doi: 10.1109/tnb.2003.816226. [DOI] [PubMed] [Google Scholar]
- 25.Saha AK, Mazumdar J, Kohles SS. Prediction of Growth Factor Effects on Engineered Cartilage Composition Using Deterministic and Stochastic Modelling. Annals of Biomedical Engineering. 2004;32(6):871–879. doi: 10.1023/b:abme.0000030262.82626.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardiner, C. W., Handbook of Stochastic Methods: For Physics, Chemistry and the Natural Sciences, Synergetics, vol.13, Springer, Berlin, 1997.
- 27.Oksendal, B., Stochastic Differential Equations, 4th edition, Springer, Berlin, 1995.
- 28.Roberts MG, Saha AK. The asymptotic behaviour of a logistic epidemic model with stochastic disease transmission. Applied Mathematical Letters. 1999;12:37–41. [Google Scholar]
- 29.Freed LE, Hollander AP, Martin I, Barry JR, Langer R, Vunjak-Novakovic G. Chondrogenesis in a Cell-Polymer-Bioreactor System. Experimental Cell Research. 1998;240:58–65. doi: 10.1006/excr.1998.4010. [DOI] [PubMed] [Google Scholar]
- 30.Vunjak-Novakovic, G., Freed, L. E., Biron, R. J., Langer, R., Effects of Mixing on the Composition and Morphology of Tissue Engineered Cartilage, Journal of the AIChE, vol. 42, pp.850–860, 1996.
- 31.Vunjak-Novakovic G, Obradovic B, Martin I, Bursac PM, Langer R, Freed LE. Dynamic Cell Seeding of Polymer Scaffolds for Cartilage Tissue Engineering. Biotechnology Progress. 1998;14(2):193–202. doi: 10.1021/bp970120j. [DOI] [PubMed] [Google Scholar]
- 32.Freed LE, Vunjak-Novakovic G, Biron RJ, Eagles DB, Lesnoy DC, Barlow SK, Langer R. Biodegradable Polymer Scaffolds for Tissue Engineering. Biotechnology. 1994;12(7):689–693. doi: 10.1038/nbt0794-689. [DOI] [PubMed] [Google Scholar]
- 33.Obradovic B, Meldon JH, Freed LE, Vunjak-Novakovic G. Glycosaminoglycan Deposition in Engineered Cartilage: Experiments and Mathematical Model. Journal of the AIChE. 2000;46:1860–1871. [Google Scholar]
- 34.Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE. Bioreactor Cultivation Conditions Modulate the Composition and Mechanical Properties of Tissue Engineered Cartilage. Journal of Orthopaedic Research. 1999;17(1):130–138. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 35.Chen P, Vukicevic S, Sampath TK, Luyten FP. Bovine Articular Chondrocytes do not Undergo Hypertrophy when Cultured in the Presence of Serum and Osteogenic Protein-1. Biochemical and Biophysical Research Communications. 1993;197:1253–59. doi: 10.1006/bbrc.1993.2612. [DOI] [PubMed] [Google Scholar]
- 36.Flechtenmacher J, Huch K, Thonar EJ, Mollenhauer JA, Davies SR, Schmid TM, Puhl W, Sampath TK, Aydelotte MB, Kuettner KE. Recombinant Human Osteogenic Protein-1 is a Potent Stimulator of the Synthesis of Cartilage Proteoglycans and Collagens by Human Articular Chondrocytes. Arthritis and Rheumatism. 1996;39(11):478–88. doi: 10.1002/art.1780391117. [DOI] [PubMed] [Google Scholar]
- 37.van Osch GJVM, van den Berg WB, Hunziker EB, Hauselmann HJ. Differential Effects of IGF-1 and TGF β-2 on the Assembly of Proteoglycans in Pericellular and Territorial Matrix by Cultured Bovine Articular Chondrocytes. Osteoarthritis and Cartilage. 1998;6:187–195. doi: 10.1053/joca.1998.0111. [DOI] [PubMed] [Google Scholar]
- 38.Kohles, S. S., Wilson, C. G., Bonassar, L. J., A Micro-Mechanical Composite Analysis of Engineered Cartilage, American Society of Biomechanics, 28th Annual Meeting, Portland, Oregon, USA, September, p.118, 2004.


