Abstract
Recombinant adenoviruses (rAds) represent a promising system for vaccine delivery but transduce dendritic cells (DC) relatively poorly. To address this concern, we used a biotin-avidin linkage to conjugate rAd vectors to ligands which bind with high affinity to selected receptors on DC (ChemR23, αvβ3 integrin, and DC-SIGN). The targeted vectors had an enhanced ability to transduce human monocyte-derived DC compared to untargeted virus. In addition, DC transduced with targeted rAd vectors were more efficient at stimulating cytokine production by autologous memory CD8+ T cells, against a vector-encoded antigen. These results expand the range of cell surface receptors that can be used to target rAd5 vectors to DC, and may facilitate future development of rAd-based vaccines.
Keywords: Adenovirus, Targeting, Dendritic Cell
1. Introduction
Dendritic Cells (DC) play an essential role in bridging the innate and adaptive immune systems (1), and in promoting the antigen-specific activation and expansion of CD8+ and CD4+ T cells (2). As a result, there is significant interest in targeting antigens and vectors to DC, in order to develop improved vaccines.
The ability to effectively transduce DC is believed to be important to the ability of many different virus vector systems, including recombinant adenoviruses (rAd), to elicit strong immune responses to encoded antigens. Indeed, rAd vectors have emerged as promising vaccine delivery systems in a range of settings, perhaps because of their inherent ability to stimulate both the innate and adaptive arms of the immune response (3-7). Despite this, it has been noted that the most widely used rAd vectors, which are based on type 5 adenovirus, are strikingly inefficient in their ability to transduce DC. This is reflected by the fact that efficient in vitro infection of human monocyte derived DC requires a very high input of rAd5 particles (8). The reason for this poor transduction efficiency is thought to be due to the lack of expression of the primary receptor for the virus, CAR, on dendritic cells (9). This observation has prompted a number of investigators to investigate whether one can increase the efficiency of rAd5-mediated gene transfer in DC by targeting the virus to alternate cell surface receptors on this cell type (10-14).
Methods for modifying the receptor specificity of rAd5 vectors include the use of bispecific single-chain antibodies, soluble CAR genetically fused to a single chain antibody or receptor ligand, and genetic modification of the fiber to include targeting peptides or ligands (15) (16, 17). These approaches have been used to target a small subset of DC-expressed surface receptors, including RGD-binding integrins and CD40 (13, 18-22), which were chosen in part because they are endocytosing molecules that are known to play a role in DC activation and maturation. RAd5 vectors that have been targeted in this manner not only have an improved capacity to transduce cultured DC, but the virally-transduced DC have also been shown to elicit enhanced immune responses in vivo (18, 21, 23).
The results summarized above establish strong proof-of-concept support for the notion that one can selectively retarget rAd5 vectors to DC, and thereby improve immune responses to vector-encoded antigens. However, there continues to be considerable interest in improving rAd vector-mediated transduction of DC, and in developing additional or improved DC-targeting strategies that may further expand the utility of this vector system for vaccine delivery. One such approach involves the development of simple, flexible approaches that may permit rapid and convenient surface modification of rAd5 vectors. With this in mind, we elected to take advantage of a recently described, genetically-modified rAd5 vector that contains a biotin acceptor peptide on the C-terminus of the fiber protein, allowing the virus to be metabolically biotinylated and subsequently bound to any ligand of interest via a biotin-avidin bridge (24).
We chose to use the biotin-avidin bridge technology to target three specific receptors on DC, which were selected on the basis of their abundant cell surface expression on dendritic cells, ability to undergo endocytosis and involvement in antigen processing/cellular activation. The receptors, αvβ3 integrin, ChemR23, DC-SIGN (9, 10, 25, 26), were targeted using high affinity binding peptides (in the case of αvβ3 integrin and Chem R23) or a monoclonal antibody (in the case of DC-SIGN). In all cases, we determined that targeting of the DC-selective receptors resulted in an increase in the efficiency of transduction of primary human monocyte-derived DC by rAd5 vectors, as measured using a GFP reporter gene. The targeted vectors also increased the expression of cell surface markers of DC maturation, as compared to unmodified controls. Finally, we constructed a rAd5 vector that encoded the immunodominant human cytomegalovirus (HCMV) pp65 protein, and targeted this to DC via each of the receptors noted above (i.e., αvβ3 integrin, ChemR23 and DC-SIGN). Each of the targeting approaches was found to result in an enhancement of the ability of vector-transduced DC to stimulate the antigen-specific activation of autologous human memory CD8+ T cells, as measured using an intracellular cytokine staining (ICS) assay. These findings have important implications for the future use of rAd-vectored vaccines in human subjects, and suggest that it may be possible to improve the effectiveness of rAd-based immunization approaches through a number of different approaches, that may collectively enhance the utility of this platform technology.
2. Materials and Methods
2.1 Cells
Cell culture reagents were purchased from Invitrogen (Carlsbad, CA). Primary human peripheral blood mononuclear cells (PBMC) were isolated from whole blood, and immature dendritic cells (DC) were generated from CD14+ monocytes as described (27). Briefly, PBMC were isolated from buffy coats after centrifugation on a lymphoprep gradient (AXIS-SHIELD, Oslo, Norway) and subsequently purified by positive selection with anti-CD14 MACS beads (Miltenyi Biotec, Auburn, CA). These monocytes were then cultured in RPMI 1640 medium supplemented with 1% autologous plasma plus 50 ng/ml human recombinant GM-CSF (R&D Systems, Minneapolis, MN) and 25 ng/ml IL-4 (R&D Systems). Media was replenished every 2 days. After 7 days of culture, DC were used for adenovirus infections. HeLa cells were cultured in Dulbecco's Modified Eagle's Medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) and 100 U/ml penicillin, 100μg/ml streptomycin and 2mM l-glutamine (1×PSG), in a humidified environment containing 5% CO2 at 37°C. HeLa cells that stably expressed DC-SIGN (HeLa-DC-SIGN cells) were generated by transfecting cells with a mammalian expression vector encoding DC-SIGN and neomycin resistance (kindly provided by Robert Doms and Carl Davis, University of Pennsylvania) and selecting stable cells resistant to Geneticin (Invitrogen, Carlsbad, CA).
2.2 Biotinylated Antibodies and ligands
Biotinylated anti-human DC-SIGN antibody (clone DCN 46) and a biotinylated isotype matched control (IgG2b,kappa) were obtained from BD Pharmingen (San Jose, CA). Biotinylated chemerin peptide (YHSFFFPGQFAFS) and a matched, alanine-substituted control peptide (YHSFFFPGQFAAS) were synthesized by Sigma-Genosys (The Woodlands, TX). Both peptides were biotinylated on the N-terminus. FNfn10-wt and FNfn10-3JCLI4 peptides were produced, purified, and biotinylated according to published methods (26).
2.3 Adenovirus Construct
The type-5 adenovirus vector used in this study is a fiber-modified Ad5 construct that was previously described by Parrott et al. (24), and which was kindly provided to us by Dr. Mike Barry of Baylor University. Briefly, the construct, referred to as Ad-Fiber-BAP-TR, contains a Biotin-Acceptor-Protein (BAP) motif within the virus fiber protein, that becomes metabolically biotinylated during production of the virus in 293A cells. The virus additionally has a mutation in the fiber knob that ablates CAR binding.
Two different adenovirus expression cassettes were used in this study. The first encodes eGFP(denoted hereafter as Ad-FBAP-GFP), while the second co-expresses eGFP in combination with the immunodominant HCMV protein pp65 (denoted hereafter as Ad-FBAP-pp65). The pp65 gene used in this construct was amplified by PCR from cell culture supernatant containing HCMV strain AD169 virus particles (ATCC VR-538), using primers pp65-A: 5′ATGCATGGTACCATGGAGTCGCGCGGTCGCCGTTG3′ and pp65-B: 5′ATGTCTAGATCAACCTCGGTGCTTTTTGGGCGTCG3′ which included a Kpn I site and XbaI site (underlined) for subsequent cloning into the adenovirus shuttle vector pAdTrack (kind gift of Drs. T-C He and B. Vogelstein, Johns Hopkins University); note that the pAdTrack vector also encodes GFP. The AdTrack vector encoding GFP and pp65 was cotransformed with AdEasy-1 into BJ5183 E. coli cells according to methods described in the AdEasy™ vector system manual (Qbiogene, Carlsbad,CA). A positive recombinant clone was then further recombined with pL29 Fiber-BAP-TR, to generate the final desired construct, as described (24).
To allow targeting of DC-SIGN, Ad-FBAP viruses (expressing GFP or pp65) were produced in 293A cells and purified on a cesium chloride gradient as described (24). Neutravidin (Molecular Probes, Eugene, OR) was then added to the metabolically biotinylated virus particles, and neutravidin (NA)-complexed virions were repurified with a second round of cesium chloride density gradient centrifugation. The neutravidin-complexed virus particles were then mixed for 1 h at 4°C with a molar excess of a biotinylated monoclonal antibody directed against DC-SIGN monoclonal or a irrelevant, isotype matched biotinylated control antibody (BD Biosciences, San Jose, CA). Free antibody was removed by subjecting the antibody-NA-rAd complex to centrifugal purification using a 300K molecular weight cutoff filter (Pall Life Sciences, Ann Arbor, MI), and the final virus preparation was resuspended in storage buffer (20mM Hepes, pH 7.8, 150mM NaCl, 500mM sucrose).
For targeting with biotinylated peptides capable of binding to ChemR23 or αvβ3 integrin, the virus was produced and purified as above and then bound to NA. After the second round of cesium chloride ultracentrifugation, the virus was divided into equal volumes and incubated with a molar excess of the biotinylated ligand at 4°C for 1h before subjecting the peptide-NA-virus complex to overnight dialysis using 50 kDa molecular weight cutoff tubing (Chemicon International, Temecula,CA).
After the final purification step, viral titers were determined by real time PCR using Taqman probe and primers that amplified a small fragment of the Adenovirus hexon gene, as described (28). Briefly, viral DNA was extracted from the protein-NA-virus complex by diluting 5 μl of the virus prep to a final volume of 100 μl in PBS, followed by digestion for 1 h at 50°C with Proteinase K (PK). PK was then inactivated by heating at 95°C for 20 min, and the 2μl of the DNA preparation was added to a PCR reaction mix containing 10 μl of Taqman Universal PCR master mix (Applied Biosystems, Foster City, CA), 900 nM of each primer and 100 nM of probe. A standard curve was generated using dilutions of the plasmid encoding for Ad-Fiber-BAP-TR. Reaction conditions were 95 °C for 10 min followed by 35 cycles of 95°C for 3 sec, 55°C for 10 sec, and 65°C for 1 min; amplification reactions were performed in a BioRad iCycler (BioRad, Hercules, CA).
2.4 pp65 Immunoblot
To detect expression of pp65, we infected 293A cells with 50 viral genome units (vg) per cell with Ad-FBAP-pp65 virus,. Forty-eight hours post-transfection cells were lysed using Cell Lytic reagent (Sigma-Aldrich, Milwaukee, WI) in the presence of protease inhibitors. Because pp65 is localized to an insoluble nuclear fraction, the insoluble pellet was collected after centrifugation of cell lysates and suspended in loading dye containing SDS and β-mercaptoethanol. After boiling and centrifugation to remove debris, the supernatant (containing pp65) was loaded onto a 4-20% Tris-HCl Ready Gel (BioRad) and subjected to electrophoresis at 150V for 1.5 h. The separated proteins were then transferred onto a nitrocellulose membrane at 100V for 1 h, and membranes were blocked for 1 h with PBS containing 10% milk, followed by three rinses of 5 minutes each in PBS-0.1% Tween. The membrane was then incubated for 2h with a 1:5000 dilution of an anti-HCMV pp65 antibody (abcam, Cambridge, UK) in PBS containing 10% milk and 0.1% Tween. The membrane was rinsed 3 times in PBS-0.1% Tween and incubated with a 1:3000 dilution of secondary antibody conjugated to horseradish peroxidase. The labeled proteins were then detected using ECL detection reagents (Amersham, Piscataway, NJ) and Biomax film (Kodak, Rochester, NY).
2.5 Immunofluorescence Assay (IFA) to visualize pp65 expression in primary human DC
Primary DC were infected with either Ad-FBAP-GFP or Ad-FBAP-GFP. Twenty four hours post infection, DC were fixed in 3.7% formaldehyde in 1XPBS, washed in PBS, and then permeabilized in 0.05% Triton X-100 in PBS. These cells were then incubated in blocking solution {2% bovine serum albumin (BSA) in PBS} for 1h. An anti-HCMV pp65 antibody (abcam) was then added at a 1:3200 dilution and incubated for 1h at room temperature. After four rinses with PBS (10 min/rinse), the cells were incubated with Alexa Fluor 594 –conjugated secondary antibodies (1:200 dilution; Molecular Probes) at room temperature for 30 min. After four more rinses in PBS, the cells were analyzed using a fluorescence microscope.
2.6 Adenovirus Transduction assays
Adenovirus particles, or protein-NA-virus complexes were added to target cells at different multiplicities of infection, as determined on the basis of virus titer calculations that were performed by real time PCR quantitation of rAd genomes in the various virus preparations. Briefly 105 cells were exposed to virus for 1h at 37°C in a volume of 200 μl of serum free media. Cells were then rinsed and serum-containing media was added. 24-48h later, the percentage of GFP positive cells was determined by flow cytometry using a FACSCalibur flow cytometer (BD, Franklin Lakes, NJ).
2.7 Immunophenotyping of infected DC
Immature human monocyte-derived DC were used at day 7 following isolation, and were incubated with media alone, LPS, or the various rAd vectors and protein-NA-virus complexes of interest. The phenotypic maturation state of the cells was determined 48 h post infection by staining with APC-conjugated monoclonal antibodies specific for a panel of cell surface markers that included CD40, CD86, and MHC Class II. Cells were analyzed both for expression of GFP (as a marker of rAd transduction) and for the indicated cell surface markers by performing flow cytometric analysis using a FACSCalibur flow cytometer (BD); data were analyzed using CellQuest software (BD).
2.8 Restimulation of CMV-specific autologous memory T cells with rAd tranduced DC
PBMC were isolated from a HCMV seropositive donor and CD14+ cells were isolated and allowed to differentiate into DC, as described above. On day 7 of culture, DC were transduced with unmodified rAd vectors or with corresponding virus-NA-protein complexes of interest. For these experiments, either Ad-FBAP-pp65, or a matched, pp65-negative control virus (Ad-FBAP-GFP) was used to transduce the DC, and cells were maintained in culture for an additional 48 h subsequent to transduction. The ability of Ad-pp65 transduced DC to activate autologous HCMV-specific T cells was then analyzed by isolating autologous PBMC and incubating them with the infected DC at a 50:1 ratio for 18h at 37°C. As a positive control for CMV-specific restimulation, PBMC were incubated with 1.75 μg of an overlapping CMV peptide pool (BD Biosciences). This pool comprises 138 peptides with an individual length of 15 amino acids and an overlap of 11 residues, which were derived from the pp65 sequence of the AD169 strain of HCMV (BD Biosciences). In order to perform intracellular cytokine staining (ICS) analysis, Golgi Plug (BD Biosciences, San Jose, CA) was added to the cultures during the final 12 h of incubation. PBMC were then permeablized and fixed with Cytofix/Cytoperm (BD Biosciences, San Jose, CA) and stained with a CD8 cocktail consisting of anti-CD8 PerCP-Cy5.5, anti-CD3 APC, anti-CD69 PE, and anti-IFN-γ FITC(BD Biosciences). Stained cells were analyzed on a LSR II flow cytometer (BD) and the flow data was subsequently analyzed using FLOWJO software (TreeStar, Ashland, OR).
2.9 Data Analysis and Statistics
Each figure portrays representative results performed in at least three independent experiments, except for Fig 4C (which was performed once). All data values were calculated from samples performed in triplicate and the data presented provides the mean value +/− the standard error of the mean. For some figures group comparisons for repeated measures were computed for ANOVA and paired t-test was performed using the SigmaPlot 2002 V8.02 software from SPSS Inc. (Chicago, IL).
Figure 4.
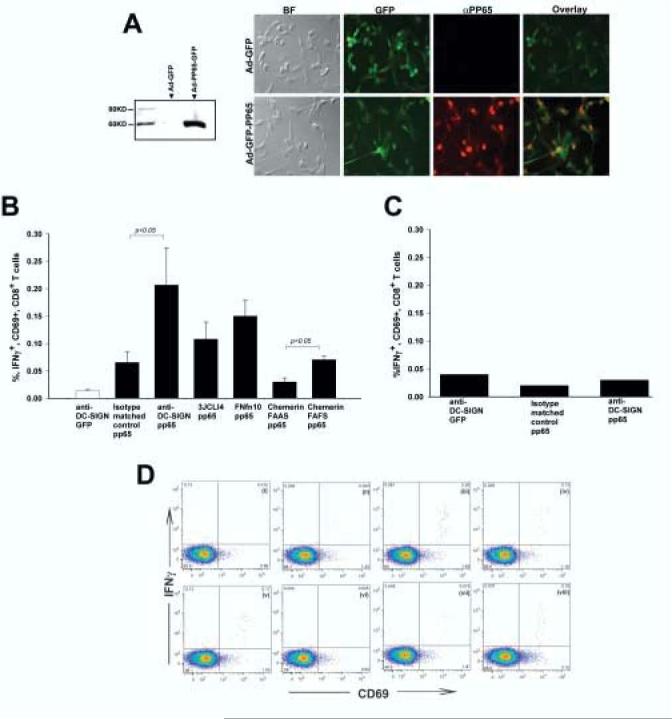
DC transduced with targeted derivatives of Ad-FBAP-pp65 are more efficient at stimulating antigen-specific autologous CD8+ T cells. (A) Efficient expression of HCMV pp65 by Ad-FBAP-pp65. (Left panel) To confirm expression of the pp65 expression cassette, HeLa cells were transduced with Ad-FBAP-pp65. Forty-eight hours thereafter cells were lysed and an immunoblot was performed on nuclear extracts with an anti-pp65 antibody. No immunoreactive protein was detected in lysate from Ad-FBAP-GFP transduced cells (lane 1), while a band of the expected 65 kDa MW was detected in lysate from cells transduced with Ad-FBAP-pp65 vector (lane 2) (Right panel) To confirm expression of pp65 in primary human DC, Ad-FBAP-pp65 or Ad-FBAP-GFP was used to infect cells and twenty-four hours later an immunofluorescence assay was performed using Alexa Fluor 594 conjugated secondary antibodies for anti-pp65. Virally-encoded GFP was also visualized (since both constructs encode GFP); overlay images for GFP and pp65 expression confirmed that nuclear pp65 was detected specifically in cells that also coexpressed GFP (as expected). BF denotes a microscopic image that was recorded under bright field illumination; final magnification in these panels is 200x. (B) Targeting of Ad-FBAP-pp65 to DC-SIGN, ChemR23 or αvβ3 integrin results in a functional increase in specific antigen presentation by vector-transduced human DC. Monocyte-derived human DC from a CMV seropositive donor were transduced with (i) anti-DC-SIGN conjugated Ad-FBAP-GFP virus (“anti-DC-SIGN GFP”), (ii) Ad-FBAP-pp65 virus that was conjugated to an irrelevant monoclonal antibody of the same isotype (“isotype matched control pp65”), (iii) anti-DC-SIGN conjugated Ad-FBAP-pp65 virus (“anti-DC-SIGN pp65”), (iv) a high- affinity and highly selective αvβ3 integrin-binding peptide (3JCLI4), (v) a low-affinity and relatively non-selective αvβ3 integrin-binding (FnFn10), (vi) a mutated derivative of a ChemR23-binding peptide, which contains an alanine substitution in a key residue (chemerin FAAS), and (vii) the wild-type version of this same peptide, which binds to ChemR23 with nanomolar affinity (chemerin FAFS). 48h following vector transduction, the DC were mixed with autologous PBMC. After a further 18h, cells were harvested and analyzed for expression of intracellular IFNγ (as well as cell surface expression of CD3, CD8 and CD69). The results shown represent mean values from three independent experiments; bars denote the standard error of the mean. Statistically significant differences are indicated with p values over the compared groups (t test). (C) Interferon-gamma responses are pp65-specific. Monocyte-derived DC from a CMV seronegative donor were transduced with (i) an anti-DCSIGN antibody conjugated to Ad-FBAP-GFP, (ii) Ad-FBAP-pp65 conjugated to an isotype matched control antibody or (iii) Ad-FBAP-pp65 conjugated to an anti-DC-SIGN antibody. The experiment was performed in the same manner as in 4B. Note that in the CMV seronegative donor, no specific enhancement of intracellular IFNγ production was observed with Ad-FBAP encoding pp65 vs. GFP, underlining the antigen specificity of the assay performed in 4B. (D) Representative intracellular cytokine staining results, corresponding to the data summarized in (B). CD69 expression is plotted on the X-axis, and intracellular IFN-γ expression on the Y-axis; all cells shown in this plot were gated to be CD3+ and CD8+. IFN-γ detection is shown from PBMC incubated with autologous, monocyte-derived DC from a CMV-seropositive donor that were transduced with (i) anti-DC-SIGN conjugated Ad-FBAP-GFP virus (negative control), or Ad-FBAP-pp65 virus that was conjugated to the following ligands: (ii) an irrelevant monoclonal antibody of the same isotype (“isotype matched control pp65”), (iii) an anti-DC-SIGN antibody (“anti-DC-SIGN pp65”), (iv) a high- affinity and highly selective αvβ3 integrin-binding peptide (3JCLI4), (v) a low-affinity and relatively non-selective αvβ3 integrin-binding (FnFn10), (vi) a mutated derivative of a ChemR23-binding peptide, which contains an alanine substitution in a key residue (chemerin FAAS), and (vii) the wild-type version of this same peptide, which binds to ChemR23 with nanomolar affinity (chemerin FAFS). (viii) displays data from PBMC that were incubated with autologous DC that had been pulsed with a pool of overlapping peptides spanning CMV pp65. Numbers in each panel denote the percentage of cells within each of the defined quadrants in the flow cytometric analysis.
3. Results
3.1 Ad-FBAP-GFP can be specifically targeted to DC-SIGN
We and others have hypothesized that it may be possible to improve the effectiveness of rAd-based vaccines by developing improved methods for directing rAd vectors to dendritic cells (11, 24). One approach to this problem is to conjugate rAd vectors to ligands that will result in more efficient virus binding and entry in DC.
Recent studies have expanded our understanding of the repertoire of cell surface receptors that are selectively or specifically expressed on human dendritic cells and their subsets (25, 29)(30-32). Many of these receptors, including the G-protein coupled receptor ChemR23 and the lectin-binding molecule DC-SIGN (29, 32-34), are present at high levels on the surface of DC, and undergo endocytosis following ligand binding. This is highly desirable from the perspective of rAd targeting, and we therefore set out to determine whether it might be possible to target rAd5 vectors to human DC using peptide and protein ligands which can interact at high affinity with a subset of these receptors (notably, ChemR23, DC-SIGN and αvβ3 integrin).
We first targeted the DC-SIGN receptor by conjugating a DC-SIGN-specific monoclonal antibody (or an irrelevant antibody of the same isotype) to the surface of a metabolically biotinylated rAd5 virus vector, using a neutravidin bridge, as previously described (24). The antibody-conjugated, GFP-encoding rAd5 vectors (Ad-FBAP-GFP) were then incubated with HeLa cells that stably expressed DC-SIGN (HeLa-DC-SIGN), or control HeLa cells (DC-SIGN negative), and GFP expression was evaluated by flow cytometry. The DC-SIGN targeted vector was substantially improved in its ability to transduce HeLa-DC-SIGN cells, relative to the control vector (targeted using the irrelevant, isotype-matched antibody), with 76% of cells being GFP positive in the former case and only 26% in the latter (not shown). Transduction of control HeLa cells by the two virus preparations was essentially identical.
The HeLa-DC-SIGN cells used in this analysis, while clonal in origin, expressed varying levels of DC-SIGN (possibly due to differences in chromatinization or methylation of the integrated plasmid encoding DC-SIGN).This allowed us to examine whether the the DC-SIGN targeted virus might be capable of discriminating between DC-SIGN positive, DC-SIGN intermediate, and DC-SIGN negative cells within the same culture. We therefore performed an experiment in which the targeted virus preparation was added to a mixed cell culture containing a preponderance of DC-SIGN negative HeLa cells and a minority of DC-SIGN positive HeLa cells (premixed in a 10:1 ratio). The results, which are shown in Fig. 1, revealed that the DC-SIGN targeted virus transduced almost 7-fold more cells with moderate levels of DC-SIGN on their surface compared to the control vector (43% vs. 6%) and 15-fold more cells with high levels of DC-SIGN on their surface (92% vs. 6%). These data suggest that the DC-SIGN conjugated vector can selectively seek out cells with high DC-SIGN on their surface in an excess of nontarget cells - suggesting that this targeting strategy may be applicable to an in vivo situation in which DC-SIGN positive cells are rare, relative to DC-SIGN negative cells.
Figure 1.
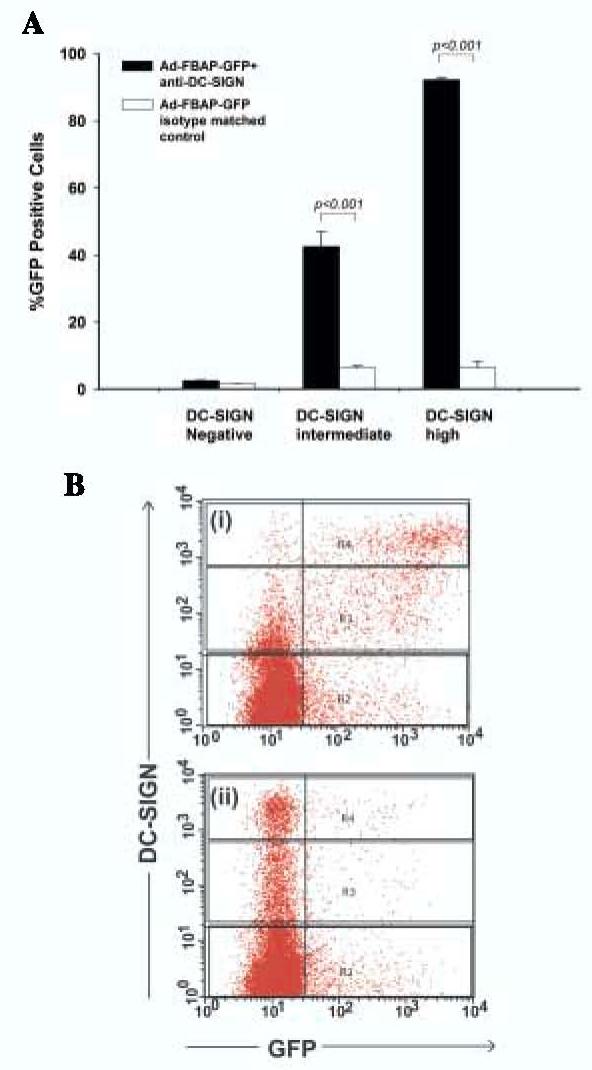
Ad-FBAP-GFP can be specifically targeted to DC-SIGN. (A) HeLa cells that were stably transfected with an empty vector (HeLa-VEC) or HeLa cells that stably expressed human DC-SIGN (HeLa-DCSIGN),were mixed in a 10:1 ratio, respectively, and then transduced with the virus-NA-protein complexes (anti-DC-SIGN antibody or isotype matched control antibody) Levels of GFP expression (% positive cells) are presented separately for the HeLa cells that expressed high levels of DC-SIGN, versus cells that expressed intermediate or undetectable levels of DC-SIGN (see panel B). Data represent mean values from three experiments; bars denote the standard error of the mean. Statistically significant differences are indicated as p values above the compared groups (t test). (B) The gating strategy used to identify high, intermediate and negative DC-SIGN expressing cells within the heterogenous population of HeLa-DCSIGN cells is shown. The representative dot plot analysis corresponds to data from one of the experiments used to generate panel A, and shows DC-SIGN expression on the Y-axis and GFP expression on the X-axis for: (i) Ad-FBAP-GFP bound to the anti-DC-SIGN antibody and (ii) Ad-FBAP-GFP bound to an isotype matched control antibody. R2 denotes the DC-SIGN negative population, while R3 and R4 denote cells that express intermediate and high levels of DC-SIGN, respectively.
3.2 Ad-FBAP-GFP targeted to DC-SIGN,ChemR23, and αvβ3 integrin receptors exhibit an increased efficiency at transducing human monocyte-derived DC
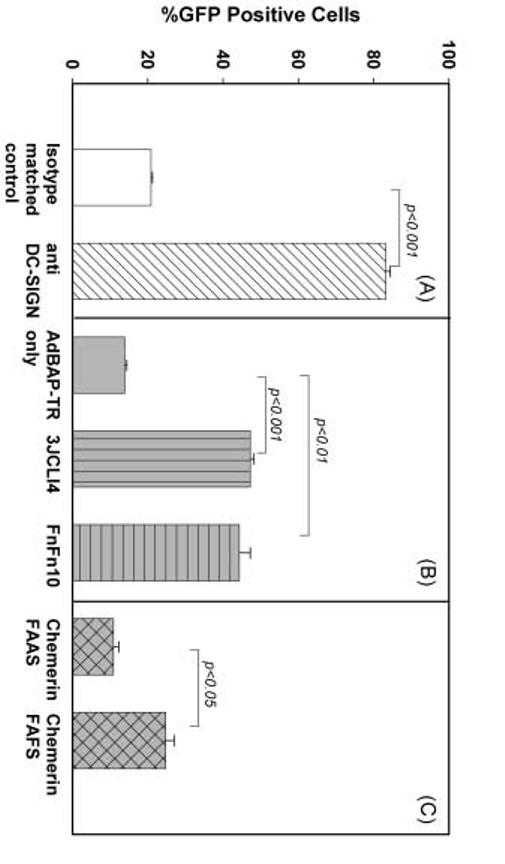
In light of the encouraging findings with HeLa cells that expressed DC-SIGN, we proceeded with experiments intended to determine whether specifically-targeted rAd5 vector conjugates might be more effective at transducing primary human monocyte-derived dendritic cells. As shown in Fig. 2A, the DC-SIGN targeted Ad-FBAP-GFP vector was approximately 4-fold more efficient at transducing primary DC, as compared to the control preparation that was conjugated to the isotype-matched control antibody (83% of DC were GFP-positive in cultures exposed to the former conjugate, versus 21% in cultures exposed to the latter conjugate). In addition, the level of GFP fluorescence in DC transduced by the DC-SIGN targeted virus was on average 2 fold brighter as compared to cells transduced by the control virus preparation (as assessed by mean fluorescence intensity; data not shown).
Figure 2.
Ad-FBAP-GFP transduction of primary human monocyte-derived DC can be enhanced by targeting to alternative receptors. Primary human monocyte-derived DC were used on day 7 following isolation of PBMC. Cells were incubated with unmodified rAd vectors (Ad-FBAP-GFP), or protein/peptide-NA-virus complexes of interest, at a multiplicity of infection of 2000 viral genome units (vg) per cell, and then GFP expression was analyzed 24-28 hours thereafter, using flow cytometry. (A) Analysis of the effect of targeting virus to DC-SIGN: GFP expression data are shown for virus that was conjugated to a DC-SIGN specific monoclonal antibody (dark bars) versus virus that was targeted using an irrelevant antibody of the same isotype (open bars). (B) Analysis of the effect of targeting virus to αvβ3 integrin. GFP expression data are shown for cultures which were transduced with virus that had been left unmodified (Ad-FBAP-GFP) or conjugated to the following peptides: (i) a low-affinity and relatively non-selective αvβ3 integrin-binding (FnFn10), (ii) a high- affinity and highly selective αvβ3 integrin-binding peptide (3JCLI4). (C) Analysis of the effect of targeting virus to ChemR23: GFP expression data are shown for virus that was conjugated to (i) a mutated derivative of a ChemR23-binding peptide, which contains an alanine substitution in a key residue (chemerin FAAS), or (ii) the wild-type version of this same peptide, which binds to ChemR23 with nanomolar affinity (chemerin FAFS). (A, B,C) Data represent mean values from 3 experiments; bars denote the standard error of the mean. Statistically significant differences are indicated as p values above the compared groups (t test).
Having demonstrated that we could enhance vector-mediated transduction of DC using a strategy that targeted the DC-SIGN receptor, we next turned our attention to the αvβ3 integrin and ChemR23 receptors. To target the αvβ3 integrin receptor we conjugated Ad-FBAP-GFP to one of two closely related peptides. These corresponded to FNFn10-wt (hereafter denoted as FnFn10) and FNfn10-3JCLI4 (hereafter denoted as 3JCLI4), which are both based on the tenth domain of fibronectin type III. FnFn10 contains the wild-type sequence of this peptide and binds with low affinity to αvβ3, while 3JCLI4 contains a mutated derivative of this sequence, selected for its ability to bind with high (800 pM) affinity to αvβ3 (26). To target the ChemR23 receptor, we used a biotinylated 13-mer peptide (hereafter denoted as Chemerin FAFS) based on the recently described active C-terminal region of Chemerin, which has been shown to bind with nanomolar affinity to its cognate receptor, ChemR23 (32, 35). We also included a biotinylated control peptide in which a critical phenylalanine residue with Chemerin FAFS was changed to alanine; this peptide (hereafter denoted as Chemerin FAAS) was used as a negative control.
Peptide-conjugated stocks of the Ad-FBAP-GFP vector were then prepared, and used to transduce monocyte-derived DC. Analysis of GFP expression by the DC 24 hours thereafter revealed that each of the targeting approaches resulted in an enhancement of vector-mediated gene transfer, relative to unmodified Ad-FBAP-GFP (Fig. 2B and 2C). Enhancement of DC transduction efficiency was greatest in the case of the two integrin-targeted virus conjugates (3JCLI4 and Fnfn10), which respectively transduced 47% and 44% of cells in the culture, as compared to only 14% in the case of cultures that were incubated with unmodified Ad-FBAP-GFP (Fig. 2B). In addition, the mean fluorescence intensity of the GFP-positive cells in cultures that were exposed to 3JCLI4- and Fnfn10- conjugated vector was, respectively, 2.6-fold and 1.96-fold greater than in the GFP-positive cells from cultures transduced with unmodified Ad-FBAP-GFP. Similar, though slightly less impressive, results were obtained with virus stocks that were conjugated to the Chemerin FAFS peptide (25% of DC in the culture became GFP+), as compared to cells that conjugated to the negative control Chemerin FAAS peptide (only 11% of cells in the culture became GFP+; Fig. 2C).
3.3 DC transduced by DC-SIGN,ChemR23, and αvβ3 integrin targeted vectors exhibit an increased level of phenotypic maturation
To ascertain whether DC transduced by each of the three targeting strategies maintained or increased their maturation status, immature human DC were transduced with Ad-FBAP-GFP bound to each of the targeting ligands described in the preceding section. Forty-eight hours later, cells were harvested and analyzed for both GFP expression (as a marker for viral transduction) and for the expression of a panel of cell surface markers, whose expression has been linked to DC maturation (CD86, CD40 and MHC Class II).
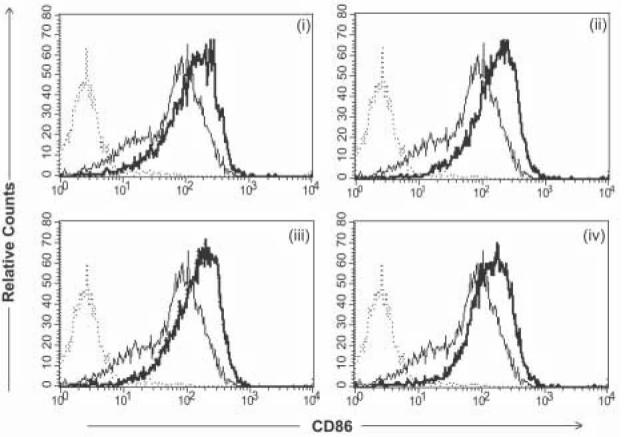
DC that were incubated with each of the targeted virus stocks exhibited a reproducible upregulation of CD86 and MHC class II expression, as measured in terms of mean fluorescence intensity (Fig. 3). There was also a modest overall increase in CD40 expression, in cultures exposed to the DC-SIGN, ChemR23 and integrin-targeting ligands (data not shown). In order to examine whether this increase in phenotypic maturation was associated with actual transduction of cells by the adenovirus vector, we analyzed the expression of CD86, CD40 and Class II on both the transduced (GFP positive) and untransduced (GFP negative) cells within the culture. In all cases, the vector-transduced cells exhibited higher levels of cell surface receptor expression – suggesting that these cells were more mature than their non-transduced counterparts, within the same culture (Table 1).
Figure 3.
Phenotypic analysis of CD86 expression following transduction with various targeted derivatives of Ad-FBAP-GFP. Primary human monocyte-derived DC were incubated with protein/peptide-NA-virus complexes of interest, at a multiplicity of infection of 2000 viral genome units (vg) per cell, and CD86 expression was analyzed by flow cytometry 48 hours later. AdFBAP-GFP virus was targeted using the following reagents: (i) a DC-SIGN specific monoclonal antibody (DC-SIGN), (ii) a nonamer peptide that binds to ChemR23 with nanomolar affinity (chemerin FAFS), (iii) a low-affinity and relatively non-selective αvβ3 integrin-binding (FnFn10), (iv) a high- affinity and highly selective αvβ3 integrin-binding peptide (3JCLI4). In each panel, the thin/light line denotes CD86 staining of unmanipulated DC (not exposed to virus) and the dark/thick line shows the CD86 expression profile of cells that were transduced with the indicated protein/peptide-NA-virus complexes. Results shown are representative of 3 independent experiments, each of which generated similar results.
Table 1.
DC transduced by Ad-FBAP-GFP exhibit an enhanced level of phenotypic maturation, as compared to untransduced DC in the same culture.
| DC Marker | Receptor Targeting | GFP+ Cells | GFP− Cells |
|---|---|---|---|
| CD40 | Untargeted | 4.16 | 3.32 |
| Isotype | 3.80 | 3.01 | |
| Anti-DC-SIGN | 3.73 | 3.11 | |
| 3JCLI4 | 3.63 | 3.34 | |
| FNfn10 | 3.67 | 2.92 | |
| Chemerin (FAAS) | 4.11 | 3.29 | |
| Chemerin (FAFS) |
4.34 |
3.5 |
|
| CD86 | Untargeted | 142 | 82 |
| Isotype | 138 | 86 | |
| Anti-DC-SIGN | 152 | 95 | |
| 3JCLI4 | 157 | 102 | |
| FNfn10 | 105 | 63 | |
| Chemerin (FAAS) | 126 | 74 | |
| Chemerin (FAFS) |
140 |
85 |
|
| Class II | Untargeted | 213 | 127 |
| Isotype | 240 | 131 | |
| Anti-DC-SIGN | 187 | 120 | |
| 3JCLI4 | 253 | 154 | |
| FNfn10 | 188 | 112 | |
| Chemerin (FAAS) | 202 | 114 | |
| Chemerin (FAFS) | 224 | 140 |
DC were transduced with Ad-FBAP-GFP targeted with the indicated molecules (see Fig. 2 legend). 48h later, cells were harvested and subjected to flow cytometric analysis using antibodies against CD40, CD86, and MHC Class II. Analysis of staining was then performed separately for gated GFP+ and GFP− cells within the same culture (i.e., cells that were transduced by the vector as well as “bystander” cells). Data shown represent mean fluorescence intensity values from three replicate measurements, taken from a single experiment. The data are representative of results from 3 independent experiments.
3.4 DC transduced by DC-SIGN,ChemR23, and αvβ3 integrin targeted vectors exhibit an enhanced ability to functionally activate antigen-specific, autologous human CD8+ T cells
The ultimate goal behind selective targeting of rAd vectors to dendritic cells is to enhance immune responses to a virally-vectored antigen. With this in mind, we decided to perform functional analyses, intended to analyze the efficiency with which vector-transduced DC could activate autologous, antigen-specific memory T cells. For this experiment, we elected to study recall responses to a highly immunodominant antigen from a ubiquitous human pathogen. We therefore selected the pp65 gene product from human cytomegalovirus (36), and identified a number of CMV-seropositive adults from whom we could isolate DC and autologous PBMC. We then constructed a pp65-encoding rAd5 vector (Ad-FBAP-pp65) and verified that the resulting virus was capable of efficiently producing pp65 in cultured mammalian cells, most notably primary human monocyte-derived DC (Fig 4A).
The Ad-FBAP-pp65 virus was then conjugated to the DC-SIGN, ChemR23 and αvβ3 integrin-specific targeting ligands, and the conjugates were used to transduce primary monocyte-derived DC. A separate aliquot of DC that were transduced with a pp65-negative vector (Ad-FBAP-GFP) served as a negative control in this experiment, while DC that were pulsed with an overlapping peptide pool derived from pp65 served as a positive control (this pool comprises 138 peptides with an individual length of 15 amino acids and an overlap of 11 residues, based on the predicted sequence of HCMV AD169 pp65). The transduced and control DC were then mixed with autologous PBMC from a CMV seropositive donor, and 18h later, the PBMC were harvested and subjected to intracellular cytokine staining for IFN-γ as well as cell surface staining for the T cell markers CD3 and CD8, and the activation marker CD69. As shown in Figs. 4B and 4D, the DC-SIGN targeted Ad-FBAP-pp65 vector was able to more efficiently stimulate antigen-specific, autologous CD8+ T cells (by approximately 3-fold), as compared to an identical vector that was conjugated to an irrelevant, isotype-matched antibody. Furthermore, the integrin- and ChemR23- targeted Ad-FBAP-pp65 vector preparations were also able to stimulate antigen-specific, autologous CD8+ T cells more efficiently (2-3 fold) than negative controls (i.e., vector conjugated to the Chemerin FAAS peptide, or Ad-FBAP-GFP vector) (Figs. 4B and 4D). To ensure that the responding CD8+ T cells were specific for HCMV pp65, we performed similar restimulation experiments using DC and autologous PBMC from a CMV seronegative donor. In this case, there was no significant difference in T cell activation by DC transduced with the Ad-FBAP-pp65 vector as compared to the irrelevant Ad-FBAP-GFP construct, regardless of the conjugation method used (Fig 4C).
4. Discussion
The results described here demonstrate that rAd5 vectors can be successfully targeted to human monocyte-derived DC, using any one of three cell surface receptors – two of which (DC-SIGN and ChemR23) have not been extensively characterized for rAd targeting. We accomplished this by using a recently described, versatile system that permits one to conjugate any biotinylated ligand of interest to a metabolically biotinylated adenovirus particle, using an avidin bridge (24). The resulting complex is highly stable, due to the extremely high affinity of the biotin-avidin interaction (10−15M), making it very appealing for in vivo use.
We first generated GFP-encoding rAd5 virus preparations that were conjugated to a monoclonal antibody specific to human DC-SIGN, or to an irrelevant, isotype-matched antibody. We then analyzed the specificity of our targeting system by adding these virus preparations to either parental HeLa cells (DC-SIGN negative) or to a HeLa subline that was engineered to stably express DC-SIGN on its surface (HeLa-DC-SIGN cells). These studies revealed that the DC-SIGN targeted vector had an approximately 3-fold enhancement in transduction of HeLa-DC-SIGN cells compared to vector bound to a biotinylated isotype-matched control antibody. Additionally in a mixed cell infection which consisted of 90% DC-SIGN negative parental HeLa cells and only 10% DC-SIGN expressing HeLa cells, the DC-SIGN targeted vector transduced 15-fold more cells with high levels of DC-SIGN on their surface, as compared to the vector that was conjugated to an irrelevant, isotype-matched control antibody. This finding is encouraging, and suggests that the DC-SIGN targeting approach might prove effective in an in vivo setting, in which the rAd5 vector is likely to encounter a small minority of cells that display very high surface levels of DC-SIGN (dendritic cells), in the context of a vast majority of cells that are DC-SIGN negative (fibroblasts, endothelial cells, etc).
These initial proof-of-concept experiments using DC-SIGN were then extended to examine the effectiveness of additional targeting strategies, including the use of high affinity ligands for other endocytosing receptors present on dendritic cells. These experiments included an evaluation of DC transduction using all three of the targeting ligands we had elected to explore (i.e., DC-SIGN, αvβ3 integrin and ChemR23 receptors).
While these studies were underway, the Curiel group reported that the results of studies in which they conjugated another DC-SIGN specific monoclonal antibody to a rAd5 vector (37). In this study, Korokhov and colleagues coupled the targeting ligand to the adenovirus using the Fc-binding domain of Staphylococcus aureus protein A, which was genetically engineered into the adenovirus fiber protein (37). Their results were broadly similar to those reported here, in that the DC-SIGN targeting approach enhanced the efficiency of vector-mediated transduction of monocyte-derived DC, while also modestly increasing DC maturation. However, some important differences exist between this earlier study and the data reported here. First, we have extended these findings by showing that rAd5 vectors which are targeted to DC-SIGN can enhance the functional ability of vector-transduced DC to support antigen-specific activation of autologous CD8+ T cells. This is important because a number of studies have suggested that DC-SIGN targeting can, under some circumstances, lead to sequestration of antigen and immune evasion (38). Second, our targeting approach has significant advantages over the method used by Korokhov and coworkers due to the use of the avidin-biotin bridge technology. This approach results in the essentially irreversible coupling to the targeting molecule to the adenovirus fiber coat protein. In contrast, the Fc-binding interaction employed in the conjugation reaction used by Korokhov is roughly one million-fold less stable, having only nanomolar affinity as compared to the femtomolar affinity of the avidin-biotin interaction. Furthermore, the Fc-based tethering approach is likely to prove unstable in vivo due to the presence of a huge excess of serum immunoglobulins that will compete for binding to the protein A subdomain to which the DC-SIGN targeting antibody was attached (37, 39).
Targeting of rAd5 vectors to cellular integrins has previously been accomplished through the genetic insertion of integrin-binding RGD motifs into the adenovirus fiber knob (10, 20, 23). These studies showed that incorporation of the RGD motif could enhance gene transfer to primary human DC, while also resulting in an increase in the phenotypic maturation of the transduced cells (10, 20). Thus, considerable precedent existed for the notion of targeting rAd5 vectors to cellular integrins, prior to the initiation of our studies. The novelty of our approach was that we used a more highly selective targeting strategy, focused on the αvβ3 integrin (as opposed to the wide range of integrins that will be targeted via RGD-containing proteins). For this purpose, we took advantage of a small, stable and high (800 pM) affinity peptide ligand for αvβ3 integrin that was previously developed in our laboratory, and which is designated here as 3JCLI4 (26). This peptide was isolated following phage display-mediated selection of a partially randomized peptide library derived from the tenth domain of fibronectin type III (Fnfn10), which also binds to the αvβ3 integrin, albeit with much lower affinity (26, 40). In the present study, we made the unexpected observation that both 3JCLI4 and Fnfn10 were effective in targeting a rAd5 vector to primary dendritic cells, enhancing DC maturation and increasing the antigen-specific immunostimulatory activity of vector-transduced DC. This unanticipated equivalence in the targeting efficiency of 3JCLI4 versus wild-type Fnfn10 suggests that these ligands might be enhancing rAd5-mediated transduction via a receptor other than αvβ3 integrin. Further studies will be required to examine this issue.
Finally, we also explored whether ChemR23 could be used as a novel target receptor for rAd5 vector-mediated transduction of primary DC. ChemR23 is a G protein coupled chemokine receptor present on human monocyte-derived DC and macrophages, as well as blood-derived plasmacytoid and myeloid DC (29, 32). The natural ligand of ChemR23, Chemerin, has been shown to serve as a potent chemotactic factor for primary DC, and truncated forms of Chemerin as short as 9 amino acids have been demonstrated to bind to ChemR23 with nanomolar affinity (34, 35). We therefore used a biotinylated 13-mer peptide derived from Chemerin to target our rAd5 vectors to primary human monocyte-derived DC. This peptide (designated here as Chemerin FAFS) was able to significantly improve the efficiency of rAd5 vector-mediated transduction of DC, while also increasing the phenotypic maturation of transduced DC and enhancing their ability to support antigen-specific activation of autologous human CD8+ T cells. Importantly, this enhancement was not observed when a mutated derivative of the Chemerin targeting peptide was used (this mutated peptide, designated here as Chemerin FAAS, contains a phenylalanine-to-alanine substitution at a reside known to be required for receptor binding; (35)). The use of the ChemR23/Chemerin-axis as a targeting strategy may also have effects on DC migration, which could represent an additional advantage of this approach – although additional studies will be needed to investigate the feasibility of this idea.
In conclusion, our results expand the repertoire of surface-expressed receptors that can be successfully used to target adenovirus vectors to human dendritic cells, and further establish the utility of metabolically biotinylated adenovirus vectors as a versatile platform for virus targeting to DC. Our data also demonstrate, for the first time, that a G-protein coupled receptor (ChemR23) can be used to direct a virus vector to human DC. In the future, it may be possible to use different targeting ligands to selectively address rAd vectors to particular DC subsets of interest - such as skin Langerhans cells, dermal and other interstitial DC, and plasmacytoid DC. Furthermore, an increased understanding of the outcome of such targeting strategies on the functional activity and maturational status of DC may make it possible to modulate (suppress or enhance) antigen-specific immune responses, for purposes of therapeutic intervention or vaccination.
ACKNOWLEDGEMENTS:
We would like to thank: George (Skip) Mercier and Mike Barry of Baylor University for the rAd vector system used in this work, for helpful advice throughout the course of our work; Robert Doms and Carl Davis for the DC-SIGN expression vector; Petra Henning and Leif Lindholm for valuable advice, discussions and assistance; Cathy Bunce, Cathy Garrett, and Julie O'Reilly of the University of Rochester Vaccine Evaluation Unit for help with phlebotomy; Birgit Bradel-Tretheway for assistance with the real time PCR protocol; Xia Jin for help in designing the pp65 restimulation assay; and John Frelinger for helpful suggestions. This work was supported by NIH grants T32 AI49815 (to C.M.), RO1 DE14194 (to R.S.), NSF REU 9986712 (to N.G.), T32 DA07232 (to S.H.R.), R21 AI058791(to S.D.) and DAMD 17-01-1-0384 (to S.D.).
References
- 1.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Foged C, Sundblad A, Hovgaard L. Targeting vaccines to dendritic cells. Pharm Res. 2002;19(3):229–38. doi: 10.1023/a:1014474414097. [DOI] [PubMed] [Google Scholar]
- 3.Shiver JW, Emini EA. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med. 2004;55:355–72. doi: 10.1146/annurev.med.55.091902.104344. [DOI] [PubMed] [Google Scholar]
- 4.Jenne L, Schuler G, Steinkasserer A. Viral vectors for dendritic cell-based immunotherapy. Trends Immunol. 2001;22(2):102–7. doi: 10.1016/s1471-4906(00)01813-5. [DOI] [PubMed] [Google Scholar]
- 5.Jooss K, Yang Y, Fisher KJ, Wilson JM. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72(5):4212–23. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowen GP, Borgland SL, Lam M, Libermann TA, Wong NC, Muruve DA. Adenovirus vector-induced inflammation: capsid-dependent induction of the C-C chemokine RANTES requires NF-kappa B. Hum Gene Ther. 2002;13(3):367–79. doi: 10.1089/10430340252792503. [DOI] [PubMed] [Google Scholar]
- 7.Higginbotham JN, Seth P, Blaese RM, Ramsey WJ. The release of inflammatory cytokines from human peripheral blood mononuclear cells in vitro following exposure to adenovirus variants and capsid. Hum Gene Ther. 2002;13(1):129–41. doi: 10.1089/10430340152712683. [DOI] [PubMed] [Google Scholar]
- 8.Offringa R, Kwappenberg K, Rabelink M, Rea D, Hoeben R. Adenoviral transduction of dendritic cells. Methods Mol Med. 2005;109:83–96. doi: 10.1385/1-59259-862-5:083. [DOI] [PubMed] [Google Scholar]
- 9.Rea D, Schagen FH, Hoeben RC, et al. Adenoviruses activate human dendritic cells without polarization toward a T-helper type 1-inducing subset. J Virol. 1999;73(12):10245–53. doi: 10.1128/jvi.73.12.10245-10253.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada N, Tsukada Y, Nakagawa S, et al. Efficient gene delivery into dendritic cells by fiber-mutant adenovirus vectors. Biochem Biophys Res Commun. 2001;282(1):173–9. doi: 10.1006/bbrc.2001.4527. [DOI] [PubMed] [Google Scholar]
- 11.Tillman BW, de Gruijl TD, Luykx-de Bakker SA, et al. Maturation of dendritic cells accompanies high-efficiency gene transfer by a CD40-targeted adenoviral vector. J Immunol. 1999;162(11):6378–83. [PubMed] [Google Scholar]
- 12.Pereboev AV, Nagle JM, Shakhmatov MA, et al. Enhanced gene transfer to mouse dendritic cells using adenoviral vectors coated with a novel adapter molecule. Mol Ther. 2004;9(5):712–20. doi: 10.1016/j.ymthe.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Brandao JG, Scheper RJ, Lougheed SM, et al. CD40-targeted adenoviral gene transfer to dendritic cells through the use of a novel bispecific single-chain Fv antibody enhances cytotoxic T cell activation. Vaccine. 2003;21(1920):2268–72. doi: 10.1016/s0264-410x(03)00050-1. [DOI] [PubMed] [Google Scholar]
- 14.Pereboev AV, Asiedu CK, Kawakami Y, et al. Coxsackievirus-adenovirus receptor genetically fused to anti-human CD40 scFv enhances adenoviral transduction of dendritic cells. Gene Ther. 2002;9(17):1189–93. doi: 10.1038/sj.gt.3301767. [DOI] [PubMed] [Google Scholar]
- 15.Heideman DA, van Beusechem VW, Offerhaus GJ, et al. Selective gene transfer into primary human gastric tumors using epithelial cell adhesion molecule-targeted adenoviral vectors with ablated native tropism. Hum Gene Ther. 2002;13(14):1677–85. doi: 10.1089/104303402760293529. [DOI] [PubMed] [Google Scholar]
- 16.Henning P, Magnusson MK, Gunneriusson E, et al. Genetic modification of adenovirus 5 tropism by a novel class of ligands based on a three-helix bundle scaffold derived from staphylococcal protein A. Hum Gene Ther. 2002;13(12):1427–39. doi: 10.1089/10430340260185067. [DOI] [PubMed] [Google Scholar]
- 17.Barnett BG, Crews CJ, Douglas JT. Targeted adenoviral vectors. Biochim Biophys Acta. 2002;1575(13):1–14. doi: 10.1016/s0167-4781(02)00249-x. [DOI] [PubMed] [Google Scholar]
- 18.Tillman BW, Hayes TL, DeGruijl TD, Douglas JT, Curiel DT. Adenoviral vectors targeted to CD40 enhance the efficacy of dendritic cell-based vaccination against human papillomavirus 16-induced tumor cells in a murine model. Cancer Res. 2000;60(19):5456–63. [PubMed] [Google Scholar]
- 19.de Gruijl TD, Luykx-de Bakker SA, Tillman BW, et al. Prolonged maturation and enhanced transduction of dendritic cells migrated from human skin explants after in situ delivery of CD40-targeted adenoviral vectors. J Immunol. 2002;169(9):5322–31. doi: 10.4049/jimmunol.169.9.5322. [DOI] [PubMed] [Google Scholar]
- 20.Okada N, Saito T, Masunaga Y, et al. Efficient antigen gene transduction using Arg-Gly-Asp fiber-mutant adenovirus vectors can potentiate antitumor vaccine efficacy and maturation of murine dendritic cells. Cancer Res. 2001;61(21):7913–9. [PubMed] [Google Scholar]
- 21.Okada N, Masunaga Y, Okada Y, et al. Dendritic cells transduced with gp100 gene by RGD fiber-mutant adenovirus vectors are highly efficacious in generating anti-B16BL6 melanoma immunity in mice. Gene Ther. 2003;10(22):1891–902. doi: 10.1038/sj.gt.3302090. [DOI] [PubMed] [Google Scholar]
- 22.Richards JL, Abend JR, Miller ML, Chakraborty-Sett S, Dewhurst S, Whetter LE. A peptide containing a novel FPGN CD40-binding sequence enhances adenoviral infection of murine and human dendritic cells. Eur J Biochem. 2003;270(10):2287–94. doi: 10.1046/j.1432-1033.2003.03596.x. [DOI] [PubMed] [Google Scholar]
- 23.Worgall S, Busch A, Rivara M, et al. Modification to the capsid of the adenovirus vector that enhances dendritic cell infection and transgene-specific cellular immune responses. J Virol. 2004;78(5):2572–80. doi: 10.1128/JVI.78.5.2572-2580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parrott MB, Adams KE, Mercier GT, Mok H, Campos SK, Barry MA. Metabolically biotinylated adenovirus for cell targeting, ligand screening, and vector purification. Mol Ther. 2003;8(4):688–700. doi: 10.1016/s1525-0016(03)00213-2. [DOI] [PubMed] [Google Scholar]
- 25.Soilleux EJ, Morris LS, Leslie G, et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71(3):445–57. [PubMed] [Google Scholar]
- 26.Richards J, Miller M, Abend J, Koide A, Koide S, Dewhurst S. Engineered fibronectin type III domain with a RGDWXE sequence binds with enhanced affinity and specificity to human alphavbeta3 integrin. J Mol Biol. 2003;326(5):1475–88. doi: 10.1016/s0022-2836(03)00082-2. [DOI] [PubMed] [Google Scholar]
- 27.Romani N, Reider D, Heuer M, et al. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. Journal of Immunological Methods. 1996;196(2):137–51. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 28.Heim A, Ebnet C, Harste G, Pring-Akerblom P. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J Med Virol. 2003;70(2):228–39. doi: 10.1002/jmv.10382. [DOI] [PubMed] [Google Scholar]
- 29.Vermi W, Riboldi E, Wittamer V, et al. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J Exp Med. 2005;201(4):509–15. doi: 10.1084/jem.20041310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12(1):71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 31.Mahnke K, Guo M, Lee S, et al. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol. 2000;151(3):673–84. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samson M, Edinger AL, Stordeur P, et al. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. Eur J Immunol. 1998;28(5):1689–700. doi: 10.1002/(SICI)1521-4141(199805)28:05<1689::AID-IMMU1689>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 33.Kwon DS, Gregorio G, Bitton N, Hendrickson WA, Littman DR. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity. 2002;16(1):135–44. doi: 10.1016/s1074-7613(02)00259-5. [DOI] [PubMed] [Google Scholar]
- 34.Wittamer V, Franssen JD, Vulcano M, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198(7):977–85. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wittamer V, Gregoire F, Robberecht P, Vassart G, Communi D, Parmentier M. The C-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J Biol Chem. 2004;279(11):9956–62. doi: 10.1074/jbc.M313016200. [DOI] [PubMed] [Google Scholar]
- 36.Wills MR, Carmichael AJ, Mynard K, et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70(11):7569–79. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korokhov N, de Gruijl TD, Aldrich WA, et al. High Efficiency Transduction of Dendritic Cells by Adenoviral Vectors Targeted To DC-SIGN. Cancer Biol Ther. 2005;4(3) doi: 10.4161/cbt.4.3.1499. [DOI] [PubMed] [Google Scholar]
- 38.Geijtenbeek TB, Van Vliet SJ, Koppel EA, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197(1):7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henning P, Andersson KM, Frykholm K, et al. Tumor cell targeted gene delivery by adenovirus 5 vectors carrying knobless fibers with antibody-binding domains. Gene Ther. 2005;12(3):211–24. doi: 10.1038/sj.gt.3302408. [DOI] [PubMed] [Google Scholar]
- 40.Koide A, Bailey CW, Huang X, Koide S. The fibronectin type III domain as a scaffold for novel binding proteins. J Mol Biol. 1998;284(4):1141–51. doi: 10.1006/jmbi.1998.2238. [DOI] [PubMed] [Google Scholar]