Abstract
Objective To compare reproductive outcomes in couples carrying a structural chromosome abnormality and non-carrier couples referred for chromosome analysis after two or more miscarriages.
Design Case-control study.
Setting Six centres for clinical genetics in the Netherlands.
Participants 278 carrier couples and 427 non-carrier couples referred for chromosome analysis between 1992 and 2000 after two or more miscarriages before 20 weeks of gestation. Couples were followed up for at least 24 months after chromosome analysis.
Main outcome measures The birth of at least one healthy child, at least one more miscarriage, and viable offspring with unbalanced chromosomal abnormalities after parental chromosome analysis.
Results Mean follow-up after chromosome analysis was 5.8 years. 120 of 247 (49%) carrier couples had one or more miscarriage after chromosome analysis compared with 122 of 409 (30%) non-carrier couples (difference 19%, 95% confidence interval 11% to 26%; P < 0.01). The percentage of couples with at least one healthy child was not significantly different in carrier couples (83%) and non-carrier couples (84%) (difference -1%, - 7% to 5%). Among 550 pregnancies in carrier couples, two viable unbalanced chromosome abnormalities were detected at prenatal diagnosis (0.4%) and the fetuses aborted and two children with an unbalanced karyotype were born (0.4%).
Conclusions Couples whose carrier status was ascertained after two or more miscarriages have a low risk of viable offspring with unbalanced chromosomal abnormalities. Their chances of having a healthy child are as high as non-carrier couples, despite a higher risk of miscarriage.
Introduction
Balanced structural chromosome abnormalities (abnormalities that involve the rearrangement of genetic material but no overall gain or loss, such as inversions and translocations) in parents can cause recurrent miscarriage. In couples with two or more miscarriages the incidence of these abnormalities varies between 3% and 6%.1-4 In carrier couples the products of conception can have a normal karyotype, the same balanced structural chromosome abnormality as the carrier, or an unbalanced structural chromosome abnormality. The last scenario can lead to the fetus being miscarried, a stillborn child, or a child born with major congenital defects and severe mental handicap. Current guidelines for the management of recurrent miscarriage recommend chromosome analysis in both partners.5-7 Once a structural chromosome abnormality has been detected, prenatal diagnosis in subsequent pregnancies and termination of pregnancy in the case of an unbalanced fetal karyotype is available.
To counsel carrier couples about their risk of viable offspring with unbalanced chromosomal abnormalities and their chances of having a healthy child or miscarriage we need to know the outcome in a population with similar abnormalities. Reports of reproductive outcome in carrier couples whose carrier status was ascertained after recurrent miscarriage provide information on only the first pregnancy after chromosome analysis or on the results of prenatal diagnosis in subsequent pregnancies, or they lack detailed information on reproductive outcome.8-13 In most studies a control group was not investigated, and they all studied small numbers of carrier couples.8-13
We aimed to investigate the long term reproductive outcome in carrier couples whose carrier status was ascertained after two or more miscarriages and to compare this outcome with that in non-carrier couples with two or more miscarriages.
Methods
Study design
We used the databases of six centres for clinical genetics in the Netherlands to identify all couples presenting for parental chromosome analysis between January 1992 and January 2001, after two or more miscarriages. Couples referred to the Academic Medical Centre, Amsterdam, have been presented elsewhere.14 When one partner was found to carry a structural chromosome abnormality we identified the couple as a carrier couple. We selected a random subset of two non-carrier couples per carrier couple by identifying the non-carrier couples tested immediately before and after the carrier couple. This matching was performed to obtain a sample balanced over time. We selected couples with at least two verified miscarriages before 20 weeks of gestation. Exclusion criteria were fewer than two miscarriages verified by a pregnancy test or ultrasonography, or if the couple did not speak Dutch, and the presence of genetic diseases in the couple that might cause chromosomal abnormalities in the fetus.
We contacted eligible couples by mail and invited them to participate in our study. After written informed consent had been obtained, we examined the medical records of the relevant department of clinical genetics and asked both partners to complete a questionnaire. Non-responders received reminders. We collected additional information from telephone interviews and the medical records of the referring doctor or midwife. Data collection focused on the reproductive outcome of both categories of couples, which was recorded for at least 24 months after chromosome analysis. The main outcome measure was a successful reproductive outcome, defined as the birth of one or more healthy (phenotypically normal) children. Other outcome measures were miscarriages and other adverse reproductive outcomes, including stillbirths, viable offspring with unbalanced chromosomal abnormalities, and viable offspring with other chromosomal or congenital abnormalities, detected either prenatally or after birth.
Cytogenetic analysis
We obtained chromosome preparations from routine peripheral blood lymphocyte cultures. At least five GTG banded metaphases (minimal 500 band level) were evaluated for each person. Karyotypes were recorded according to the recommendations of the international standing committee on human cytogenetic nomenclature 1995.15 We did not classify individuals with sex chromosome aneuploidy, chromosomal polymorphism, or low level mosaicism as carriers.
Statistical analysis
We tested differences between carrier couples and non-carrier couples with the Student's t test for normally distributed continuous variables, the Mann-Whitney U test for nonparametric continuous variables, and the χ2 test for categorical variables. P values < 0.05 were considered significant. All statistical analyses were performed using SPSS version 11.5.1.
Results
Baseline characteristics
Between January 1992 and January 2001, 11 971 couples were referred for parental chromosome analysis to the six participating centres after two or more miscarriages. A structural chromosome abnormality was found in 382 couples (3.2%). We invited 1148 couples to participate in our study: all 382 carrier couples and 766 non-carrier couples. Of those invited, 61% were eligible for inclusion: 278 couples with a balanced structural chromosome abnormality (73%) and 427 couples with normal parental karyotypes (56%). Reasons for non-participation were exclusion, refusal to participate, non-response, and unknown address.
Table 1 lists the baseline characteristics of the couples. A total of 320 couples (45%) had undergone chromosome analysis after two miscarriages, 263 couples (37%) after three miscarriages, and 122 couples (17%) after four or more miscarriages. The mean duration of follow-up was 5.8 (range 2.0-11.4) years.
Table 1.
Baseline characteristics of couples carrying a structural chromosome abnormality and non-carrier couples referred for parental chromosome analysis after two or more miscarriages. Values are numbers (percentages) of couples unless otherwise indicated
| Carrier couples (n=278) | Non-carrier couples (n=427) | P value | |
|---|---|---|---|
| Maternal age (years) | |||
| Mean (SD) | 31.8 (4.3) | 32.7 (5.0) | |
| Median (interquartile range) | 32 (29-35) | 32 (29-37) | 0.02 |
| Pregnant | 73 (26) | 111 (26) | 0.56 |
| Number of previous miscarriages | |||
| 2 | 108 (39) | 212 (50) | |
| 3 | 111 (40) | 152 (36) | |
| ≥4 | 59 (21) | 63 (15) | 0.01 |
| Mean | 3.0 | 2.8 | <0.01 |
| Number of healthy children | |||
| 0 | 154 (55) | 207 (49) | |
| 1 | 98 (35) | 156 (37) | |
| ≥2 | 26 (9) | 64 (15) | 0.05 |
| Mean | 0.6 | 0.7 | 0.04 |
| Number of handicapped, stillborn, or diseased children | |||
| No previous abnormal offspring | 252 (91) | 384 (90) | |
| ≥1 abnormal offspring | 26 (9) | 43 (10) | 0.75 |
We found significant differences between carrier couples and non-carrier couples. Women who were carriers were younger at the time of chromosome analysis (mean 31.8 v 32.7 years, difference -0.9 years, 95% confidence interval -1.6 to -0.2 years), had experienced more miscarriages (3.0 v 2.8), and had a lower mean number of healthy children (0.6 v 0.7).
The 278 recorded structural chromosome abnormalities consisted of 177 reciprocal translocations (64%), 43 robertsonian translocations (15%), 21 pericentric inversions (8%), 21 paracentric inversions (8%), and 16 other structural chromosome abnormalities (6%). The sex distribution of carriers was unequal: 176 (63%) carriers were women.
Follow-up
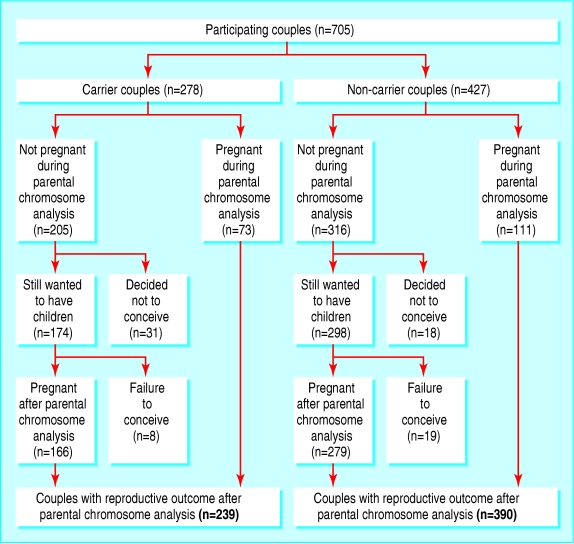
The figure shows the follow-up of all couples entered in our study. After the results of chromosome analysis became available, 49 couples decided not to conceive (31 carrier couples (15%) and 18 non-carrier couples (6%)). In carrier couples the main reasons were the risk of having a child with congenital abnormalities (n = 17) and not wanting to have more miscarriages (n = 11). In non-carrier couples the main reasons were advanced maternal age (n = 10), fear of further miscarriages (n = 5), and other (n = 7).
Pregnancy occurred at least once after chromosome analysis in 239 carrier couples and 390 non-carrier couples. Table 2 shows the outcome of these pregnancies. A significantly greater proportion of carrier couples than non-carrier couples had one or more miscarriage after the analysis (120 of 249, 49% v 122 of 409, 30%; difference 19%, 95% confidence interval 11% to 26%).
Table 2.
Reproductive outcome after parental chromosome analysis in couples with recurrent miscarriage.* Values are numbers (percentages) of couples unless otherwise indicated
| Reproductive outcome | Carrier couples (n=247) | Non-carrier couples (n=409) | Difference in % (95% CI)§ | P value |
|---|---|---|---|---|
| Failure to conceive | 8 (3.2) | 19 (4.6) | −1.4 (−4.4 to 2.0) | 0.38 |
| One or more miscarriages | 120 (48.6) | 122 (29.8) | 18.8 (11.1 to 26.3) | <0. 01 |
| One or more terminated pregnancies | 6 (2.4) | 8 (2.0) | 0.5 (−1.8 to 3.4) | 0.69 |
| One or more ectopic pregnancies | 3 (1.2) | 13 (3.2) | −2.0 (−4.3 to 0.7) | 0.11 |
| One or more stillbirths | 3 (1.2) | 6 (1.5) | −0.3 (−2.1 to 2.2) | 0.79 |
| One or more children who died postpartum | 1 (0.4) | 4 (1.0)† | −0.6 (−2.1 to 1.4) | 0.41 |
| One or more ill or handicapped children | 2 (0.8) | 11 (2.7)‡ | −1.9 (−4.0 to 0.5) | 0.09 |
| One or more healthy children | 205 (83.0) | 344 (84.1) | −1.1 (−7.2 to 4.6) | 0.71 |
Limited to couples who still wanted to conceive after chromosome analysis and those pregnant at the time of chromosome analysis.
One couple with two children who died after birth.
One couple with two ill or handicapped children.
Calculated difference might be different from the crude percentages owing to rounding off of numbers.
The success rate—defined as the birth of a healthy child—was lower in carrier couples than in non-carrier couples for both the first pregnancy and second pregnancy after parental chromosome analysis (table 3). After the second pregnancy the rate of successful pregnancies was not significantly different in the two groups. At least one healthy child was born to 83% of the carrier couples and 84% of the non-carrier couples (difference -1%, -7% to 5%; P = 0.047%), and adverse pregnancy outcomes were similar in the two sets of couples.
Table 3.
Successful reproductive outcome after parental chromosome analysis in couples with two or more miscarriages.* Values are numbers (percentages) of couples unless otherwise indicated
|
Success rate per pregnancy†
|
Cumulative success rate‡
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Carries (n=239) | Non-carriers (n=390) | Difference in % (95% CI) | P value | Carriers (n=247) | Non-carriers (n=409) | Difference in % (95% CI)§ | P value | |
| Pregnancy after chromosome analysis
|
|
|
|
|
|
|
|
|
| 1st | 148/239 (62) | 280/390 (72) | −10 (−18 to −2) | 0.01 | 148 (60) | 280 (68) | −9 (−16 to −1) | <0.01 |
| 2nd | 66/151 (44) | 119/215 (55) | −12 (−22 to −1) | 0.03 | 173 (70) | 324 (79) | −9 (−16 to −2) | <0.01 |
| 3rd | 45/85 (53) | 35/87 (40) | 13 (−2 to 27) | 0.12 | 194 (79) | 332 (81) | −3 (−9 to 4) | 0.41 |
| 4th | 14/40 (35) | 18/48 (38) | −3 (−22 to 17) | 0.63 | 200 (81) | 339 (83) | −2 (−8 to 4) | 0.54 |
| 5th | 10/23 (43) | 6/23 (26) | 17 (−10 to 41) | 0.22 | 205 (83) | 342 (84) | −1 (−7 to 5) | 0.84 |
| 6th | 2/12 (17) | 4/16 (25) | −8 (−36 to 24) | 0.60 | 205 (83) | 342 (84) | −1 (−7 to 5) | 0.84 |
| Total follow-up | — | — | — | 205 (83) | 344 (84) | −1 (−7 to 5) | 0.71 | |
Success rate defined as the birth of at least one healthy child.
Limited to couples with at least one pregnancy after chromosome analysis.
Limited to couples pregnant during chromosome analysis or who still wanted to conceive after the analysis, or both (including couples with failure to conceive after chromosome analysis).
Calculated difference might be different from the crude percentages owing to rounding off of numbers.
Among the carrier couples, 85 of 157 (54%) with reciprocal translocations had one or more miscarriages compared with 18 of 37 (49%) with inversions, 13 of 38 (34%) with robertsonian translocations, and 4 of 15 (27%) with other types of abnormality. Proportions of couples giving birth to one or more healthy child during the follow-up period were similar in the various types of structural chromosome abnormality: 83% (131 of 157) for reciprocal translocations, 82% (31 of 38) for Robertsonian translocations, 78% (29 of 37) for inversions, and 93% (14 of 15) for other abnormalities.
Six pregnancies were terminated in carrier couples: three for social reasons; one because of trisomy 21, not related to the parental structural chromosome abnormality; and two because of an unbalanced karyotype resulting from a structural chromosome abnormality in the carrier. In these last two cases the parental structural chromosome abnormality had been ascertained after two or more miscarriages. In the first of these cases the fetal karyotype was 46,XY,der(18)t(3;18)(q27;p11.1) and the parental karyotype was 46,XY,t(3;18)(q27;p11.1). In the second case the fetal karyotype was 46,XY,der(9)t(3;9)(q25.3;p24) and the parental karyotype was 46,XX,t(3;9)(q25.3;p24).
Figure 1.
Follow-up after chromosome analysis of 705 couples with two or more miscarriages
Three stillbirths occurred in carrier couples after carrier status had been established. In all three cases the couple had not had prenatal diagnosis. In one case the karyotype of the child was not determined after birth. In another case the karyotype was uncertain owing to culture failure. In a third case culture also failed, but comparative genomic hybridisation showed no signs of an unbalanced karyotype. Congenital abnormalities were not found in any of the cases.
Two children with an unbalanced karyotype were born to carrier couples after parental chromosome analysis. In the first case the parental karyotype was 46,XX,t(16;22)(p13;q11.2). The unbalanced chromosome abnormality 46,XY,der(22)t(16;22)(p13;q11.2) was detected when amniocentesis was performed at 19 weeks of gestation. A severely handicapped child with Potter's syndrome and weighing 1500 g was born at 43 weeks; the child died immediately after birth. In the second case, the parents decided to refrain from prenatal diagnosis after the ultrasound scan in the second trimester was normal. The parental karyotype was 46,XX,t(6;8)(q26;q24.1). A child weighing 3900 g who had multiple congenital abnormalities and a 46,XY,der(6)t(6;8)(q26;q24.1) karyotype was born at 38 weeks of gestation.
One child with oesophageal atresia was born to a carrier couple. The karyotype of this child was not established because the abnormality was thought not to be related to the parent's chromosome abnormality.
In total, we found four unbalanced karyotypes: two were detected at prenatal diagnosis and followed by induced abortion, one was detected at prenatal diagnosis but not followed by pregnancy termination, and one was found in a severely handicapped child. All four unbalanced karyotypes resulted from a reciprocal translocation in one of the parents: three resulted from a translocation in the mother and one in the father.
Discussion
The risk of viable offspring with chromosomal abnormalities was low in carrier couples whose carrier status was ascertained after two or more miscarriages. Their chances of having a healthy child were as high as non-carrier couples, despite a higher risk of a subsequent miscarriage.
Comparison with related research
The incidence of structural chromosome abnormalities in our study (3.2%) was at the low end of the range of incidences found in previous studies (3-6%).1-4 This might be because we used restrictive selection criteria for structural chromosome abnormalities, as recommended by the international standing committee on human cytogenetic nomenclature.15 We did not include individuals with a chromosomal polymorphism (such as inversion 9), low level mosaicism, or sex chromosome aneuploidy, abnormalities that are included in many other series describing the incidence of structural chromosome abnormalities in couples with recurrent miscarriage.
In agreement with two recent studies, we found that the birth of a healthy child at first pregnancy after chromosome analysis was lower in carrier couples (59%) than non-carrier couples (72%). Carp et al found that a parental chromosomal abnormality decreased the chance of a live birth in the subsequent pregnancy: 45% of pregnancies in 73 carrier couples compared with 55% of pregnancies in 588 non-carrier couples, although this decrease was not significant.8 Sugiura-Ogasawara et al reported a significantly increased rate of miscarriage in the first pregnancy after chromosome analysis: 52% in 49 carrier couples compared with 28% in 1184 non-carrier couples.9
Limitations
Out of a total of 550 pregnancies after parental chromosome analysis in couples whose carrier status was ascertained after recurrent miscarriage, only two cases of viable offspring with chromosomal abnormalities were detected at prenatal diagnosis (0.4%) after which the pregnancies were terminated. In two other cases severely handicapped children with an unbalanced structural chromosome abnormality were born (0.4%). Even though the response rate among carrier couples was good (73%), a selection bias could have occurred: couples with viable offspring with unbalanced chromosome abnormalities may have been more likely to refuse to participate in our study, thus leading to an under-representation of such abnormalities.
Implications
The two earlier studies had small numbers of carrier couples and limited their observations to the pregnancy immediately after parental chromosome analysis.8,9 We recorded successive pregnancy outcomes during a long follow-up period (mean duration of 5.8 years) to obtain more accurate information on long term reproductive outcome. In our cohort, 83% of the carrier couples and 84% of the non-carrier couples gave birth to at least one healthy child after chromosome analysis; this finding could have implications for the counselling of couples with recurrent miscarriage due to chromosome abnormalities. However, a subgroup of women who repeatedly miscarry (four or more miscarriages) may have a worse prognosis because other factors might contribute to their miscarriages.
Currently, counselling couples about their risk of having a child with an unbalanced karyotype is based mainly on empirical risk estimates or databases that lack exact data on reproductive history or outcome, or both.16-18 The risk of viable offspring with chromosomal abnormalities depends on the chromosome segment involved, the sex of the carrier parent, and the mode of ascertainment. In general, carrier couples ascertained after the birth of an affected child are at the highest risk of having viable offspring with chromosomal abnormalities (20-22%), whereas couples ascertained after recurrent miscarriage have an estimated risk of 2% to 5% (derived from data obtained by prenatal diagnosis after parental chromosome analysis).19,20
In our cohort, less than 2% of carrier couples had viable offspring with unbalanced chromosomal abnormalities: two cases were detected at prenatal diagnosis after which the pregnancies were terminated (0.4%) and two severely handicapped children (0.4%) were born. In the 278 carrier couples in our study, structural chromosome abnormalities more commonly resulted in miscarriage rather than viable offspring with unbalanced chromosomal abnormalities. However, more than 10% of carrier couples decided not to conceive after parental chromosome analysis, so there may be a case for changing the guidance to these couples.
Preimplantation genetic diagnosis has been proposed as an option to reduce the occurrence of offspring with chromosomal abnormalities in carrier couples and further miscarriages in carrier couples with recurrent miscarriage, although its efficiency has not yet been established.21,22 Our findings of the good reproductive outcome in these couples bring into question whether an assisted reproductive technique is desirable. Preimplantation genetic diagnosis is an expensive intervention, which requires an in vitro fertilisation procedure and therefore bears the risk of serious complications.
Conclusion
The risk of viable offspring with chromosomal abnormalities is low in carrier couples whose carrier status was ascertained after two or more miscarriages. Their chances of having a healthy child are as high as non-carrier couples, despite a higher risk of a subsequent miscarriage. The more accurate risk information provided by our study should help carrier couples when deliberating between the risk of another miscarriage, a handicapped child, and the chance of a healthy child.
What is already known on this topic
Couples who carry structural chromosome abnormalities, whose carrier status is ascertained after recurrent miscarriage, are at risk of having a child with severe congenital abnormalities
What this study adds
The risk of viable offspring with chromosomal abnormalities is low in carrier couples whose carrier status is ascertained after two or more miscarriages
Their chances of having a healthy child are as high as for non-carrier couples (over 80%), but they have a higher risk of a subsequent miscarriage
The more accurate risk information provided by our study should help carrier couples when deliberating between the risk of another miscarriage, a handicapped child, and the chance of a healthy child
We thank the clinical cytogeneticists from the participating departments of clinical genetics for supplying data: AC Knegt, Academic Medical Centre, Amsterdam; K Madan, V U Medical Centre, Amsterdam; R Hochstenbach, University Medical Centre, Utrecht; KBJ Gerssen-Schoorl, University Medi-cal Centre, Groningen; CH Wouters, Erasmus Medical Centre, Rotterdam; and KBM Hansson, Leiden University Medical Centre. We also thank A Melim, research nurse, for her participation and collection of data.
Contributors: FvdV, NJL, and PMMB were the principal investigators and designed the protocol. MG was the coordinating investigator and supervised its progress. MTMF collected the data. MTMF and JCK did the statistical analysis. MTMF wrote the initial draft and all authors took part in the further preparation of the paper. MG is the guarantor.
Funding: Supported by ZonMW, the Netherlands Organisation for Health Research and Development (945-02-032).
Competing interests: None declared.
Ethical approval: Review board of University of Amsterdam.
References
- 1.Tharapel AT, Tharapel SA, Bannerman RM. Recurrent pregnancy losses and parental chromosome abnormalities: a review. Br J Obstet Gynaecol 1985;92: 899-914. [DOI] [PubMed] [Google Scholar]
- 2.Braekeleer M de, Dao TN. Cytogenetic studies in couples experiencing repeated pregnancy losses. Hum Reprod 1990;5: 518-28. [DOI] [PubMed] [Google Scholar]
- 3.Clifford K, Rai R, Regan L. An informative protocol for the investigation of recurrent miscarriage: preliminary experience of 500 consecutive cases. Hum Reprod 1994;9: 1328-32. [DOI] [PubMed] [Google Scholar]
- 4.Franssen MT, Korevaar JC, Leschot NJ, Bossuyt PM, Knegt AC, Gerssen-Schoorl KB, et al. Selective chromosome analysis in couples with two or more miscarriages. BMJ 2005;331: 137-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutch Society of Obstetricians and Gynecologists. Habitual abortion. Utrecht: NVOG, 1999. Guideline no. 20.
- 6.American College of Obstetricians and Gynecologists. Management of recurrent early pregnancy loss. ACOG practice bulletin. Int J Gynaecol Obstet 2002;78: 179-90. [DOI] [PubMed] [Google Scholar]
- 7.Royal College of Obstetricians and Gynecologists. The investigation and treatment of couples with recurrent miscarriage. London: RCOG, 2003. Guideline no. 17.
- 8.Carp H, Feldman B, Oelsner G, Schiff E. Parental karyotype and subsequent live births in recurrent miscarriage. Fertil Steril 2004;81: 1296-301. [DOI] [PubMed] [Google Scholar]
- 9.Sugiura-Ogasawara M, Ozaki Y, Sato T, Suzumori N, Suzumori K. Poor prognosis of recurrent aborters with either maternal or paternal reciprocal translocations. Fertil Steril 2004;81: 367-73. [DOI] [PubMed] [Google Scholar]
- 10.FitzSimmons J, Jackson D, Wapner R, Jackson L. Subsequent reproductive outcome in couples with repeated pregnancy loss. Am J Med Genet 1983;16: 583-7. [DOI] [PubMed] [Google Scholar]
- 11.Sachs ES, Jahoda MG, Van Hemel JO, Hoogeboom AJ, Sandkuyl LA. Chromosome studies of 500 couples with two or more abortions. Obstet Gynecol 1985;65: 375-8. [PubMed] [Google Scholar]
- 12.Fortuny A, Carrio A, Soler A, Cararach J, Fuster J, Salami C. Detection of balanced chromosome rearrangements in 445 couples with repeated abortion and cytogenetic prenatal testing in carriers. Fertil Steril 1988;49: 774-9. [DOI] [PubMed] [Google Scholar]
- 13.Portnoï MF, Joye N, van den AJ, Morlier G, Taillemite JL. Karyotypes of 1142 couples with recurrent abortion. Obstet Gynecol 1988;72: 31-4. [PubMed] [Google Scholar]
- 14.Goddijn M, Joosten JH, Knegt AC, Veen F van der, Franssen MT, Bonsel GJ, et al. Clinical relevance of diagnosing structural chromosome abnormalities in couples with repeated miscarriage. Hum Reprod 2004;19: 1013-7. [DOI] [PubMed] [Google Scholar]
- 15.ISCN 1995: recommendations of the international standing committee on human cytogenetic nomenclature. Basel: Karger, 1995.
- 16.Stene J, Stengel-Rutkowski S. Genetic risks of familial reciprocal and robertsonian translocation carriers. In: Daniel A, ed. The cytogenetics of mammalian autosomal rearrangements. New York: Alan R Liss, 1988: 3-72.
- 17.Barisic I, Zergollern L, Muzinic D, Hitrec V. Risk estimates for balanced reciprocal translocation carriers—prenatal diagnosis experience. Clin Genet 1996;49: 145-51. [DOI] [PubMed] [Google Scholar]
- 18.Cans C, Cohen O, Lavergne C, Mermet MA, Demongeot J, Jalbert P. Logistic regression model to estimate the risk of unbalanced offspring in reciprocal translocations. Hum Genet 1993;92: 598-604. [DOI] [PubMed] [Google Scholar]
- 19.Boue A, Gallano P. A collaborative study of the segregation of inherited chromosome structural rearrangements in 1356 prenatal diagnoses. Prenat Diagn 1984;4: 45-67. [DOI] [PubMed] [Google Scholar]
- 20.Daniel A, Hook EB, Wulf G. Risk of unbalanced progeny at amniocentesis to carriers of chromosome rearrangements: data from United States and Canadian laboratories. Am J Med Genet 1989;33: 14-53. [DOI] [PubMed] [Google Scholar]
- 21.Munne S, Sandalinas M, Escudero T, Fung J, Gianaroli L, Cohen J. Outcome of preimplantation genetic diagnosis of translocations. Fertil Steril 2000;73: 1209-17. [DOI] [PubMed] [Google Scholar]
- 22.Carp HJA, Dirnfeld M, Dor J, Grudzinskas JG. ART in recurrent miscarriage: preimplantation genetic diagnosis/screening or surrogacy? Hum Reprod 2004;19: 1502-5. [DOI] [PubMed] [Google Scholar]